Abstract
Mitochondrial dysfunction has been associated with insulin resistance, obesity and diabetes. Hyperinsulinaemia and hyperlipidaemia are hallmarks of the insulin-resistant state. We sought to determine the contributions of high insulin and saturated fatty acid exposure to mitochondrial function and biogenesis in cultured myocytes. Differentiated C2C12 myotubes were left untreated or exposed to chronic high insulin or high palmitate. Mitochondrial function was determined assessing: oxygen consumption, mitochondrial membrane potential, ATP content and ROS (reactive oxygen species) production. We also determined the expression of several mitochondrial genes. Chronic insulin treatment of myotubes caused insulin resistance with reduced PI3K (phosphoinositide 3-kinase) and ERK (extracellular-signal-regulated kinase) signalling. Insulin treatment increased oxygen consumption but reduced mitochondrial membrane potential and ROS production. ATP cellular levels were maintained through an increased glycolytic rate. The expression of mitochondrial OXPHOS (oxidative phosphorylation) subunits or Mfn-2 (mitofusin 2) were not significantly altered in comparison with untreated cells, whereas expression of PGC-1α (peroxisome-proliferator-activated receptor γ co-activator-1α) and UCPs (uncoupling proteins) were reduced. In contrast, saturated fatty acid exposure caused insulin resistance, reducing PI3K (phosphoinositide 3-kinase) and ERK (extracellular-signal-regulated kinase) activation while increasing activation of stress kinases JNK (c-Jun N-terminal kinase) and p38. Fatty acids reduced oxygen consumption and mitochondrial membrane potential while up-regulating the expression of mitochondrial ETC (electron chain complex) protein subunits and UCP proteins. Mfn-2 expression was not modified by palmitate. Palmitate-treated cells also showed a reduced glycolytic rate. Taken together, our findings indicate that chronic insulin and fatty acid-induced insulin resistance differentially affect mitochondrial function. In both conditions, cells were able to maintain ATP levels despite the loss of membrane potential; however, different protein expression suggests different adaptation mechanisms.
Keywords: fatty acid, insulin, insulin resistance, mitochondrion, membrane potential, myocyte, peroxisome-proliferator-activated receptor γ co-activator-1α
Abbreviations: ANT, ATP/ADP translocator; Cox2 etc., cytochrome c oxidase subunit 2 etc.; CR, calorie restriction; DCF-DA, 2′,7′-dichlorofluorescein diacetate; DMEM, Dulbecco's modified Eagle's medium; ECAR, extracellular acidification rate; ERK, extracellular-signal-regulated kinase; ERRα, oestrogen-related receptor α; ETC, electron chain complex; FCCP, carbonyl cyanide p-trifluoromethoxyphenylhydrazone; FOXO, forkhead box O; GptxI, glutathione peroxidase; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; Mfn-2, mitofusin 2; mtDNA, mitochondrial DNA; NRF1, nuclear respiratory factor-1; OCR, oxygen consumption rate; OXPHOS, oxidative phosphorylation; PPAR, peroxisome-proliferator-activated receptor; PGC-1, PPARγ co-activator-1; PI3K, phosphoinositide 3-kinase; Prdx III, peroxiredoxin; ROS, reactive oxygen species; SOD, superoxide dismutase; TCA, trichloroacetic acid; Tfam, mitochondrial transcripton factor A; UCP, uncoupling protein
INTRODUCTION
Mitochondrial dysfunction in skeletal muscle has been associated with insulin resistance that occurs in states of obesity and Type 2 diabetes [1–3]. In fact, insulin-resistant individuals have reduced expression of mitochondrial gene mRNAs and lower protein expression of respiratory chain subunits [4–8], decreased mtDNA (mitochondrial DNA), reduced oxidative enzyme activity [5] and decreased mitochondrial size and density [3,5,7,9]. Furthermore, several studies have shown impaired mitochondrial respiration in patients with Type 2 diabetes and in their lean insulin-resistant offspring [1,10–13], although this has not been observed in other studies [14,15].
