Abstract
DP (dermal papilla) is a mesenchyme-derived structure situated at the base of the HF (hair follicle) that plays an important role in embryonic hair morphogenesis and maintenance of the hair growth cycle. hMSCs (human mesenchymal stem cells) have gained widespread attention in the field of tissue engineering, but not much is known about the differentiation of hMSCs into DP cells. hMSCs involved in HF formation were examined in our previous study. Here, we have explored the differentiation potential of hMSCs into DP cells by co-culturing hMSCs with DP cells, which proved to be the case. During the differentiation process, the expression of versican, CD133, SCF (stem cell factor), ET-1 (endothelin-1) and bFGF (basic fibroblast growth factor) increased. Compared with hMSCs alone, the aggregate number clearly increased when co-cultured with DP cells. The expression in vivo of HLA-I (human leucocyte antigen class I) was confined to DP of the newly formed HF. The data suggest that hMSCs possess the potential to differentiate into DP cells in vivo and in vitro.
Keywords: co-culture, dermal papilla cells, differentiation, hair follicle, human mesenchymal stem cells
Abbreviations: α-SMA, α-smooth muscle actin; bFGF, basic fibroblast growth factor; DMEM, Dulbecco's minimum essential medium; DP, dermal papilla; ET-1, endothelin-1; FBS, fetal bovine serum; H&E, haematoxylin and eosin; HF, hair follicle; HLA-I, human leucocyte antigen class 1; hMSC, human mesenchymal stem cell; RT–PCR, reverse transcription–PCR; SCF, stem cell factor
1. Introduction
DP (dermal papilla) is located in the lower area of the HF (hair follicle), where it induces HF development, maintains hair shaft growth and control hair-cyclic activity (Jahoda et al., 1984; Matsuzaki and Yoshizato, 1998). DP cells are believed to be the source of the dermal-derived signalling molecule(s) involved in HF development and embryogenesis and, later, in postnatal hair cycling (Rendl et al., 2008; Kwack et al., 2009). One character of DP cells is the pronounced aggregative behaviour when cultured in vitro (Jahoda and Oliver, 1984). Along with the increase in the passages and prolonging of culture time, aggregative behaviour disappeared (Young et al., 2009). However, if co-cultured with primary DP cells, the aggregative behaviour could be observed again. Another property of DP cells is the co-expression of SCF (stem cell factor), ET-1 (endothelin-1) and bFGF (basic fibroblast growth factor) (Lu et al., 2006). Versican secretion by DP is implicated in the induction of hair morphogenesis and the initiation of hair regeneration (Kishimoto et al., 1999; Feng et al., 2011) also play important roles in the maintenance of hair growth in mouse species (Soma et al., 2005). A previous report has demonstrated that CD133 is a novel surface marker useful for collecting DP cells (Ito et al., 2007).
MSCs (mesenchymal stem cells) are a population of multi-potential cells that can proliferate and differentiate into multiple mesodermal tissues. However, very little research has been conducted on DP cells from MSCs. hMSCs (human mesenchymal stem cells) were obtained from 4–6 week old embryos from volunteers who had termination of pregnancy with RU486 antiprogesterone compound. The identification of hMSC was previously reported (Wu et al., 2006). hMSCs can proliferate and differentiate into multiple tissues including bone, cartilage, muscle, ligament, tendon, fat and stroma, and can give rise to bone, cartilage, fat, muscle and neurons. hMSCs involved in HF formation were also previously reported (Wu et al., 2006).
Here, we have employed a co-culture system to investigate the effect of DP cells on hMSCs, and examined the alterations in the cytokine secretion profile: (i), SCF, (ii) ET-1 and (iii) bFGF. Expression of ET-1, bFGF and SCF by hMSCs markedly increased in hMSCs/DP co-cultures. Expression of CD133 and versican was increased, and the aggregate number per square inch of hMSCs co-cultured with DP cells was clearly increased. In vivo experiments showed that the expression of HLA-I (human leucocyte antigen class I) was confined to DP of the newly formed HF. The data suggest that hMSCs possess the potential to differentiate into DP cells in vivo and in vitro.
