Abstract
In most somatic tissues, ASCs (adult stem cells) are crucial for the maintenance of tissue homoeostasis under normal physiological state and recovery from injury. LRC (label retaining cell) assay is a well-known method of identifying possible somatic stem/progenitor cells and their location both in situ and in vivo. BrdU (bromodeoxyuridine) was used here to tag the possible CSCs (cardiac stem cells)/CPCs (cardiac progenitor cells) in newborn pups, followed by a trace period of up to 24 months. In addition, we have used our newly developed ‘KAL’ method to rapidly Kill proliferating cells in adult heart tissues, then, Activate and Label the surviving CSCs/CPCs. LRCs that definitively exist in the heart tissues of adult mice, and some LRCs express the stem cell marker, Sca-1 or c-Kit, and are located primarily in the myocardium and vascular endothelial regions. Moreover, the number of LRCs remains nearly constant during the lifespan of the mouse. After injury induced by 5-fluorouracil, the proliferating cells were almost completely cleared on day 3, and the activated CSCs/CPCs retained their BrdU label after regeneration was complete. A small percentage of the CSCs/CPCs express Sca-1 or c-Kit. Furthermore, the LRC method together with KAL may be used to identify and locate possible CSCs/CPCs, which has potential clinical application.
Keywords: cardiac stem/progenitor cells, histological location, label retaining cell, regeneration
Abbreviations: ASC, adult stem cell; BrdU, bromodeoxyuridine; CPC, cardiac progenitor cell; CSC, cardiac stem cell; DAPI, 4′,6-diamidino-2-phenylindole; 5-Fu, 5-fluorouracil; HSC, haemopoietic stem cell; LRC, label retaining cell; N-cad, N-cadherin; Sca-1, stem cell antigen-1; TAC, transit-amplifying cell
1. Introduction
Traditionally, the heart has been viewed as a post-mitotic organ, which is incapable of repairing any form of damage (Cantin et al., 1981). However, this dogma has been challenged recently by progress in stem cells in the heart, and accumulating evidence suggests there are stem and progenitor cells resident in an adult heart (Urbanek et al., 2006; Bearzi et al., 2007, 2009; Meinhardt et al., 2011). Furthermore, these cells are in charge of turnover and division in response to myocardial injury (Poss et al., 2002; Beltrami et al., 2003; Oh et al., 2003; Laugwitz et al., 2004; Messina et al., 2004; Bollini et al., 2011). In addition, candidate CSCs (cardiac stem cells)/CPCs (cardiac progenitor cells) were identified through cell surface markers expressed by stem cells in other tissues, such as Sca-1 (stem cell antigen-1) (Matsuura et al., 2004), c-Kit (Miyamoto et al., 2010) and N-cad (N-cadherin; Frisch et al., 2008). However, direct identification of definite CSCs/CPCs has been problematic because of the lack of specific stem cell antigens. Given that the heart in adult mammals is a quiescent organ, it is difficult to identify and locate tissue-resident CSCs/CPCs. LRC (label retaining cell) assay is a viable method used to locate stem cells and identify them as slow dividing, quiescent or segregating chromosomes asymmetrically (Karpowicz et al., 2005; Shinin et al., 2006; Rando, 2007). ASCs (adult stem cells) can be labelled by DNA synthesis precursor analogues such as 3H-TdR or BrdU (bromodeoxyuridine) during development or tissue regeneration. Early studies showed that these labels are diluted by TACs (transit-amplifying cells) after a prolonged chase. In contrast, ASCs will retain these labels for an extended period (Cotsarelis et al., 1990). Accordingly, long-term label retention by a cell would reflect ‘stemness’, and has been used to identify tissue-resident stem cells and assess turnover of stem cell population in other somatic tissues (Smith, 2005; Foudi et al., 2009). More importantly, LRCs have even been detected in adult renal papilla (Oliver et al, 2004) and liver (Li et al., 2010) with infrequent cell division, even by this method in an adult mouse heart (Urbanek et al., 2006). In order to identify and locate CSCs/CPCs in the heart of normal adult mice, we performed label retention experiments in newborn mice, and LRCs were detected using the immunohistochemical method in vivo.
The drug 5-Fu (5-fluorouracil) is toxic to cells that are synthesizing DNA. Early studies indicated that more primitive stem cells could be enriched following 5-Fu administration (Hodgson and Bradley, 1979); subsequent studies have confirmed the earlier study (Van, 1984). Here, 5-Fu was used to kill rapidly proliferating cells and activate the surviving CSCs/CPCs, which make the further label experiment possible.
Thereafter, we developed a combination of the label retention method and rapid KAL method, which was used effectively to identify and locate possible CSCs/CPCs.
2. Materials and methods
2.1. Animals
ICR strain mice (4–5 weeks old) were used in the experiments, and all the mice had free access to food and water. All animal experimentation was carried out in accordance with current institutional guidelines for the care and use of experimental animals.
2.2. Antibodies and 5-Fu
The following antibodies were used in the study: mouse anti-BrdU monoclonal antibody (1:500–1:1000, Amersham), mouse anti-sca-1 monoclonal antibody (1:50–1:100, BD Pharmingen), rabbit anti-c-kit polyclonal antibody (1:50–1:100, Santa Cruz); while secondary antibodies were purchased from Jackson Immuno Research and Invitrogen Company; 5-Fu (20 mg/ml, life-technologies).
