Abstract
The effects of four elicitors, including 100 μmol/l MeJA (methyl jasmonate), 40 μl/l hydrogen peroxide (30%, w/w), 80 mg/l SA (salicylic acid) and 0.4 g/l F3 (fungal elicitor), on suspension cultures of Taxus cuspidata were studied. After addition of the above four elicitors, the enzyme activity of 10-DBAT (10-deacetylbaccatin III-10-O-acetyltransferase) was induced and reached its maximum of 5.47, 0.97, 3.30 and 6.82 U, respectively. After elicitation, the concentrations of cytochrome P450 monooxygenase were also induced to its maximum values of 0.069, 0.336, 0.321 and 0.193 nmol/ml, respectively. In addition, under the elicitation, the change in 10-DBAT activity was similar to that of cytochrome P450 monooxygenase concentration. The products of these two enzymes changed after the variety of the enzymes, and the taxol content increased through the cultivation.
Keywords: 10-deacetylbaccatin III, 10-deacetylbaccatin III-10-O-acetyl transferase, baccatin III, cytochrome P450 monooxygenase, Taxol, Taxus cuspidata
Abbreviations: 6-BA, 6-benzylaminopurine; 10-DAB III, 10-deacetylbaccatin III; 10-DBAT, 10-deacetylbaccatin III-10-O-acetyltransferase; DTT, dithiothreitol; DW, dry weight; F3, fungal elicitor; GGPP, geranylgeranyl diphosphate; MeJA, methyl jasmonate; SA, salicylic acid
1. Introduction
Taxol (paclitaxel) is one of the most effective antineoplastic drugs currently available and is used to treat a range of cancers (Wani et al., 1971). Plant cell culture is a promising method to produce taxol and related taxane compounds (Gibson et al., 1993). After 17 years study, the productivity of Taxus cell culture has been increased, but the content of taxol in the cells is still too low to reach industrial application. Also, the production of the taxol produced by Taxus cell culture on a large scale still has some engineering problems, such as the contamination of the Taxus cells by bacteria during long-term cultivation. To solve these problems, the establishment of Taxus cell cultivation with rapid cell growth rate and high yield of the products is needed.
In plant cell culture, the addition of precursors and elicitors plays an important role in enhancing the secondary metabolism of cells (Dornenburg and Knorr, 1995). It has been reported that elicitation using biotic and abiotic elicitors is one of the most effective methods to enhance the production or accelerate the synthesis of taxol in Taxus cells, such as MeJA (methyl jasmonate), SA (salicylic acid), hydrogen peroxide and fungal extract (Stierle et al., 1993; Ciddi et al., 1995; Yukimune et al., 1996; Yu et al., 2001).
The biosynthesis pathway of Taxol consists of the synthesis of taxane tricyclic diterpene core and N-debenzoyl phenylisoserine side chain. Taxol core derives from the cyclation and the modification of GGPP (geranylgeranyl diphosphate) synthesized from four IPP (isopentenyl diphosphate) molecules through the function of GGPP synthase (Guo et al., 2006). In this pathway, several cytochrome P450 monooxygenases and acetyltransferases are involved in the biosynthesis of taxol core, and 10-DBAT (10-deacetylbaccatin III-10-O-acetyl transferase), which functions in the last step of Taxol core synthesis is the most important enzyme (Frense, 2007). At present, the gene encoding 10-DBAT has been cloned and expressed in Escherichia coli (Walker and Croteau, 2000a), and the molecular weight of this enzyme is about 49 kDa. The specificity of 10-DBAT has been proven by the substrate competition reaction of crude enzyme extracted from Taxus media.