2. Materials and methods
2.1. Isolation, culture and identification of DP cells
Six cell strains of human DP cells, established from scalp follicles, were cultured for 5 or more passages. Normal specimens were taken from patients without systemic disease who were undergoing plastic surgery, having given informed consent. DP cells were cultured in DMEM (Dulbecco's minimum essential medium) with 20% FBS (fetal bovine serum), 100 units/ml penicillin/streptomycin and 50 ng/ml amphotericin B (Sigma Co.).
The identification of DP cells was done through RT–PCR (reverse transcription–PCR), immunohistochemistry and morphological tests. The morphological characteristic of DP cells shows aggregative behaviour. RT–PCR methods were used to detect the expression of SCF, ET-1 and bFGF (Table 1). α-SMA (α-smooth muscle actin) in DP cells was done by indirect immunofluorescence. Immunohistochemistry methods were used to detect the expression of versican. The positive incidence of CD133 was detected by flow cytometry.
Table 1. Primers used for quantitative real-time PCR.
2.2. hMSCs isolation and culture
hMSCs were obtained from 4–6 week old embryos. Four human embryos aged 4–6 weeks were obtained from voluntary termination of pregnancy with RU486 antiprogesterone compound according to the guidelines and with the approval of our national ethics committee. hMSCs were isolated and cultured in vitro in DMEM supplemented with 10% FBS (PAA Laboratories GmbH) and 1% penicillin/streptomycin (Gibco), and 2 mmol/l l-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin (Gibco) as a standard medium and incubated in a humidified atmosphere of 95% (v/v) air and 5% (v/v) CO2 at 37°C. Expression of specific antigens, SH2, CD29, CD44 and OCT4, was determined for each cell strain.
2.3. hMSCs co-cultured with DP cells
There were 3 experimental groups: Group 1, DP cells co-cultured with hMSCs; Group 2, DP cells co-cultured with fibroblasts; and Group 3, hMSCs cultured single. DP cells were plated (5×104 cells/well) in transwell chambers (Costar) and the inserts placed in a freshly prepared 6-well plate containing the culture medium of DP cells. hMSCs (passage 9) and fibroblast (105 cells/well) were plated in 6-well dishes.
DP cells were cultured on inserts for 3 days; hMSCs and fibroblasts were cultured in 6-well dishes for 1 day prior to incubation in co-culture. At the time of insert transfer, half of the hMSCs medium was extracted and replaced with DP cells media. hMSCs were used for the following study after being co-cultured with DP cells for 7 days.
Real-time PCR, immunohistochemistry and flow cytometry methods were used to examine the expression of SCF, ET-1, bFGF, versican and CD133 in hMSCs co-cultured with DP cells. Morphological observations were also made.
2.4. hMSCs differentiate into DP cells in vivo
The experimental animals were male BALB/c-nu/nu mice, 4–6 weeks of age and 16–20 g in weight (Shanghai Experimental Animal Center, Chinese Academy of Sciences, China). They were housed in sterile conditions, placed in the GLP (Good Laboratory Practice) Laboratory of the Second Military Medical University. Thirty mice were divided equally into five groups: blank control group, DMEM intracutaneous injection group, fibroblast intracutaneous injection group, keratinocyte intracutaneous cell injection group and hMSCs intracutaneous injection group. Cell number in each group was 104.
Skin tissue near the injection site was collected 7 days after intracutaneous injection. Tissues to be fixed and processed were cut to a size not larger than 5-mm-thick for indirect immunofluorescence. The primary antibodies used were HLA-I (Sigma Co) and keratin (Sigma Co). The subcutaneous tissue between the 5 groups was compared after H&E (haematoxylin and eosin) staining.
2.5. Antibodies
The primary antibodies were HLA-I, (Sigma Co) and α-SMA staining (Santa Cruz Biotechnology). The secondary antibodies were FITC and TRITC (tetramethylrhodamine β-isothiocyanate) conjugated antibodies (Kangcheng Co.), which were diluted 1:100. H2O2 oxidoreductase-conjugated secondary antibodies were purchased from Santa Cruz Co. (Santa Cruz Biotechnology).