2.3. BrdU labelling newborn mice
Newborn mice (7 days old) were injected subcutaneously with BrdU (Sigma, pH 7.4) twice a day for 3–5 consecutive days (50–100 mg/kg per injection for each mouse). The labelled mice were killed at 2 weeks (n = 2 mice), 3 months (n = 2 mice), 6 months (n = 2 mice) and 24 months (n = 2 mice) (Figure 1A) to trace and locate the LRCs throughout their lifespan. The traced time was calculated from the initial administration of BrdU. According to Urbanek et al. (2006), cells with BrdU label can be divided into ‘BrdU bright’ cells and ‘BrdU dim’ cells.
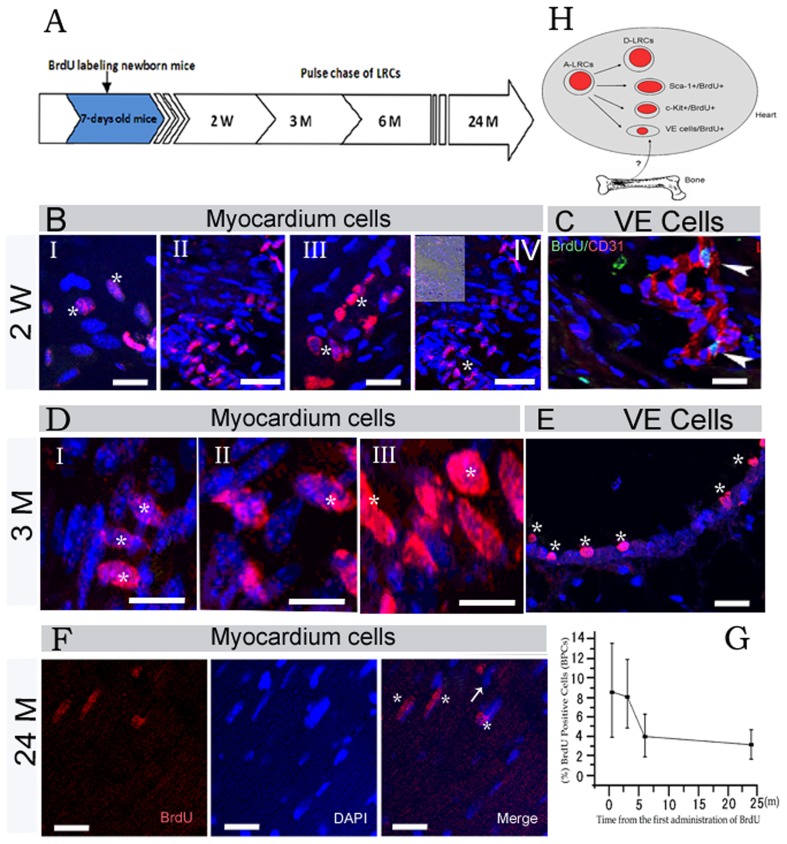
Figure 1. Existence and turnover of LRCs in cardiac tissues of normal adult mice.
(A) Shows schematic outlines of the BrdU pulse chase. w: week, m: month. (BI–IV) Show distinct locations of LRCs in the cardiac tissues after a 2 weeks chase, and the asterisk in I–IV indicates BrdU positive cells (asterisk), and the photo in the top left corner of IV contains white light. (C) Shows immunohistostaining results of BrdU (green) and CD31 (red) for cardiac tissues, and arrowhead indicates the location of LRCs after a 2 weeks chase. (D) Shows LRCs are mainly myocardium cells after a 3 months chase, and the asterisk in I–III shows BrdU (red) positive cells. (E) Shows LRCs located in the vicinity of vascular endothelial region with varied embedding after 3 months chase, and 6 asterisks indicate BrdU positive cells. (F) Shows identified LRCs in cardiac tissues after 24 months chase, and asterisk indicates BrdU positive myocardium cells, however, an arrow indicates ‘BrdU light’ myocardium cells. (G) Shows statistical analysis of BrdU label-retaining cells. The black boxes represent the mean values of LRCs (n = 2 mice at each time point). The error bars represent standard derivations. P = 0.026<0.05 shows there was significant difference (2 weeks versus 6 months), however, P = 0.103>0.05 indicates there was no significant difference (6 months versus 24 months). (H) Shows a model of LRCs in heart after birth, a part of activated-LRCs turn into d-LRCs. Scale bars: BI, BIII, C, E and F = 20 μm; DI–DIII = 30 μm; BII, BIV = 40 μm.
2.4. 5-Fu induced injury plus BrdU pulse-chase experiment
The mice were treated through the tail vein with a single injection of 5-Fu (Life-Technologies) at 100–160 mg/kg at pH 7.4. The day of injection was designated day 0. The same age, sex and strain mice (n = 2) in control group were injected intraperitoneally with BrdU (50–100 mg/kg per injection).
To study the kinetics of possible CSCs/CPCs that entered S-phase following 5-Fu-induced administration, the mice (n = 3) were injected intraperitoneally with a single pulse of BrdU (50–100 mg/kg per injection) on days 3, 4, 5 and 7. The mice were killed 3 h after injection. In addition, to chase the tagged CSCs/CPCs, heart tissues of the mice (n = 3) were obtained every 12 h on day 4.