At the cellular level, one of the main cellular manifestations of insulin resistance is an impairment of insulin signalling in peripheral tissues, particularly liver, skeletal muscle, heart and adipose tissue. Insulin binding to its receptors at the cell surface triggers the initiation of signalling cascades that regulate cell metabolism (including glucose, amino acid and lipid metabolism), cell proliferation and cell survival. Several pieces of experimental evidence suggest that insulin signal transduction pathways are also important for mitochondrial function and that insulin resistance has a negative impact on the mitochondria. First, in vitro, insulin administration to cultured myoblasts has been shown to regulate transcription of mitochondrial genes such as Mfn, and the subunits I and IV of the cytochrome c oxidase (MTCOI and NCOIV) [16,17]. In Drosophila, the insulin-regulated transcription factor dFOXO (Drosophila forkhead box O; orthologue of mammalian FOXO1a) regulates the expression of many nutrient responsive genes, including the homologue of the mammalian PGC-1 [PPAR (peroxisome-proliferator-activated receptor) γ co-activator-1], a transcriptional co-activator implicated in controlling mitochondrial gene expression in mammals [18]. These data strongly suggest that mitochondrial biogenesis is linked to insulin signalling via dFOXO-mediated repression of a PGC1 homologue. Furthermore, in vivo studies performed in humans showed that administration of insulin to non-diabetic insulin-sensitive subjects caused an increase in the MAPR (mitochondrial ATP production rate), an effect that was not seen in diabetic patients [19]. It has also been reported that muscle mitochondrial protein synthesis is stimulated by insulin treatment [19,20] and that this process is also impaired in diabetic subjects [21]. The PI3K (phosphoinositide 3-kinase) pathway is responsible for many of the metabolic actions of insulin and represents an important pathway for insulin signalling in muscle. Furthermore, activation of the PI3K pathway is required for the phosphorylation and activation of the serine/threonine kinase Akt, which has been reported to translocate to the mitochondria in response to insulin [22,23].
Similarly, nutrient oversupply, particularly of fatty acids, can induce changes in mitochondrial activity and may play a role in the development of mitochondrial dysfunction. For example, switching animals to a high-fat diet for a few days was sufficient to rapidly reduce the mitochondrial rate of ATP synthesis [24]. In a separate study, genes coding for proteins involved in OXPHOS (oxidative phosphorylation) and mitochondrial biogenesis were down-regulated in response to high-fat feeding [25]. Furthermore, increased fatty acid exposure, as seen in obesity, leads to the production and intracellular accumulation of diacylglycerol and ceramides, which also lead to a reduction in PI3K signalling in muscle, thus leading to increased insulin resistance [26].
In order to clarify the contribution of factors related to the insulin-resistant state (high insulin or high lipids) to mitochondrial dysfunction, we examined the effects of chronic insulin treatment on mitochondrial function and biogenesis and compared that with saturated fatty acid exposure in a cultured cell system. We demonstrate that chronic insulin and fatty acid exposure contribute differently to mitochondrial function.
MATERIALS AND METHODS
Materials
All chemicals and reagents were purchased from Sigma–Aldrich or Thermo Fisher Scientific unless otherwise stated.
Cell culture
C2C12 cells were grown in a humidified atmosphere in 95% air and 5% CO2 at 37°C in DMEM (Dulbecco's modified Eagle's medium) containing 25 mM glucose supplemented with 10% (v/v) FBS (fetal bovine serum), penicillin (100 units/ml), streptomycin (100 μg/ml) and Hepes (10 mM) until they reached 90% confluence. At this point, differentiation and fusion to myotubes were initiated by changing to the fusion medium, which was DMEM supplemented with 2% (v/v) horse serum and penicillin, streptomycin and Hepes of the same concentration as in the growth medium. Myotubes between days 3 and 5 after differentiation were used for experiments. For chronic insulin treatment cells were treated with 100 nM insulin for 24 or 48 h in the fusion medium as indicated. Fresh medium with insulin was changed every 24 h. For the fatty acid treatments sodium palmitate (Sigma) was bound to 12% (w/v) fatty-acid-free BSA in the DMEM medium to make a stock solution of 2 mM palmitate. This stock was then diluted to obtain fatty acid concentrations ranging from 0.2 to 1.6 mM in 4.8% (w/v) BSA as indicated in the Figure legends.
Western blot analysis
Whole-cell lysates and immunoblotting was performed as previously described [27]. Antibodies used were purchased as follows: phospho-Akt (Ser473), Akt, p44/42 MAP and phospho-p44/42 MAPK (mitogen-activated protein kinase) (ERK1/2) (Thr202/Tyr204), insulin receptor β, and phospho-SAPK (stress-activated protein kinase)/JNK (Thr183/Tyr185) were from Cell Signaling; Mfn-2 (mitofusin 2) antibody was from Abcam; anti-Porin 31HL was from Calbiochem; anti-β-tubulin and actin were from Sigma–Aldrich. Antibodies against Complex I–V were from Invitrogen. HRP (horseradish peroxidase)-conjugated secondary antibodies were from Thermo Fisher Scientific. Densitometric analysis was performed using the Image J software (NIH).