MeJA, SA, hydrogen peroxide and fungal extract have been reported to be capable of inducing the production of taxol (Stierle et al., 1993; Ciddi et al., 1995; Yukimune et al., 1996; Yu et al., 2001), but the mechanisms of these elicitors have not been completely understood. Especially, little has been reported on the relation between the above elicitors and the activity of cytochrome P450 monooxygenases or acetyltransferases. As mentioned above, 10-DBAT, which catalyses the formation of the important diterpene intermediate of baccatin III in the taxol biosynthesis pathway, and cytochrome P450 monooxygenase, which is also involved in the synthesis of taxol intermediate, are two important enzymes in the biosynthesis of the taxol (Walker and Croteau, 2000b). In order to improve the productivity of taxol by Taxus baccata cell cultures, the effects of MeJA, SA, hydrogen peroxide and fungal extract on the activity of 10-DBAT, the content of cytochrome P450 monooxygenase, biosynthesis of baccatin III, 10-DAB III (10-deacetylbaccatin III) and the production of Taxol were investigated in this study.
2. Materials and methods
2.1. Cells and culture conditions
T5 strain of the Taxus cuspidata cell line was established by our laboratory. Solid and liquid modified SSS media (pH 5.6–5.8) were prepared. The solid medium consisting of 1 mg/l NAA (α-naphthalene acetic acid), 0.5 mg/l 6-BA (6-benzylaminopurine), 10 mg/l vitamin B1, 30 g/l sucrose and 2 g/l gellan gum was used for cell growth and subculture. For solid medium cultivation, 2 g fresh cells were inoculated in 250-ml flasks containing 30 ml solid media and cultivated at 25°C in the dark for 28 days. The liquid medium consisted of 1 mg/l NAA, 0.5 mg/l 6-BA, 30 g/l sucrose and elicitors for the biosynthesis of taxol. The inoculated cells in the liquid medium were cultivated at 25°C in dark on a shaker at 100 rev./min for 4 days.
2.2. Induction by MeJA, SA and hydrogen peroxide
MeJA, SA and hydrogen peroxide (30%, w/w) were added to 50 ml sterilized liquid SSS medium containing 5 g subcultured fresh cells. The combination of the elicitors in the medium was prepared with 100 μmol/l MeJA, 80 mg/l SA and 40 μl/l hydrogen peroxide. These concentrations were confirmed to be the respective optimal ones when separately used in the previous experiments.
2.3. Induction by F3
The fungal strain was screened from endophytic fungi of yew bark by our lab and was named F3. For the preparation of F3, 0.56 g mycelium was scraped from the solid medium and added to a small quantity of distilled water. The mycelium solution was autoclaved at 125°C for 15 min and then added to 1.4 l plant cell culture medium. F3 concentration was 0.4 g/l, which was an optimum concentration confirmed in the previous experiments.
2.4. Extraction of crude enzymes
The extraction method was modified based on that described by Zocher et al. (1996) and Walker et al. (2000). The lyophilized cells (∼1 g) were pulverized in a mortar and then extracted with 20 ml of 30 mmol/l Hepes buffer (pH 7.4) containing 3 mmol/l DTT (dithiothreitol) and 0.8 g cross-linked PVP (polyvinylpyrrolidone). The mixture was slowly ground for 30 min and then filtered through four layers of gauze to remove the solids. The filtrate was centrifuged at 1500 rev./min at 4°C for 30 min to remove the cell debris then at 15,000 rev./min (15580 g) at 4°C for 75 min. The supernatant was followed by filtration through 0.45 μm nylon membrane, and the filtrate was desalted using a Sephadex G-25 column, which was previously equilibrated with the same buffer (without DTT). The desalinated solution was used as the crude enzyme for the activity assay. All the extraction procedures were carried out at 4°C.
2.5. Analysis
2.5.1. Cell concentration
The cell concentration was measured by dry weight. For this measurement, cells were harvested and washed with distilled water three times to remove the residual medium, then lyophilized to constant weight using a vacuum-freeze drier.