3. Results and discussion
3.1. DP cells identification
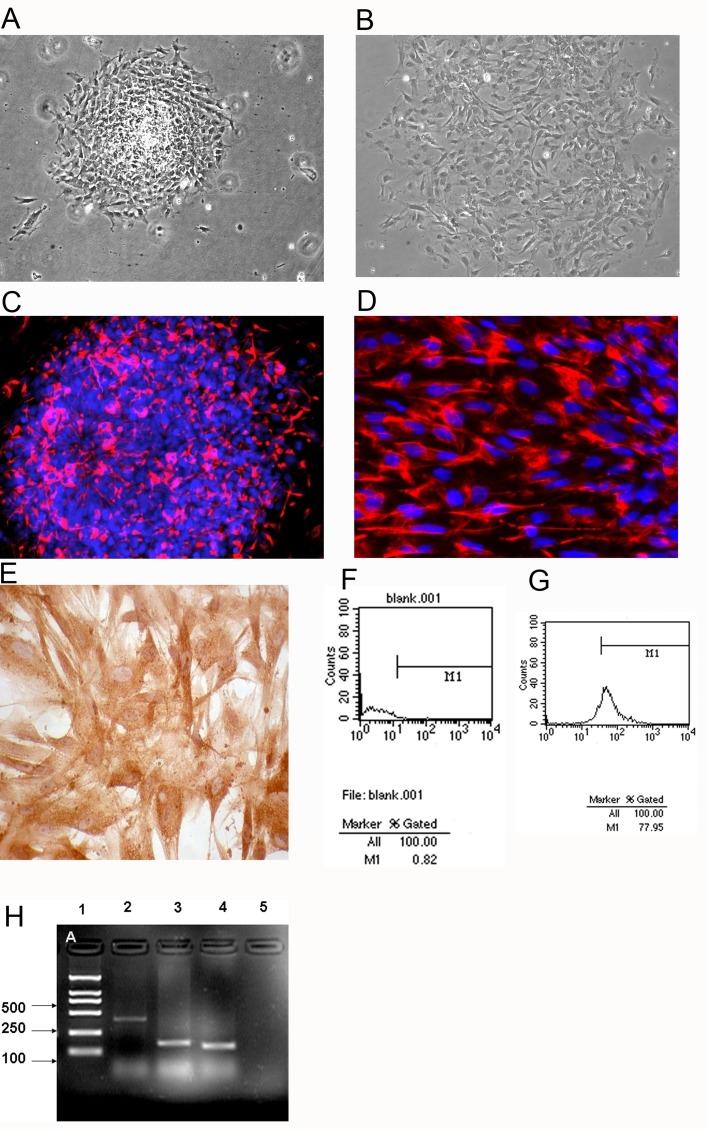
DP cells were initially grown on plastic. DP cells grow out after culturing for 48 h (Figure 1A). Three days later, 40–50% of attached cells showed fusion. The cells continued to move away from the DP until confluence was attained, after which they were sub-cultured. DP cells outgrowths were sub-cultured using 0.25% trypsin in PBS/EDTA (0.2 mg/ml). No difference in behaviour was observed between DP cells on plastic, but a prerequisite for cell outgrowth was that the DP had attached. Cells were cultured beyond passage 8 for 6 human DP cell strains. The aggregation behaviour was maintained over 6 passages, and then slowly disappeared as the cultural time prolonged (Figure 1B).
Figure 1. DP cells isolation and identification.
(A) cells outgrown from DP after 2 days culture. (B) Passage 8 DP cell culture, where the aggregation behaviour has disappeared. (C, D) DP cells positive for α-SMA, indicating mesoblast origin. (E) Versican distribution in DP cells. (F, G) Flow cytometry was used to determine the proportion of CD133-positive cells from passage 3 DP cells. (F) Negative control. (G) CD133 detection, the positives were 78%. (H) RT–PCR analysis of passage 3 DP cells. From left to right: 1 lane: marker; 2 lane: SCF; 3 lane: ET-1; 4 lane: bFGF; 5 lane: no RT control. A, B, C: ×100; D: ×200; E: ×400.
Since DP cells are specialized mesenchymal cells that are related to dermal fibroblasts, it is expected that α-SMA is expressed in DP cells (Figures 1C and 1D).
Versican was strongly expressed in the cytoplasm of the DP cells (Figure 1E). The positives for CD133 in DP cells (passage 3) were 78% (Figure 1G). Passage 3 DP cells co-expressed SCF, ET-1 and bFGF at a high level (Figure 1H).