Real-time PCR
Total RNA was extracted from mature myotubes using TRIzol® reagent (Invitrogen), following the manufacturer's instructions. cDNA was generated using SuperScript® VILO™ cDNA synthesis kit (Invitrogen). Real-time PCR was performed using the StepOnePlus™ Real-Time PCR System (Applied Biosystems) with cDNA template (6.25 ng), primers (100–400 nM each), and SYBR Green qPCR Master Mix. Primers were designed using Primer3 Software and obtained from Sigma–Aldrich/Genosys. Quantifications of gene expression were normalized to 18S or cyclophilin A. The primer pairs for each gene are shown in Supplementary Table S1 (at http://www.bioscirep.org/bsr/032/bsr0320465add.htm).
Mitochondrial isolation
Cells were washed with PBS and homogenized in isolation buffer containing 2.5% (w/v) BSA, 220 nM mannitol, 70 mM sucrose, 1 mM EDTA, 10 mM Tris/HCl, pH7.4, using a Dounce homogenizer. Cell lysates were centrifuged twice (700g, for 5 min, at 4°C) and the supernatants further centrifuged at 7000 g, for 10 min, at 4°C.The pellets were resuspended in isolation buffer and the centrifugation step repeated twice. The pellet containing crude mitochondria was resuspended in 20 μl of buffer and used in further biochemical assays.
Analysis of inner mitochondrial membrane potential
Cells were incubated in DMEM without serum for 2 h before they were stained with JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine, 10 μg/ml; Molecular Probes/Invitrogen) for 10 min at 37°C. Cells were then treated with or without insulin (100 nM) for 30 min before they were trypsinized, resuspended in the growth medium and transferred to the flow cytometer (FACSCalibur; Becton Dickinson). JC-1 was excited at 488 nm and the monomer signal (green) was analysed at 525 nm (FL1). Simultaneously, the aggregate signal (red) was analysed at 590 nm (FL2). Data were acquired and analysed by using the BD CellQuest™Pro Software (BD Biosciences). Fluorescence values were determined on 10000 cells for each condition in each experiment. The results show the average of three to five independent experiments.
Determination of cellular ATP
Cellular ATP content was determined with a luciferin/luciferase luminometry ATP measurement kit (Sigma) according to manufacturer's instructions. Briefly, cell lysates were prepared in 1% (w/v) TCA (trichloroacetic acid) and incubated for 15 min before centrifugation (11300 g, 10 min, 4°C). The supernatant containing ATP was neutralized by Tris/HCl buffer (50 μl, 0.1M, pH 9.0) before it was mixed with the bioluminescent solution and read by a luminometer (Berthold Tube Master; Berthold Technologies). ATP concentrations in cell lysates were then determined from a standard curve, and then normalized for the total protein concentrations.
Determination of ROS production
Cells were washed in PBS and incubated with DCF-DA [(2′,7′-dichlorofluorescein diacetate); Invitrogen] at a final concentration of 2 μM at 37°C in the dark for 30 min. Cells were then washed in warmed PBS and left in the dark for 20 min before imaging on a Leica DMI6000 microscope under a 488 nm excitation filter. Mean fluorescence for control or treated cells was quantified and analysed over 5 min time intervals using Leica LAF software. Average fluorescence values were calculated from at least three independent experiments each with triplicate cell plates per conditions, with 50–180 cells.
OCR (oxygen consumption rate) and ECAR (extracellular acidification rate)
Respirometry of cultured muscle cells was performed using the Seahorse Bioscience XF 24 platform. Briefly, C2C12 cells were grown and differentiated on Seahorse Bioscience XF24 plates. Cells were then left untreated (controls) or treated with 100 nM insulin or with 0.25 mM palmitate. The instrument was calibrated the day before the experiment as per the instructions of the manufacturer. The injection ports were loaded with appropriate mitochondrial inhibitors as specified in the Figure legends. Oxygen consumption and medium acidification values were normalized by protein content.