2.5.2. Taxanes content
To extract taxanes from the cells, the lyophilized cells (∼50 mg) were dissolved in 10 ml methanol and stirred at 120 rev./min in the dark for 24 h at room temperature and then filtered. This operation was repeated twice, and all the extracts were collected together. After the methanol in the extracts was evaporated, the residue was extracted with 20 ml distilled water and 20 ml methylene chloride, and the organic phase was then collected. This operation was repeated twice, and the organic fractions were then mixed together. The methylene chloride was evaporated, and the remaining taxanes were re-dissolved in 5 ml methanol.
Taxanes in the above sample were detected by HPLC (Shimadzu 10Avp) with a Shim-pack CLC-phenyl column and UV detector at 227 nm, using a mobile phase of methanol/acetonitrile/water (20:33:47 by volume). The elution rate was 1 ml/min. The Taxol standard was obtained from the Chinese Medical Institute, baccatin III and 10-DAB III standards were purchased from Sigma.
2.5.3. 10-DBAT enzyme assay
10-DBAT activity was measured by the modified method described by Zocher et al. (1996) and Walker et al. (2000). For the cell-free acetylation experiments, 100 μl of the above crude enzyme was mixed with 400 μl Hepes buffer containing 5 mmol/l MgCl2, 20 μmol/l 10-DAB III and 2 μl vinyl acetate. After incubation for 1 h at 25°C, the reaction was terminated by adding 2 ml distilled water, and the mixture was extracted with 2 ml of chloroform twice. The organic phase was evaporated, and the residue was dissolved in 2 ml methanol (HPLC grade). The authenticity of the enzymatic products was detected by HPLC. One unit (U) of 10-DBAT activity was defined as the amount of the enzyme that converted 1 nmol 10-DAB III into baccatin III per hour under the above conditions.
2.5.4. Cytochrome P450 monooxygenase
The cytochrome P450 monooxygenase content was analysed according to the method of Omura and Sato (1964). Protein quantification was determined by Bradford's method using bovine albumin fraction V as the standard.
3. Results and discussion
3.1. Effect of MeJA on the enzyme and production of taxanes during the cell cultivation
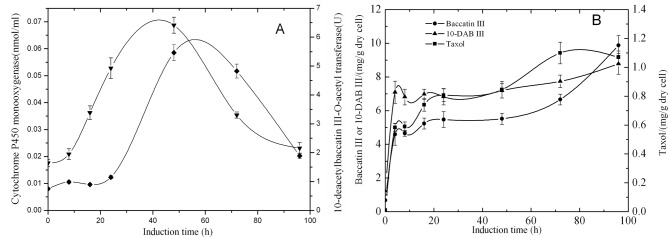
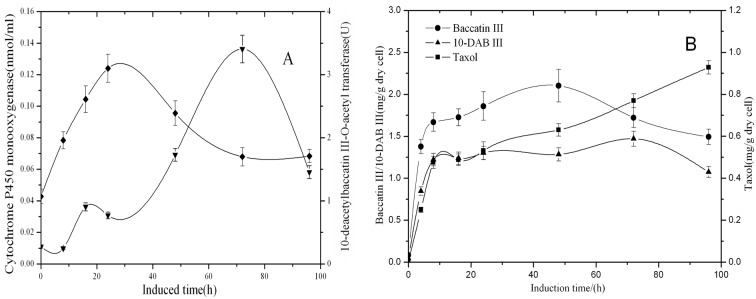
MeJA was an important elicitor found to increase taxol production in Taxus cell cultures effectively (Mirjalili and Linden, 1996; Yukimune et al., 1996; Wang and Wu, 2005). Our previous experiment showed that 100μM MeJA was the optimum concentration for elicitation of taxol production. The results of the enzyme activity of 10-DBAT and contents of cytochrome P450 monooxygenase, taxol, baccatin III and 10-DAB III after the addition of 100 μmol/l MeJA into the culture are shown (Figures 1A, 1B). After inoculating the cells into the induction medium, the concentration of cytochrome P450 monooxygenase increased quickly on the addition of MeJA. At the 48th hour, it reached maximal value and then decreased quickly. At the beginning of the experiment, the activity of 10-DBAT increased slowly. The enzyme activity of 10-DBAT began to increase rapidly at the 24th hour and reached a maximum of 5.47 U at the 48th hour, then decreased to near the level before the elicitation at the 96th hour (Figure 1A).