2.5. Immunohistostaining and examination of sections by confocal laser scanning
Three times 5 min immersions of liver tissue sections (5 μm) in xylene were followed by rehydration through a descending series of ethanol concentrations. Antigen was retrieved with 0.01 M citrate buffer (pH 6.5) at 70°C for 1 h. After cooling for 30 min at room temperature, the sections were treated with 0.1% Tween-20 in PBS for 5 min, and blocked in 3% BSA in PBS for 10 min at room temperature. Subsequently, the sections were digested with DNase I (1:50–1:100 dilution in PBS) for 20 min at room temperature, followed by incubation with anti-BrdU monoclonal antibody and other specific antibodies overnight at 4°C. The sections were washed with PBS for 10 min followed by incubation with the appropriate fluorescent secondary antibodies for 1 h at room temperature. DAPI (4′,6-diamidino-2-phenylindole; Molecular Probes) was used for nuclear staining. Finally, the slides were extensively washed 3×5 min with PBS and mounted. The sections were examined and photographed using a laser scanning confocal microscope.
2.6. Statistical analysis
Random fields (>8 fields) in each area were examined by a blinded operator for appropriate assessment. The data were quantified by Definiens Tissue Studio software (Munich Germany) and the results were analysed by Origin 5.0 software. All the data are reported as means±S.D. The paired 2-tailed Student's t test was used to determine statistical significance; P<0.05 was considered significant.
3. Results
3.1. Identification of CSCs/CPCs and their turnover in adult mice
Newborn animals have a high rate of cell proliferation, so most cells including stem/progenitor cells were initially labelled with BrdU during S phase. The BrdU label was gradually diluted at each division. However, only infrequently or slowly dividing cells (putative stem cells) retain most of the BrdU for a prolonged period. We determined whether candidate CSCs/CPCs reside in heart tissues using a pulse-chase assay (Figure 1A), and where they might reside.
Two weeks after the initial pulse (Figures 1B and 1C), the labelled cells were mainly myocardium cells with various size and oval-like cells located in the vicinity of the vascular endothelia region, and most of the labelled cells were brightly stained with BrdU. These cells were located primarily in the apex (Figure 1BI), interventricular septum (Figure 1BII), left ventricle (Figure 1BIII), cardiac valves (Figure 1BIV) and vascular endothelial region (Figure 1C). Moreover, they appeared in clusters. After 3 months of chasing, bright labelled BrdU (BrdU bright) cells were mainly myocardium cells, located in the cardiac valves (Figure 1D) and vascular endothelial region (Figure 1E). The labelled cells still appeared in cluster; however, they showed a trend to a more scattered distribution after prolonged chasing. However, BrdU positive cells were not found between the myocardial layers. Interestingly, several ‘BrdU bright’ cells appeared in the vicinity of the vascular region with varied embedding, and their nuclei were oval-shaped, which suggested the existence of vascular endothelium progenitor cells with the property of retaining BrdU label. In addition, a part of the LRCs also expressed the Sca c-Kit or Sca-1 (Figures 4A and 4B).
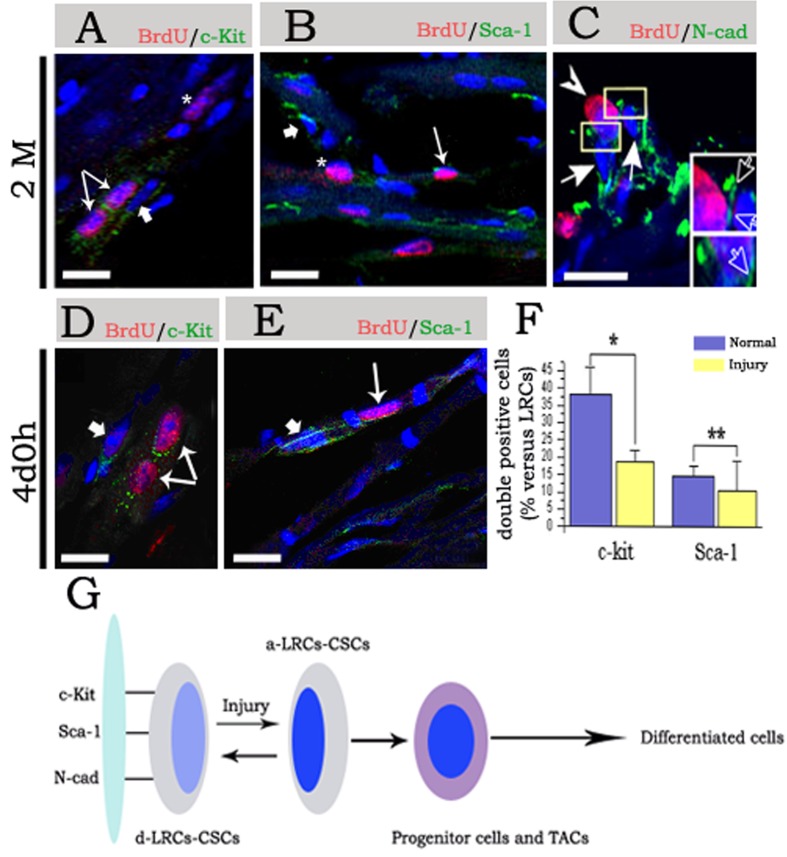
Figure 4. Molecular characteristics of CSCs/CPCs during chasing and post-injury.