Statistical analysis
Results are presented as means±S.E.M. Comparisons between treatment groups were performed by Student's t test or ANOVA (GraphPad Prism; GraphPad Software) as appropriate. P<0.05 was considered statistically significant (*P<0.05, **P<0.01 and ***P<0.001).
RESULTS
Chronic insulin treatment causes insulin resistance and mitochondrial uncoupling
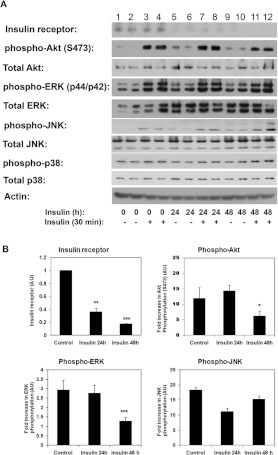
In order to investigate the role of impaired insulin signalling in mitochondrial function we first generated a model of insulin resistance in cultured C2C12 myotubes, by treating cells chronically with insulin (100 nM) for up to 2 days. As shown in Figure 1, chronic insulin treatment decreased the expression of the insulin receptor, which was evident following 24 h of insulin treatment. Quantification of three to five independent experiments showed a reduction of 60% of insulin receptor protein after 24 h insulin exposure and up to 75–80% after 48 h in comparison with untreated cells (Figure 1B). Concomitantly, 48 h of insulin treatment reduced the subsequent insulin-stimulated phosphorylation of the serine/threonine kinase Akt (Figure 1A, lanes 11 and 12 in comparison with control cells, lanes 3 and 4, and Figure 1B). Activation of ERKs (p44/p42) following an acute insulin treatment was also reduced in cells chronically treated with insulin for 48 h (Figure 1B). Basal activation of ERK p44/p42 was also increased after insulin treatment of cells. However, basal activation of the stress kinases p38 MAPK or JNK was not different in the insulin-treated group in comparison with control non-treated cells. We observed no changes in the phosphorylation levels of p38 MAPK in the insulin-treated cells in comparison with control cells. These data suggest that 48 h of chronic insulin treatment causes insulin resistance by reducing signalling through PI3K/Akt and p44/p42 MAPK without affecting p38 or JNK activation.
Figure 1. Chronic insulin treatment induces insulin resistance and inhibits PI3K signalling.
(A) C2C12 myotubes were left untreated (lanes 1–4) or treated with 100 nM insulin for 24 h (lanes 5–8) or 48 h (lanes 9–12) before they were serum-deprived in DMEM for 4 h and either left untreated (lanes 1, 2, 5, 6, 9 and 10) or treated with insulin (100 nM) for 30 min (lanes 3, 4, 7, 8, 11 and 12). Whole-cell extracts were prepared and samples separated by SDS/PAGE and immunoblotted with the indicated antibodies. A representative experiment is shown. (B) Quantification of the effects of chronic insulin treatment on insulin signalling. Results are means±S.E.M. [AU (arbitrary units)] from three to five experiments. Data for individual proteins were normalized to loading controls (actin, Akt, ERK, JNK or p38). Non-treated cells were used as reference. Statistical analysis: one-way ANOVA (*P<0.05, **P<0.01 and ***P<0.001).
Next, we examined whether the mitochondrial function was impaired in chronically insulin-treated cells. To this end, we examined the OCR and ATP levels in cells chronically treated with insulin for 2 days. Insulin-treated cells showed a higher OCR and higher maximal respiratory capacity [in the presence of the uncoupler FCCP (carbonyl cyanide p-trifluoromethoxyphenylhydrazone)] than the control untreated cells (Figure 2A). Despite this, the ATP levels of the cells were not different than controls (Figure 2B).
Figure 2. Mitochondrial function in C2C12 myotubes chronically treated with insulin.