Figure 1. Time course of enzyme activity of 10-DBAT (♦), the concentration of cytochrome P450 monooxygenase (▾), the contents of 10-DAB III (▾), baccatin III (•) and Taxol (▪) after the induction with 100 μM MeJA, in T. cuspidata cell suspension culture.
Samples were taken 0, 8, 16, 24, 48, 72 and 96 h after the induction. Values are means of triplicate results and error bars represent the S.D.
The contents of taxol, baccatin III and 10-DAB III changed similarly after the addition of MeJA (Figure 1B). At the fourth hour after the inoculation, the contents of taxol, baccatin III and 10-DAB III accumulated quickly, which was related to the strong induction effect of MeJA, then the content of taxol continued to increase, but the accumulation of baccatin III and 10-DAB III increased slowly. After 72 h, taxol accumulated rapidly and reached a maximum of 1.1 mg/g [DW (dry weight)], then started to decline. The contents of baccatin III and 10-DAB III continued to increase until the 96th hour, which might be caused by the decline of the content of taxol.
This result revealed that the addition of MeJA first affected cytochrome P450 monooxygenase, which pertained to the biosynthesis of 10-DAB III and stimulated the formation of 10-DAB III. The result also showed that 10-DAB III accoumulated rapidly, but then tended to be stable, which was related with its transformation to taxol and baccatin. In the first 4 h of the experiment, although the activity of 10-DBAT was low, the content of baccatin III showed a rapid accumulation, which might be due to the high catalytic activity of 10-DBAT. It can be seen that the contents of cytochrome P450 monooxygenase and the activity of 10-DBAT reach their maximum value at the 48th hour, but at that moment the accumulation of three taxanes does not achieve its highest value, which shows the lag effect between the metabolite accumulation and the contents or the activities of related enzymes (Figure 1A).
In contrast, although the enzyme activity of 10-DBAT and the concentration of cytochrome P450 monooxygenase without induction also trended to increase, it was still on a quite low level. The corresponding contents of 10-DAB III and baccatin III had a slow upward trend, indicating a relevant relationship. Synthesis of taxol in 24 h and 72 h declined, which was the same as the enzyme activity of 10-DBAT. This kind of relationship indicated that the enzyme activity of 10-DBAT was closely related to taxol synthesis. In addition, the three metabolites reached the maximal value at the 72th hour and then declined. This trend was related to the activities of the two enzymes.
3.2. Effect of hydrogen peroxide on the enzyme and production of taxanes
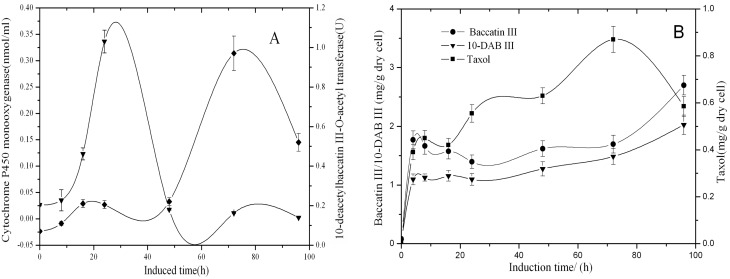
Hydrogen peroxide is one of the signal molecules in plant defense responses (Scheel, 1998). A previous experiment showed that taxol content in T. cuspidata cell suspension culture was also increased with the addition of hydrogen peroxide. The results of hydrogen peroxide elicitation during the cell culture are shown (Figures 2A, 2B). After the addition of hydrogen peroxide, the concentration of cytochrome P450 monooxygenase increased significantly and reached its highest value of 0.336 nmol/ml at the 24th hour and then decreased quickly. 10-DBAT had a slow increase in the first 16 h and, after that, remained almost constant until the 48th hour and then increased quickly and reached a maximum of 0.97 U at the 72th hour.