(A)–(C) Show staining of cardiac tissues in 2 months chasing. (A) indicates staining of BrdU and c-Kit, the 2 fine arrows point to double positive cells, and the asterisk indicates BrdU+/c-Kit− cells (asterisk); however, the thick arrow points to BrdU−/c-Kit+ cells (arrow). (B) Indicates staining of BrdU and Sca-1, a fine arrow points to double positive cells, and the asterisk indicates BrdU+ and Sca-1− cells; however, the thick arrow points to BrdU−/Sca-1+ cells (arrow). (C) Indicates staining of BrdU and N-cad, the arrowhead indicates BrdU+/N-cad+ cells adhering to BrdU−/N-cad+ spindle cells (arrow), the low right corner in (C) are magnification of rectangular region, and the hollow arrow indicate N-cad expression between the BrdU+ cells and BrdU− cells. (D and E) Show staining of cardiac tissues at day 4 post-injury; (D) indicates staining of BrdU and c-Kit. The 2 fine arrow point to double positive cells; however, the thick arrow points to BrdU−/c-Kit+ cells (arrow). (E) Indicates staining of BrdU and Sca-1, fine arrow point to double positive cells, however, the thick arrow points to BrdU−/Sca-1+ cells (arrow). Nuclei were staining with DAPI. (F) Shows statistical of double positive cells. *P<0.01 show most significance and **P>0.05 shows no significance. (G) Shows a dynamic model of CSCs/CPCs regeneration post-injury. The d-CSCs/CPCs pool (d-LRC-CSCs/CPCs), circulate only a few times over the life span of the mouse in a homoeostatic situation, but is activated (a-LRC-CSCs/CPCs) and participated in replenishment of the cardiac tissues post-injury (scale bars: A, B, D and E = 20 μm; C = 10 μm).
After 24 months of chasing, most of the LRCs were still myocardial cells with long spindle nuclei, with a scattered distribution in aging heart tissues (Figure 1F).
The dynamics of LRCs in the cardiac tissues from youth to aging is shown in Figure 1(G). There was a sharp decline in the percentage of the BrdU positive cells between 2 weeks and 6 months, ranging from 8.7 to 3.9% (P<0.05). The percentage of LRCs kept nearly constant from 6 to 24 months, and the dynamics of LRCs in the cardiac tissues are indicated in a model shown in Figure 1(H). Some activated-CSCs/CPCs, which were tagged with BrdU after birth can turn into d-CSCs/CPCs (dormant-CSCs/CPCs) at a certain specific time, and a portion of them were responsible for proliferation and differentiation during heart development. Therefore, the identification of LRCs provides functional evidence of resident CSCs/CPCs in the adult cardiac tissue, which may be the ‘stem cell pool’ for regeneration of heart tissue. In addition, LRCs have a slower turnover than other somatic tissues.
3.2. Activation and kinetics of CSCs/CPCs at different times after injury
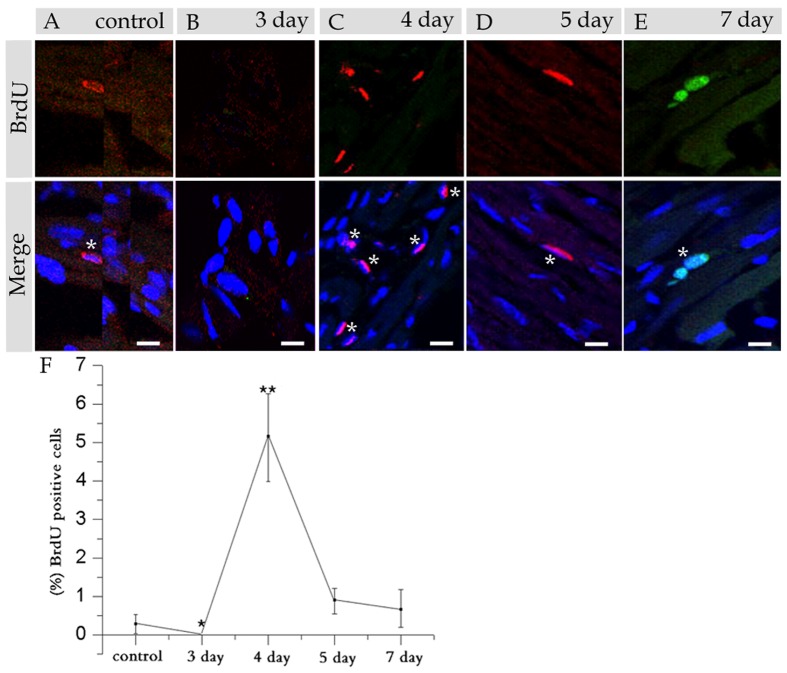
We determined whether d-CSCs/CPCs can be activated and regenerate injured heart tissue, and also the kinetics of the activated CSCs/CPCs at various times post-injury. BrdU was administrated on days 3, 4, 5 and 7 to chase the activated CSCs/CPCs post-injury. The mice were killed 3 h after BrdU injection. After immunohistostaining of BrdU and DAPI, a few of the BrdU positive cells appeared in the myocardium in the control group (Figure 2A). On day 3 (Figure 2B), BrdU positive cells barely appeared in the heart tissues; however, on day 4, BrdU positive cells with variable shapes increased sharply, which suggested CSCs/CPCs were activated significantly from stem cell pool. After BrdU had been administrated on day 5 and day 7 post-injury, only a few BrdU positive cells appeared in heart tissues, and the number of LRCs gradually returned to the baseline level (Figures 2D and 2E), which suggested that the tissue-resident CSCs/CPCs were controlling the regeneration of the injured heart, and the activated-CSCs/CPCs returned to dormancy again after regeneration had been completed.