3.2. Analysis of hMSCs co-cultured with DP cells and fibroblast
hMSCs have some interesting properties, such as multipotency, easy isolation and culture, a highly expansive potential and immunosuppression (Fierro et al., 2004; Baksh and Tuan 2007; Jones et al., 2007). These cells may be an attractive therapeutic tool for regenerative medicine and tissue engineering (Aggarwal and Pittenger, 2005; Risbud and Shapiro, 2005; Yoo et al., 2010). Therefore, more recent studies have focused on adult stem cells for future clinical applications (Crop et al., 2009; Gaebel et al., 2011). hMSCs, when transplanted systemically, can migrate to sites of injury in animals (Mylotte et al., 2008). hMSCs are multipotent stem cells that can differentiate into a variety of cell types, for example, osteoblasts, chondrocytes, myocytes, adipocytes and β-pancreatic islet cells (Mylotte et al., 2008; Donzelli et al., 2011).
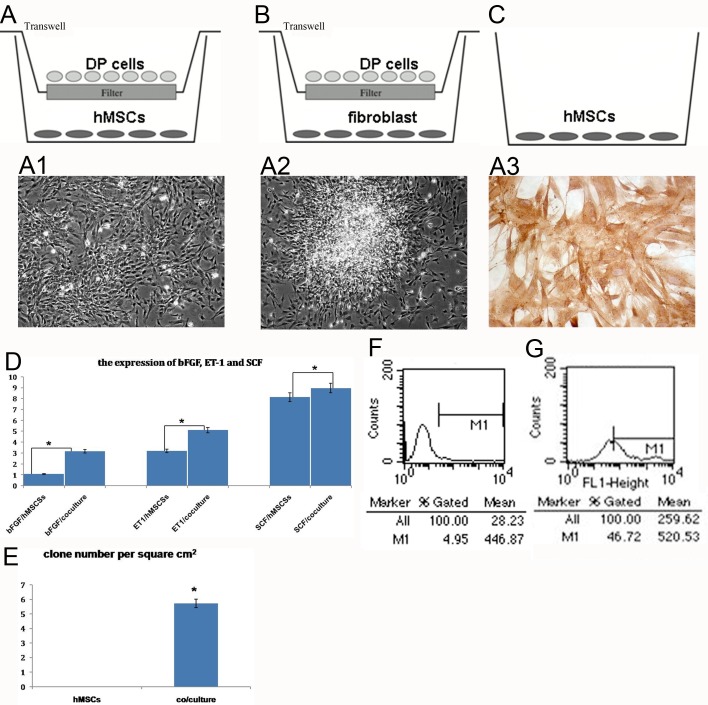
A cell co-culture system was used to investigate the intercellular interaction and cells differentiation potential. Photomicrographs of normal hMSCs show fibroblast morphology. Confluent hMSCs form whirlpool-like fibroblasts (Figure 2A1). After 7 days of co-culturing in indirect contact with DP cells, the hMSCs showed aggregation (Figure 2A2). The aggregate numbers per cm2 in hMSCs and hMSCs co-cultured with DP cells were counted, and the data are shown in Figure 2(E), with hMSCs co-cultured with DP cells being greater than hMSCs cultured alone.
Figure 2. hMSCs co-cultured with DP cells.
(A–C) The co-culture system. (A) Experimental group. (B, C) Control groups. A1: hMSCs cultured alone. A2: hMSCs co-cultured with DP cells for 7 days. Cell morphology changed from fibroblastic to aggregative behaviour. A3: detection of versican in hMSCs co-cultured with DP cells, cytoplasm positive. (D) Detection of SCF, ET-1 and bFGF expression in hMSCs co-cultured with DP cells. Quantification of secretion bFGF, ET-1 and SCF where 100% corresponds to the amount of bFGF, ET-1 and SCF secreted after hMSCs were co-cultured with DP cells for 7 days. (E) The aggregate number of per cm2 of hMSCs and hMSCs co-cultured with DP cells for 7 days. Flow cytometry was used to determine the proportion of CD133-positive cells from hMSCs co-cultured with DP cells. (F) Negative control. (G) CD133 detection, positives were 47%. A1, A2: ×200; A3: ×400. *P<0.05
After 7 days culture in DP medium, hMSCs retained the whirlpool-like formation (Figure 2C). For fibroblast co-cultured with DP cells for 7 days, no aggregation occurred (Figure 2B). The analyses of fibroblast co-cultured with DP cells and hMSCs cultured in DP medium alone showed no morphological changes.