Figure 2. Time course of enzyme activity of 10-DBAT (♦), the concentration of cytochrome P450 monooxygenase (▾), the contents of 10-DAB III (▾), baccatin III (•) and taxol (▪) after the induction with 40 μl/l hydrogen peroxide (30%, w/w) in T. cuspidata cell suspension culture.
Samples were taken 0, 8, 16, 24, 48, 72 and 96 h after the induction. Values are means of triplicate results, and error bars represent the S.D.
In the first 4 h after inoculation, the contents of 10-DAB III, baccatin III and taxol showed a rapid accumulation. The content of Taxol increased continuously and reached the maximum value at the 72nd hour. The contents of 10-DAB III and baccatin III increased quickly after 72 h. As 10-DAB III and baccatin III were the direct precursors of taxol, and the synthesis of taxol declined after 72 h, therefore, 10-DAB III and baccatin III started to accumulate and increased after 72 h. The accumulation of taxol is faster than 10-DAB and baccatin, possibly due to the latter two being transformed into taxol (Figure 2).
It can be seen that the concentration of cytochrome P450 monooxygenase increases quickly and reaches the maximal value at the 24th hour and then declines significantly, which may be concerned with the oxidative burst of hydrogen peroxide (Figures 2A, 2B). The content change of Taxol is similar to the activity of 10-DBAT; they all reach their peak value at the 72nd hour and then decrease. 10-DBAT was the key enzyme in the synthesis of baccatin III, which was the direct precursor of taxol, so the content of taxol was indirectly related with the activity of 10-DBAT.
3.3. Effect of SA on the enzyme and production of taxanes
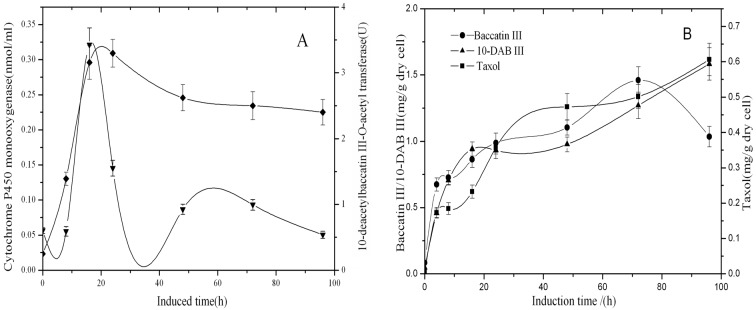
SA was added as an elicitor to improve Taxol production (Yu et al., 2001; Wang et al., 2007). Our previous experiment showed that the optimal concentration of SA was 80 mg/l. The results of the SA elicitation experiment is shown (Figures 3A, 3B). After the addition of SA, both cytochrome P450 monooxygenase and 10-DBAT immediately began to increase rapidly and reached the respective maximal values of 0.321 nmol/ml and 3.30 U at the 16th and 24th hours, respectively. After that, both of them started to decline, and cytochrome P450 monooxygenase declined more quickly.
Figure 3. Time course of enzyme activity of 10-DBAT (♦), the concentration of cytochrome P450 monooxygenase (▾), the contents of 10-DAB III (▾), baccatin III (•) and taxol (▪) after the induction with 80 mg/l SA in T. cuspidata cell suspension culture.
Samples were taken 0, 8, 16, 24, 48, 72 and 96 h after the induction. Values are means of triplicate results, and error bars represent the S.D.
Meanwhile, baccatin III, 10-DAB III and taxol accumulated after the elicitation, and the content of baccatin III reached a maximum at the 72nd hour, while the content of 10-DAB III continued to increase during the whole cultivation. The content of Taxol increased continuously like 10-DAB III and reached 0.81 mg/g (DW) at the 96th hour after elicitation. The decrease of baccatin III content after 72 h was attributed to the lower activity of 10-DBAT and was also related to the biosynthesis of taxol.