Figure 2. Immunohistostaining with BrdU and DAPI of the control and the mice at various days post 5-Fu exposure.
(A) Shows staining of a control heart section. (B) Shows staining on day 3. (C) Shows staining on day 4. (D) Shows staining on day 5. (E) Shows staining on day 7. (F) Shows the statistical analysis of BrdU positive cells at each time point (n = 3 mice). Nuclei were stained with DAPI (blue), and the asterisks indicate ‘BrdU bright’ cells. *P<0.01 (control versus day 3 post-injury) and **P<0.01 (control versus day 4 post-injury) indicates most significant differences (scale bar = 20 μm).
From a statistical point of view, CSCs/CPCs were seen on day 3, which suggested that the proliferation cells have been extensively destroyed at this time, while a great number of activated-CSCs/CPCs were observed on day 4 (Figure 2F), and these cells were responsible for the regeneration of heart tissues post-injury, indicating the functionality of these cells.
3.3. Chase and kinetics of activated CSCs/CPCs on day 4 post-injury
To chase the activated-CSCs/CPCs further in vivo, 5-Fu induced injury plus BrdU pulse was subsequently designed, and a chase of every 12 h was done through day 4 (Figure 3A). Immunohistostaining of BrdU and N-cad of cardiac sections was carried out on the material collected. At the initial BrdU pulse (0 h), most of the BrdU bright cells were located at the myocardium, including the endothelial region (Figure 3B), which suggested that the endothelial progenitor cells were located in the vascular endothelial region and they could take part in the regeneration of the injured heart. After a 12 h chase, most LRCs still appeared in the myocardium with scattered distribution (Figure 3C). Furthermore, the nuclear morphology of the BrdU bright cells at this time was similar to that in 0 h. However, after a 24 h chase, some LRCs with nuclear morphology of mature cardiomyocytes appeared in the myocardium (Figure 3D). After a 36 h chase, some LRCs together with surrounding cardiomyocytes had formed an embellished dish connection (Figure 3E).
Figure 3. Chasing and kinetics of activated CSCs/CPCs every 12 h on day 4 post-injury.
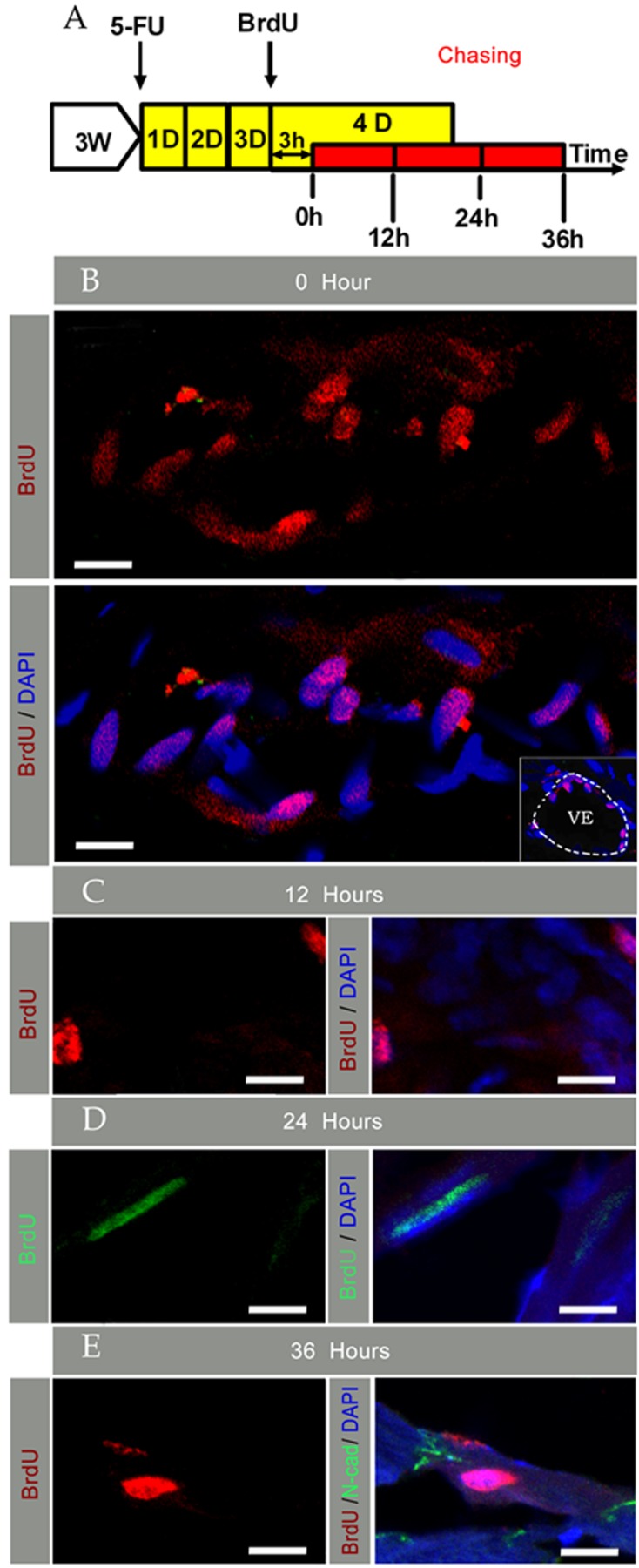
(A) Shows schematic outlines of 5-Fu-induced injury plus BrdU pulse-chase assays. (B) Shows staining at the initiation of pulse chase, and the right corner photo in (B) shows vascular endothelial location of ‘BrdU bright’ cells; the dotted line indicates the VE region. (C) Shows staining after a 12 h chase. (D) Shows staining after a 24 h chase. (E) Shows staining after a 36 h chase. Nuclei were stained with DAPI (scale bar = 10 μm).