(A) Oxygen consumption. Cells were either left untreated (controls) or treated with 100 nM insulin for 48 h. Oxygen consumption was determined using a Seahorse XF24 Flux analyser and following the manufacturer's instructions. Data were normalized by protein content. As indicated the following mitochondrial inhibitors were used: oligomycin A 10 μg/ml, FCCP 10 μM). Data are means±S.E.M. for five independent experiments. Statistical analysis: Student's t test (*P<0.05, **P<0.01 and ***P<0.001) (B) Cellular ATP content. C2C12 myotubes were left untreated or treated with insulin (100 nM) for 48 h. ATP content was measured using a luciferin/luciferase kit (Sigma) and following the manufacturer's instructions. The concentrations of ATP were normalized for the total protein concentrations (μmol/μg of protein) and the results presented as the percentage respective to the control. Results are means±S.E.M. for four independent experiments each measured in triplicate. (C) Mitochondrial membrane potential (Δψ). C2C12 myotubes were left untreated or treated with insulin (100 nM) for 48 h before they were serum-starved in DMEM without serum for 2 h and stained with JC-1 dye as described in the Material and Methods section. Acute treatment of insulin was for 30 min. Cells were trypsinized and analysed by flow cytometry. Data are presented as the percentage of the control; significance was analysed using a Student's t test (***P<0.001). (D) ECAR. Cells were either left untreated (controls) or treated with 100 nM insulin for 48 h. Proton excretion as a measure of glycolysis was determined using a Seahorse XF24 Flux analyser and following the manufacturer's instructions. Data were normalized by protein content. As indicated the following mitochondrial inhibitors were used: oligomycin A 10 μg/ml, FCCP 10 μM). Data are means±S.E.M. for five independent experiments. Statistical analysis: data were analysed using a Student's t test (*P<0.05). (E) ROS production. C2C12 myotubes were either left untreated or treated with insulin (100 nM, 48 h). Cells were washed in PBS and stained with DCF-DA as detailed in the Materials and Methods section. Cells were visualized in a Leica DMI6000 microscope under 488 nm excitation filter and fluorescence was quantified over a 5 min time intervals with Leica LAF software. The means±S.E.M. of fluorescence for n=41–59 cells are shown, analysed by Student's t test (***P<0.001).
We next determined the mitochondrial membrane potential by flow cytometry. Cells were stained with JC-1, a cationic dye that selectively accumulates in the mitochondrial inter-membrane space in a manner proportional to the proton gradient. JC-1 exhibits green (525 nm) fluorescence in its monomeric state, but in energized mitochondria it forms aggregates that shift its emission fluorescence from green (525 nm) to the far-red (590 nm) emission wavelength. Thus, quantification of the ratio between the red/green-emitted lights provides a measure of the mitochondrial membrane potential. As observed in Figure 2(C) cells exposed to chronic insulin for 2 days, exhibited the reduced mitochondrial membrane potential in comparison with control untreated cells. Concomitantly with this, ROS was lower in the insulin-treated cells (Figure 2E).
Insulin-resistant cells also displayed a higher ECAR (Figure 2D), which suggests a higher glycolytic rate in order to maintain ATP levels.
Overall these data suggest that insulin-induced insulin resistance causes mitochondrial uncoupling decreasing mitochondrial efficiency, which is compensated by increased glycolysis.
Chronic insulin treatment alters the expression of transcripts encoding for mitochondrial proteins or proteins involved in mitochondrial biogenesis
To evaluate further the impact of chronic insulin treatment on mitochondrial function, we sought to determine whether chronic insulin treatment alters the expression of mitochondrial genes or genes associated with mitochondrial biogenesis.
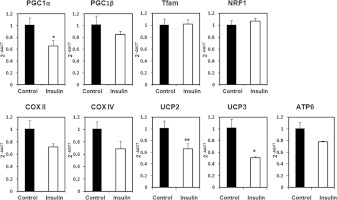
To this end we determined the mRNA levels of the transcriptional co-activators PGC-1α and PGC-1β, which have been found to regulate mitochondrial biogenesis. We found that PGC-1α mRNA levels were markedly decreased in insulin-treated cells in comparison with controls (Figure 3), but no changes were detected in PGC-1β. Despite the changes in PGC-1α, no changes were detected in the mRNA levels of the downstream transcriptional factors NRF1 (nuclear respiratory factor-1) or Tfam (mitochondrial transcription factor A) (Figure 3).
Figure 3. Mitochondrial gene expression in C2C12 myotubes treated with chronic insulin treatment.
C2C12 myotubes were left untreated or treated with insulin for 48 h. Total mRNA was extracted and the mRNA abundance of genes coding for mitochondrial proteins examined by quantitative real-time PCR. Results were analysed by the ΔΔCT method. Data are means±S.E.M. for three independent experiments analysed by Student's t test (*P<0.05 and **P<0.01).
We also determined the levels of mRNA coding for several mitochondrial proteins. The expression of mRNAs coding for Cox 2 (cytochrome c oxidase subunit 2), Cox4 and ATP-synthase subunits showed a tendency to decrease after insulin treatment, albeit it did not reach statistical significance. We observed, however, a significant reduction in the mRNA levels coding for UCP (uncoupling protein) 2 and UCP3 in cells treated with insulin.