Although DP cells play key roles in HF development, DP cell culture supernatant was used to see if it induced HF regeneration. However, isolation and multiplication of DP cell culture was difficult (Randall VA, 1996; Inamatsu et al., 1998).
Real-time PCR analysis results of hMSCs co-cultured with DP cells in indirect contact showed co-expression of SCF, ET-1 and bFGF (Figure 2D). After 7 days co-culturing in indirect contact with DP cells, SCF, ET-1 and bFGF expression clearly increased (Figure 2D).
Expression of versican was localized in the cytoplasm of the hMSCs co-cultured with DP cells (Figure 2A3). Positives for CD133 changed from 4.9 to 46.7% in the hMSCs co-cultured with DP cells for 7 days (Figures 2F and 2G). These observations are in line with the hypothesis that hMSCs possess the potential to differentiate into DP cells. They indicate that hMSCs are a good choice for hair regeneration. Thus, hMSCs are capable of differentiating into DP cells.
3.3. hMSCs differentiate into DP cells in vivo
For animal experiments, the observation time was 1–30 days, and also 2 months and 3 weeks observation. The subcutaneous tissue structure was analysed quantitatively to follow the morphological changes of skin tissue.
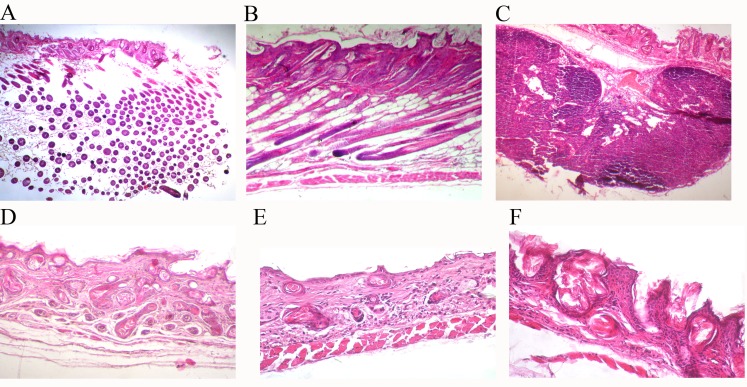
H&E sections of the subcutaneous tissue structure of the hMSCs injection group and control mice showed many differences (Figure 3). HF appeared in the hMSCs injection group (Figures 3A and 3B). For the keratinocyte injection group, a hard cell mass formed at the injection site (Figure 3C). Compared with the hMSCs injection group, no HF formed in fibroblast or DMEM injection groups (Figures 3D and 3E).
Figure 3. H&E analysis of morphological difference.
(A) 1 day after injection and (B) 30 days after injection show the subcutaneous tissues of hMSCs intracutaneous injection group mice. (C) Shows subcutaneous tissues of 7 days after keratinocyte intracutaneous injection. (D, E) 7 days after fibroblast and DMEM injection, respectively. (F) Shows the subcutaneous tissues of normal BALB/c-nu/nu mice. (A) ×100; (D, F) ×200; (B, C and E) ×400.