3.4. LRCs and lineage molecular characteristics during chasing and post-injury
Expression of several lineage characteristics by LRCs was assessed by double immunohistostaining of cardiac tissue sections after 2 months chasing and on day 4 post-injury (2 months). The heart sections were stained for BrdU and 3 markers related to the stem cell. Interestingly, at 2 months of chasing (Figures 4A and 4B), not all LRCs expressed markers related to the stem cells, and only some of them expressed c-Kit or Sca-1. Several of the LRCs also expressed N-cad, and the N-cad positive cells and BrdU negative cells were spindle cells. Moreover, these cells have not formed intercalated discs, but adhesion connections, which suggested potential niches can adhere to CSCs/CPCs.
A similar result was observed on day 4 post-injury (Figures 4D, 4E and 3E). BrdU+/c-Kit+ cells appeared in the vicinity of BrdU−/c-Kit+ cells, and BrdU+/Sca-1+ cells appeared in the vicinity of BrdU−/Sca-1+ cells.
From a statistical point of view, the percentage of double positive cells (BrdU+/c-Kit+ or BrdU+/Sca-1+) was ∼38 and 15% respectively at 2 months of chasing, 18 and 11% on day 4 post-injury (Figure 4F), which suggests that several types of cardiac stem/progenitor cells retain the BrdU label and take part in the regeneration of the injured heart. Compared with a normal state, the number of BrdU+/c-Kit+ cell was markedly reduced, but interestingly, the number of BrdU+/Sca-1+ cell remained relatively constant post-injury.
We have also shown a dynamic model that d-CSCs/CPCs possess most of the multi-lineage long-term self-renewal activity, and they were efficiently activated in response to 5-Fu induced heart injury (Figure 4G). Subsequently, the activated-CSCs/CPCs are responsible for the regeneration of the injured heart, and after re-establishment of homoeostasis, activated-CSCs/CPCs returned to dormancy again.
4. Discussion
The data provide immunohistochemical evidence of a population of rare cardiac cells, which can retain a bright BrdU label for 24 months in vivo. This not only convincingly indicates that LRCs (possible CSCs/CPCs) exist in adult mouse heart, but also confirms and expands data from 2 previous BrdU pulse-chase experiments in adult rodents (Urbanek et al., 2006; Gonzalez et al., 2008). Our work also demonstrates that LRCs preferentially reside in the apex, left ventricular, cardiac valves and vascular endothelial regions of the heart, although several putative anatomically CSCs/CPCs compartments have been put forward (Urbanek et al., 2006; Meinhardt et al., 2011).
From the BrdU labelling procedure, we showed that ∼4% of cardiac cells were BrdU bright after a 24 months chase, in contrast with previous data in adult mice (∼1.3% of cardiac cells were BrdU bright cells after 10 weeks (Urbanek et al., 2006) and ∼0.3% of cardiac cells were BrdU bright cells after 12 months chase (Meinhardt et al., 2011) and rats (∼1% of cardiac cells were BrdU positive cells after a 12 weeks chase; Gonzalez et al., 2008). It is also not clear whether the LRCs identified in their studies show a link between LRCs and cardiac regeneration. There are some reasons for this disagreement. One possibility is that the difference in ages and strains of animals, or LRCs in our research, is more primitive than the cells in their studies. More importantly, our results reflect an actual size of the total pool of slow-cycling cells in the heart. Another possibility is that the LRCs identified in the other studies might be only TACs, and they are not bona fide cardiac stem/progenitor cells. The number of CSCs/CPCs labelled by BrdU was more than that in the adult mice, but further experiments may elucidate whether the results will be affected by differences in the dose of BrdU. Our BrdU positive cells could be found in normal mice at 4–5 weeks of age after a single BrdU pulse, the percentage being ∼0.3% (Figure 2A). Moreover, after these TACs were killed following 5-Fu administration (Figure 2B), the tissue-resident CSCs/CPCs were activated and participated in the regeneration of the injured heart. They could also retain the BrdU label after regeneration had been completed (Figures 2C and 3B–3E).