Similar to hydrogen peroxide, SA was also a strong oxidant. Owing to the stress effect, it can stimulate the expression of these two enzymes and promote the synthesis of these three metabolites. Because of its shorter effect than hydrogen peroxide, its ability to promote metabolite synthesis was limited.
3.4. Effect of fungal elicitor F3 on the enzyme and production of taxanes
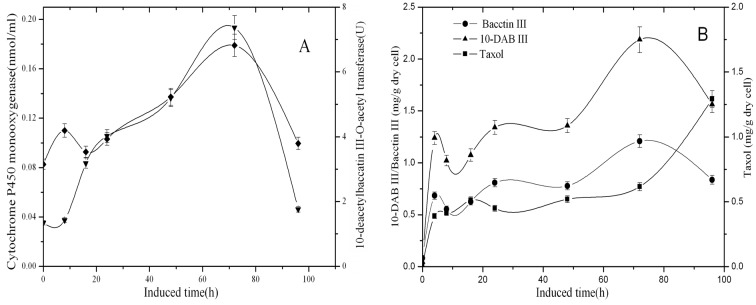
It has been reported that elicitation induced by F3 is one of the most powerful ways to enhance the taxol production in Taxus cells (Qin and Lan, 2004). In the present work, F3 was originally isolated from the inner bark of T. cuspidata by our lab. The effect of 0.4 g/l F3 on T. cuspidata cell suspension culture is shown (Figures 4A, 4B). Both cytochrome P450 monooxygenase and 10-DBAT began to increase quickly after the fungal extract was added into the medium and reached the maximum of 0.193 nmol/ml and 6.82 U by 72 h, respectively.
Figure 4. Time course of enzyme activity of 10-DBAT (♦), the concentration of cytochrome P450 monooxygenase (▾), the contents of 10-DAB III (▾), baccatin III (•) and taxol (▪) after the induction with fungal elicitor F3 in T. cuspidata cell suspension culture.
Samples were taken 0, 8, 16, 24, 48, 72 and 96 h after the induction. Values are means of triplicate results, and error bars represent the S.D.
The content change trends of baccatin III and 10-DAB III were similar to the changes in the enzyme activity and concentration; the contents reached the peak values by 72 h and, thereafter, decreased. The content of taxol increased continuously and reached 1.72 mg/g (DW) by 96 h after elicitation. This trend was related to the changes of both cytochrome P450 monooxygenase and 10-DBAT, which indicated a relationship with the synthesis of baccatin III and 10-DAB III. Owing to the complexity of elicitation of fungus, the change trend of taxol was different with those of MeJA, hydrogen peroxide and SA. The culture results demonstrated that elicitor-induced taxol production was closely related to the plant defense response (Yu et al., 2001). It was possible that different elicitor signals induced different parts of the defense response (Zhang et al., 2000), and some generalities existed in the defense response signal transmission pathway induced by these elicitors. Accordingly, addition of fungal F3 elicitation may activate the defense system of Taxus cells, therefore, it promoted taxol synthesis.
3.5. Effect of combination of elicitors on the enzyme and production of taxanes
The effects of the combination of the elicitors of MeJA, SA and hydrogen peroxide on the enzyme and production of taxanes (Khosroushahi et al., 2006) are shown (Figures 5A, 5B). Though the concentration of cytochrome P450 monooxygenase changed similarly to those when a single elicitor of MeJA and F3 was added, the absolute value was lower. The activity of 10-DBAT reached a maximum value by 24 h ,which was similar to the SA elicitation, and also the absolute value of the combination of the elicitors was lower than those under the elicitation of MeJA and F3. This may be because of the offset effect when blending several elicitors.
Figure 5. Time course of enzyme activity of 10-DBAT (♦), the concentration of cytochrome P450 monooxygenase (▾), the contents of 10-DAB III (▾), baccatin III (•) and taxol (▪) after the induction of combination of MeJA, SA and hydrogen peroxide elicitor in T. cuspidata cell suspension culture.