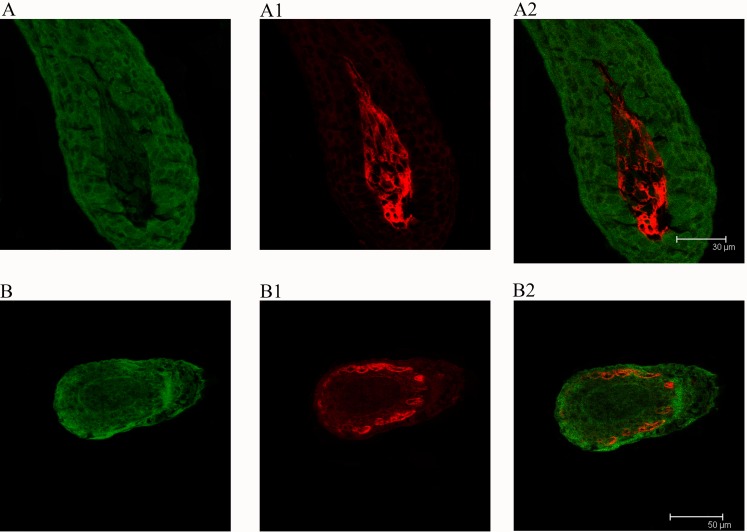
hMSCs were confirmed in HF formation by HLA-I detection (Figures 4A and 4B). In some HF, HLA-I was expressed in the sites of HF (Figure 4B), but for other HF only the DP was intensely stained in the lower portion of HFs (Figure 4A).
Figure 4. HLA-I and keratin expression during HF formation (7 days after intracutaneous injection).
Green, keratin; red: HLA-I. A: Scale bar = 30 μm. B: scale bar = 50 μm.
Animal experiments also showed that hMSCs are non-tumourigenic. In hMSCs injection mice, HLA-1 had been detected 1 month later (Wu et al., 2006). Thus, the survival time of hMSCs in mice is >1 month. However, in fibroblast injection mice, no HF was found. As a terminal differentiation cell with no further differentiation potential, it is normal that HF formation is not induced. No morphological changes were observed after DMEM had been injected.
DP cells can induce HF formation (Hamada and Randall, 2006). The cells with aggregative behaviour and secreting SCF, ET-1, bFGF, versican and CD133 have a greater inductive ability (Kwack et al., 2009). We found that hMSCs have the potential to differentiate into DP cells both in vivo and in vitro, which means they have promise for future hair regeneration.
Footnotes
This work was supported by the National Natural Science Foundation of China [grant numbers 90208026 and 30428001] and Major Program of The Eleventh Five-Year Plan of PLA Medicine [grant number 06G62].
Author Contribution
Houqi Liu conceived and designed the experiments; Minjuan Wu and Xiaocan Guo performed the experiments; Minjuan Wu analysed the data and Minjuan Wu and Qing Sun wrote the paper.
References
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Baksh D, Tuan RS. Canonical and non-canonical Wnts differentially affect the development potential of primary isolate of human bone marrow mesenchymal stem cells. J Cell Physiol. 2007;212:817–26. doi: 10.1002/jcp.21080. [DOI] [PubMed] [Google Scholar]
- Crop M, Baan C, Weimar W, Hoogduijn M. Potential of mesenchymal stem cells as immune therapy in solid-organ transplantation. Transpl Int. 2009;22:365–76. doi: 10.1111/j.1432-2277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- Donzelli E, Lucchini C, Ballarini E, Scuteri A, Carini F, Tredici G, Miloso M. ERK1 and ERK2 are involved in recruitment and maturation of human mesenchymal stem cells induced to adipogenic differentiation. J Mol Cell Biol. 2011;3:123–31. doi: 10.1093/jmcb/mjq050. [DOI] [PubMed] [Google Scholar]
- Feng M, Yang G, Wu J. Versican targeting by RNA interference suppresses aggregative growth of dermal papilla cells. Clin Exp Dermatol. 2011;36:77–84. doi: 10.1111/j.1365-2230.2010.03917.x. [DOI] [PubMed] [Google Scholar]
- Fierro FA, Sierralta WD, Epuñan MJ, Minguell JJ. Marrow-derived mesenchymal stem cells: role in epithelial tumor cell determination. Clin Exp Metastasis. 2004;21:313–9. doi: 10.1023/b:clin.0000046130.79363.33. [DOI] [PubMed] [Google Scholar]
- Hamada K, Randall VA. Inhibitory autocrine factors produced by the mesenchyme-derived hair follicle dermal papilla may be a key to male pattern baldness. Br J Dermatol. 2006;154:609–18. doi: 10.1111/j.1365-2133.2006.07144.x. [DOI] [PubMed] [Google Scholar]
- Inamatsu M, Matsuzaki T, Iwanari H, Yoshizato K. Establishment of rat dermal papilla cell lines that sustain the potency to induce hair follicles from a follicular skin. J Invest Dermatol. 1998;111:767–75. doi: 10.1046/j.1523-1747.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- Ito Y, Hamazaki TS, Ohnuma K, Tamaki K, Asashima M, Okochi H. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J Invest Dermatol. 2007;127:1052–60. doi: 10.1038/sj.jid.5700665. [DOI] [PubMed] [Google Scholar]