Besides the confirmatory findings, our study has two novel aspects, one in providing more comprehensive data on the kinetics of label dilution with beyond 1 year of chasing (Figure 1G); and also in providing a model of LRCs turnover from birth to aging. A second is that it provides more convincing evidence on the regeneration of tissue-resident CSCs/CPCs in heart post-injury, when these TACs were killed. In the whole stem cell pool of the heart, only a small number of cardiac cells derived from LRCs (∼0.3%) was transiently proliferating.
Cairns (1975) first put forward the hypothesis that ASCs maintain an ‘immortal DNA strand’ by asymmetric division. This has since been supported by a great deal of evidence (Karpowicz et al., 2005; Foudi et al., 2009; Li et al., 2010), although there is evidence that supports an alternative point of view (Kiel et al., 2007). In our study, the BrdU bright cells were found in the myocardium of the heart after a 24-month chase (Figure 1F), which demonstrates that LRCs exist in the hearts of adult mice which may have contained immortal DNA strands. The results indicated that the optimal time for CSCs/CPCs labelling occurred in the 7-day mouse.
The number of LRCs remained nearly constant with aging, and its percentage being ∼4. Moreover, the presence of ‘BrdU bright’ cells in a 24-month chase indicated that the LRCs divided asymmetrically, which allowed them to retain the label after a prolonged chase. Therefore, there is no relationship between the number of LRCs and cell division times. From the data above, we can infer that a combination of symmetric and asymmetric cell division may occur in the heart tissues of postnatal mice, while LRCs (CSCs/CPCs) divide asymmetrically to retain the ‘immortal’ DNA strands for reducing their mutation rates.
As mentioned, the previous studies have identified or isolated c-Kit, Sca-1 positive cells from the heart of rodents (Beltrami et al., 2003; Oh et al., 2003; Messina et al., 2004; Matsuura et al., 2004; Urbanek et al., 2006), even human heart (Bearzi et al., 2007), and identified N-cad+ cells from bone marrow (Frisch et al., 2008). c-Kit or Sca-1 was first reported as one of the cell surface markers in HSCs (haemopoietic stem cells; van de Rijn et al., 1989; Okada et al., 1991). In addition, N-cad is expressed in the surface of long-term HSCs (Frisch et al., 2008) and forms an adherent junction through N-cad on the surface of the osteoblasts, comprising the HSCs niches in the bone marrow of adult animals. A similar analysis was performed in our study; interestingly, the percentage of BrdU/c-Kit and BrdU/Sca-1 double positive cells was ∼38 and ∼15% and disagree with the results from another group (Meinhardt et al., 2011) (∼90% of LRCs were Sca-1) during the normal state. Moreover, c-Kit or Sca-1 positive cells were not distributed in clusters and were located in interstitial spaces near the cardiomyocytes, which is in contradiction with the previous result (Urbanek et al., 2006; Bearzi et al., 2007; Meinhardt et al., 2011), which needs to be resolved.
In addition, one sub-population of N-cad+ LTRs (long-term BrdU retaining cells) has been identified in a normal adult heart, and, these N-cad positive cells did not form intercalated disc structures with the adjacent cardiomyocytes, but formed an adherent junction with the neighbouring cells (Figure 4C), which suggests the potential niches can adhere to CSCs/CPCs.
For the 5-Fu induced heart injury, the activated-CSCs/CPCs distributed in the cluster at 0 h on day 4, and another 2 populations of c-Kit or Sca-1 positive cells appeared, which lacked expression of N-cad. More importantly, the percentage of these cells was ∼15 and ∼11% respectively (Figures 4D and 4E); however, the percentage of BrdU and Sca-1 positive cells remained almost constant. One possibility is that c-Kit+/BrdU+ cells have a long proliferation time, and BrdU+/Sca-1+ cells might be along the differentiation pathway. In addition, expression of N-cad was not found from 0 to 24 h on day 4 post-injury. Subsequently, some LRCs together with the surrounding cardiomyocytes form an embellished dish connection at 36 h (Figure 3C). Therefore, our results suggest that there are several different subpopulations of CSCs/CPCs in adult mouse hearts, and they cooperate together to repair the injured heart under suppressive conditions.
After the TACs were killed by the administration of 5-Fu, the more primitive CSCs/CPCs were activated and labelled. Moreover, other subpopulations of CSCs/CPCs also take part in the regeneration of the injured heart, besides the c-kit/BrdU and Sca-1/BrdU cell subpopulations (the total percent of the 2 populations is <50%). The results from the transient BrdU pulse indicated that the change in the number of CSCs/CPCs is remarkable post-injury. Moreover, the chase experiment also suggests that the times of maximum rate of DNA synthesis occur at 0 h on day 4 post-injury (Figure 3B). More serious injuries may lead to shorter G1/S transition times. At the same time, 5-Fu-induced injury probably synchronized the cell cycles and rapid symmetric cell division of CSCs/CPCs. Apparently, the CSCs/CPCs were mobilized by 5-Fu exposure and divided symmetrically to expand the stem cell population for regenerating the injured heart. Finally, the heart tissues completely regenerate themselves in a relatively short period of time. Further experiments are expected to elucidate whether the variant in the injected doses for 5-Fu and BrdU will have an effect on the results in the future.