Samples were taken 0, 8, 16, 24, 48, 72 and 96 h after the induction. Values are means of triplicate results, and error bars represent the S.D.
The prompt enhancement of the activities of cytochrome P450 monooxygenase and 10-DBAT led to the rapid accumulation of 10-DAB III and baccatin III within 8 h. After reaching the peak value, the subsequent decrease in the contents of 10-DAB III and baccatin III was also attributed to the lowered activities of cytochrome P450 monooxygenase and 10-DBAT. The content of taxol increased continuously after the induction and reached a maximum by 96 h. This phenomenon showed the opposite trend between taxol and 10-DAB III or baccatin III. In addition, the combination of elicitors did not reach the expected objective from the results, which may be due to the amount of elicitors added to the culture medium, and a combination of the elicitors always had more strong stress effect than a single elicitor. If the amount of each elicitor was reduced, the results may be more ideal.
According to the above results, the different effects of the four elicitors on 10-DBAT and cytochrome P450 monooxygenase were detected, which suggested that the induction pattern of the four elicitors may be different. It may conclude that induction of taxol production and activation of 10-DBAT and cytochrome P450 monooxygenase by elicitors might involve two different signalling pathways; also, it would be helpful to understand the elicitation mechanism and the combination of elicitors to enhance Taxol production in the cell suspension cultures.
In addition, the experimental results showed that, in the process of Taxus cell culture without any elicitors, the concentration of cytochrome P450 monooxygenase and the maximum activity of 10-DBAT was 0.03 nmol/ml and 0.2 U (data not shown) respectively, and baccatin III and Taxol were not detected. Therefore, in the process of Taxus cell culture, adding elicitors was extremely important for the expression of cytochrome P450 monooxygenase and the 10-DBAT and the synthesis of taxol.
4. Conclusion
The four elicitors applied in this study can increase activities of 10-DBAT and cytochrome P450 monooxygenase and promote taxol synthesis. F3 was the best elicitor, and then MeJA, hydrogen peroxide and the combination of elicitor and SA. The synthesis of taxol closely correlated with the activities of 10-DBAT and cytochrome P450 monooxygenase.
Acknowledgements
We thank Mr Ruiqiang Sun for his technical support and helpful discussion for the preparation of this manuscript and Professor Xin-Hui Xing of Tsinghua University for his valuable comments on the manuscript.
Footnotes
We acknowledge support from the 11th Five-Year Plan [grant number 2008BAI63 800].
Author contribution
The research was completed under the guidance of Zhi-Gang Guo. The majority of the experiments were performed by Jian-Feng Zhang, and Shan Gong wrote the manuscript.