- Jahoda CA, Oliver RF. Vibrissa dermal papilla cell aggregative behaviour in vivo and in vitro. J Embryol Exp Morphol. 1984;79:211–24. [PubMed] [Google Scholar]
- Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–2. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- Jones BJ, Brooke G, Atkinson K, McTaggart SJ. Immunosuppression by placental indoleamine 2,3-dioxygenase: a role for mesenchymal stem cells. Placenta. 2007;228:1174–81. doi: 10.1016/j.placenta.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Kishimoto J, Ehama R, Wu L, Jiang S, Jiang N, Burgeson RE. Selective activation of the versican promoter by epithelial–mesenchymal interactions during hair follicle development. Proc Natl Acad Sci USA. 1999;96:7336–41. doi: 10.1073/pnas.96.13.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwack MH, Shin SH, Kim SR, Im SU, Han IS, Kim MK. l-ascorbic acid 2-phosphate promotes elongation of hair shafts via the secretion of insulin-like growth factor-1 from dermal papilla cells through phosphatidylinositol 3-kinase. Br J Dermatol. 2009;160:1157–62. doi: 10.1111/j.1365-2133.2009.09108.x. [DOI] [PubMed] [Google Scholar]
- Lü ZF, Cai SQ, Wu JJ, Zheng M. Biological characterization of cultured dermal papilla cells and hair follicle regeneration in vitro and in vivo. Chin Med J (Engl) 2006;119:275–81. [PubMed] [Google Scholar]
- Matsuzaki T, Yoshizato K. Role of hair papilla cells on induction and regeneration processes of hair follicles. Wound Repair Regen. 1998;6:524–30. doi: 10.1046/j.1524-475x.1998.60605.x. [DOI] [PubMed] [Google Scholar]
- Mylotte LA, Duffy AM, Murphy M, O'Brien T, Samali A, Barry F. Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells. 2008;26:1325–36. doi: 10.1634/stemcells.2007-1072. [DOI] [PubMed] [Google Scholar]
- Gaebel R, Furlani D, Sorg H, Polchow B, Frank J, Bieback K. Cell origin of human mesenchymal stem cells determines a different healing performance in cardiac regeneration. PLoS ONE. 2011;6:1–14. doi: 10.1371/journal.pone.0015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall VA. The use of dermal papilla cells in studies of normal and abnormal hair follicle biology. Dermatol Clin. 1996;14:585–94. doi: 10.1016/s0733-8635(05)70386-7. [DOI] [PubMed] [Google Scholar]
- Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–57. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbud MV, Shapiro IM. Stem cells in craniofacial and dental tissue engineering. Orthod Craniofac Res. 2005;8:54–9. doi: 10.1111/j.1601-6343.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- Soma T, Tajima M, Kishimoto J. Hair cycle-specific expression of versican in human hair follicles. J Dermatol Sci. 2005;39:147–54. doi: 10.1016/j.jdermsci.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Wu M, Yang L, Liu S, Li H, Hui N, Wang F. Differentiation potential of human embryonic mesenchymal stem cells for skin-related tissue. Br J Dermatol. 2006;155:282–91. doi: 10.1111/j.1365-2133.2006.07357.x. [DOI] [PubMed] [Google Scholar]
- Yoo B-Y, Shin Y-H, Yoon H-H, Seo Y-K, Song K-Y, Park J-K. Optimization of the reconstruction of dermal papilla like tissues employing umbilical cord mesenchymal stem cells. Biotechnol Bioprocess Eng. 2010;15:182–90. [Google Scholar]
- Young TH, Tu HR, Chan CC, Huang YC, Yen MH, Cheng NC. The enhancement of dermal papilla cell aggregation by extracellular matrix proteins through effects on cell-substratum adhesivity and cell motility. Biomaterials. 2009;30:5031–40. doi: 10.1016/j.biomaterials.2009.05.065. [DOI] [PubMed] [Google Scholar]