These above findings together with earlier observations (Urbanek et al., 2006) support the notion of a heterogeneous stem cells pool, in which d-CSCs/CPCs raised various cardiac progenitor cells. More importantly, the existence of LRCs in the heart after 5-Fu-induced injury also reveals that LRCs play an important role as a regeneration cell pool.
We have also provided a model of CSCs/CPCs in normal and injury state (Figures 1H and 4G): in normal state, with the development after birth, a part of the activated-CSCs/CPCs would turn into d-CSCs/CPCs that are located in the compartments. However, these d-CSCs/CPCs would be activated and turned into activated-CSCs/CPCs again, with these activated-CSCs/CPCs in charge of the regeneration of the injured heart.
In summary, although these experiments are only a small step in the study of CSCs/CPCs, the data prove conclusively that the LRCs of the heart are stem cells that are responsible for the regeneration of the injured heart. The experiments support the hypothesis put forward by Cairns, and produce direct evidence for LRCs (CSCs/CPCs) existing in the heart tissues of adult mice and containing immortal DNA strands. The LRCs are primarily located in the apex, left ventricular, cardiac valves and vascular endothelial regions of the mouse heart tissue, which indicate the heart is an organ regulated by several stem cell compartments. Similar to other tissues, heart tissues contain multiple compartments that have distinct responses to different types of injuries rather than a single location of CSCs/CPCs. The label retention method together with the KAL method provides a direct and rapid way to identify CSCs/CPCs and evaluate new stem cell markers within the heart.
Footnotes
This work was partly supported by the Changzhou Research Program of Application Foundation [grant number 20111089] and the Changzhou Science and Technology Supporting Project [grant number 20112158].
Author contribution
Leilei Lu, Fusheng Li and Jingjing Lu designed the research. Leilei Lu and Fusheng Li analysed the data and wrote the manuscript.
References
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–6. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearzi C, Leri A, Lo Monaco F, Rota M, Gonzalez A, Hosoda T. Identification of a coronary vascular progenitor cell in the human heart. Proc Natl Acad Sci USA. 2009;106:15885–90. doi: 10.1073/pnas.0907622106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bollini S, Smart N, Riley PR. Resident cardiac progenitor cells: at the heart of regeneration. J Mol Cell Cardiol. 2011;50:296–303. doi: 10.1016/j.yjmcc.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Cantin M, Ballak M, Beuzeron-Mangina J, Anand-Srivastava MB, Tautu C. DNA synthesis in cultured adult cardiocytes. Science. 1981;214:569–70. doi: 10.1126/science.7291996. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–37. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Frisch BJ, Porter RL, Calvi LM. Hematopoietic niche and bone meet. Curr Opin Support Palliat Care. 2008;2:211–7. doi: 10.1097/SPC.0b013e32830d5c12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foudi A, Hochedlinger K, Buren VD, Schindler JW, Jaenisch R, Carey V. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotech. 2009;27:84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- Hodgson GS, Bradley TR. Properties of haematopoietic stem cells surviving 5-ffi uorouracil treatment: evidence for a pre-CFU-S cell? Nature. 1979;281:381–82. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- Karpowicz P, Morshead C, Kam A, Jervis E, Ramunas J, Cheng V. Support for the immortal strand hypothesis: neural stem cells partition DNA asymmetrically in vitro. J Cell Biol. 2005;170:721–32. doi: 10.1083/jcb.200502073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–42. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2004;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FS, Lu LL, Lu JJ. Identification and location of label retaining cells in mouse liver. J Gastroenterol. 2010;45:113–21. doi: 10.1007/s00535-009-0139-2. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–91. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- Messina E, Angelis LD, Frati G, Morrone S, Chimenti S, Fiordaliso F. Isolation and expression of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Kawaguchi N, Ellison GM, Matsuoka R, Shin'oka T, Kurosawa H. Characterization of long-term cultured c-Kit+ cardiac stem cells derived from adult rat hearts. Stem Cells Dev. 2010;19:105–16. doi: 10.1089/scd.2009.0041. [DOI] [PubMed] [Google Scholar]
- Meinhardt A, Spicher A, Roehrich ME, Glauche I, Vogt P, Vassalli G. Immunohistochemical and flow cytometric analysis of long-term label-retaining cells in the adult heart. Stem Cells Dev. 2011;20:211–22. doi: 10.1089/scd.2009.0203. [DOI] [PubMed] [Google Scholar]
- Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Nishikawa S, Miura Y. Enrichment and characterization of murine hematopoietic stem cells that express c-kit molecule. Blood. 1991;78:1706–12. [PubMed] [Google Scholar]
- Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, Keating MT. heart regeneration in zebrafish. Science. 2002;298:2188–90. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- Rando TA. The immortal strand hypothesis: segregation and reconstruction. Cell. 2007;129:1239–43. doi: 10.1016/j.cell.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Smith GH. Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development. 2005;132:681–7. doi: 10.1242/dev.01609. [DOI] [PubMed] [Google Scholar]
- Shinin V, Gayraud-Morel B, Gome ‘s D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol. 2006;8:677–87. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA. 2006;103:9226–31. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van ZG. Studies of hematopoietic stem cells spared by 5-fluorouracil. J Exp Med. 1984;159:679–90. doi: 10.1084/jem.159.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn M, Heimfeld S, Spangrude GJ, Weissman IL. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci USA. 1989;86:4634–8. doi: 10.1073/pnas.86.12.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]