References
- Ciddi V, Srinivasan V, Shuler ML. Elicitation of Taxus sp cell cultures for production of taxol. Biotechnol Lett. 1995;17:1343–46. [Google Scholar]
- Dornenburg H, Knorr D. Strategies for the improvement of secondary metabolite production in plant-cell cultures. Enzyme Microb Technol. 1995;17:674–84. [Google Scholar]
- Frense D. Taxanes: perspectives for biotechnological production. Appl Microbiol Biotechnol. 2007;73:1233–40. doi: 10.1007/s00253-006-0711-0. [DOI] [PubMed] [Google Scholar]
- Gibson DM, Ketchum REB, Vance NC, Christen AA. Initiation and growth of cell lines of Taxus brevifolia (Pacific yew). Plant Cell Rep. 1993;12((9)):479–82. doi: 10.1007/BF00236091. [DOI] [PubMed] [Google Scholar]
- Guo BH, Kai Y, Jin HB, Tang KX. Taxol synthesis. African J Biotechnol. 2006;5:15–20. [Google Scholar]
- Khosroushahi AY, Valizadeh M, Ghasempour A, Khosrowshahli M, Naghdibadi H, Dadpour MR, Omidi Y. Improved Taxol production by combination of inducing factors in suspension cell culture of Taxus baccata. Cell Biol Int. 2006;30:262–69. doi: 10.1016/j.cellbi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mirjalili N, Linden JC. Methyl jasmonate induced production of taxol in suspension cultures of Taxus cuspidata: ethylene interaction and induction models. Biotechnol Prog. 1996;12:110–18. doi: 10.1021/bp9500831. [DOI] [PubMed] [Google Scholar]
- Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes II: solubilization, purification, and properties. J Biol Chem. 1964;239:2379–85. [PubMed] [Google Scholar]
- Qin WM, Lan WZ. Fungal elicitor-induced cell death in Taxus chinensis suspension cells is mediated by ethylene and polyamines. Plant Sci. 2004;166:989–95. [Google Scholar]
- Scheel D. Resistance response physiology and signal transduction. Curr Opin Plant Biol. 1998;1:305–10. doi: 10.1016/1369-5266(88)80051-7. [DOI] [PubMed] [Google Scholar]
- Stierle A, Strobel G, Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of pacific yew. Science. 1993;260:214–16. doi: 10.1126/science.8097061. [DOI] [PubMed] [Google Scholar]
- Walker K, Croteau R. Molecular cloning of a 10-deacetylbaccatin III-10-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Proc Natl Acad Sci USA. 2000a;97:583–87. doi: 10.1073/pnas.97.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K, Croteau R. Taxol biosynthesis: molecular cloning of a benzoyl-CoA: taxane 2 alpha-O-benzoyltransferase cDNA from Taxus and functional expression in Escherichia coli. Proc Natl Acad Sci USA. 2000b;97:13591–96. doi: 10.1073/pnas.250491997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K, Schoendorf A, Croteau R. Molecular cloning of a taxa-4(20),11(12)-dien-5 alpha-ol-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Arch Biochem Biophys. 2000;374:371–80. doi: 10.1006/abbi.1999.1609. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wu JY. Nitric oxide is involved in methyl jasmonate-induced defense responses and secondary metabolism activities of Taxus cells. Plant Cell Physiol. 2005;46:923–30. doi: 10.1093/pcp/pci098. [DOI] [PubMed] [Google Scholar]
- Wang YD, Wu JC, Yuan YJ. Salicylic acid-induced taxol production and isopentenyl pyrophosphate biosynthesis in suspension cultures of Taxus chinensis var. mairei. Cell Biol Int. 2007;31:1179–83. doi: 10.1016/j.cellbi.2007.03.038. [DOI] [PubMed] [Google Scholar]
- Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. Isolation and structure of Taxol a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–27. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- Yu LJ, Lan WZ, Qin WM, Xu HB. Effects of salicylic acid on fungal elicitor-induced membrane-lipid peroxidation and taxol production in cell suspension cultures of Taxus chinensis. Process Biochem. 2001;37:477–82. [Google Scholar]
- Yukimune Y, Tabata H, Higashi Y, Hara Y. Methyl jasmonate-induced overproduction of paclitaxel and baccatin III in Taxus cell suspension cultures. Nat Biotechnol. 1996;14:1129–32. doi: 10.1038/nbt0996-1129. [DOI] [PubMed] [Google Scholar]
- Zhang CH, Mei XG, Liu L, Yu LJ. Enhanced paclitaxel production induced by the combination of elicitors in cell suspension cultures of Taxus chinensis. Biotechnol Lett. 2000;22:1561–64. [Google Scholar]
- Zocher R, Weckwerth W, Hacker C, Kammer B, Hornbogen T, Ewald D. Biosynthesis of taxol: enzymatic acetylation of 10-deacetylbaccatin-III to baccatin-III in crude extracts from roots of Taxus baccata. Biochem Biophys Res Commun. 1996;229:16–20. doi: 10.1006/bbrc.1996.1751. [DOI] [PubMed] [Google Scholar]