Chronic insulin treatment does not alter the expression of electron transfer proteins or Mfn-2
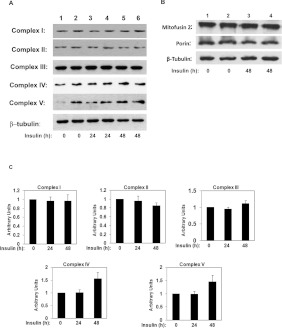
To investigate further the impact of chronic insulin on mitochondrial function we examined by Western blotting the expression of ETC (electron chain complex) protein subunits Mfn-2, a protein involved in mitochondrial fusion, and porin in these cells. Chronic insulin exposure did not significantly alter the expression of ETC protein Complexes I–V (Figure 4A) or the levels of Mfn-2 (Figure 4B). The expression levels of porin were also unchanged in insulin treated cells in comparison with controls (Figure 4B). In any case these data indicate that insulin-induced insulin resistance does not compromise the expression of mitochondrial OXPHOS proteins.
Figure 4. Chronic insulin treatment does not alter expression of OXPHOS protein complexes porin or Mfn-2.
(A) C2C12 myotubes were left untreated (lanes 1 and 2) or treated with insulin for 24 h (lanes 3 and 4) or for 48 h (lanes 5 and 6). Whole-cell lysates were obtained, and separated by SDS/PAGE and immunoblotted with specific antibodies as indicated. A representative experiment is shown. (B) C2C12 myotubes were left untreated (lanes 1 and 2) or treated with insulin for 48 h (lanes 3 and 4). Whole-cell lysates were obtained, and separated by SDS/PAGE and immunoblotted with specific antibodies as indicated. A representative experiment is shown. (C) Quantification of effects of chronic insulin treatment on OXPHOS complex protein expression [a.u. (arbitrary units)]. Data from three experiments were normalized to actin. Non-treated cells were used as reference. Statistical analysis: one-way ANOVA.
Saturated fatty acid exposure reduces PI3K signalling, increases JNK and p38 activity and decreases mitochondrial membrane potential
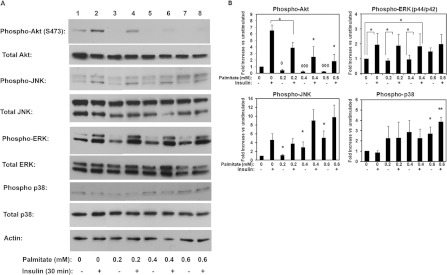
Chronic treatment of cells with the saturated fatty acid palmitate induced insulin resistance in a dose-dependent manner, as evidenced by the attenuated Akt activation following an acute treatment of cells with insulin for 30 min (Figure 5). As the concentrations of the fatty acid increased, we observed a marked decline in the capacity of insulin to phosphorylate Akt and a decrease in the basal activation of Akt. Concomitantly with this, as the concentration of palmitate increased we observed increased basal activation of the stress kinases JNK and p38 (Figures 5A and 5B). In subsequent experiments, we chose to examine mitochondrial function at 0.2 mM palmitate, since at this concentration the activation of Akt was significantly reduced (to approx. 45% of that seen in control cells, i.e. insulin-treated in the absence of fatty acid, without compromising cell viability (cell viability was determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] assay and at this concentration of fatty acid it was not significantly different to control untreated (results not shown).
Figure 5. Chronic saturated fatty acid exposure inhibits PI3K signalling in C2C12.
(A) C2C12 myotubes were treated with increasing concentrations of palmitate in DMEM/4.8% BSA or DMEM/4.8% BSA alone for 24 h as described in the Materials and methods section. Cells were then serum starved for 3–4 h and subsequently either left untreated or treated with insulin for 30 min as indicated. Cell lysates were obtained, and separated by SDS/PAGE and immunoblotted with specific antibodies as indicated. A representative experiment is shown. (B) Quantification of the effects of fatty acid exposure on insulin signalling. Data for individual proteins were normalized to loading controls (Akt, ERK, JNK or actin). Control cells were used as reference. Data shown are means±S.E.M. for three to five experiments. Statistical analysis: ANOVA with Bonferroni post-hoc test (*or ◇P<0.05, ** or ◇◇P<0.01 and ◇◇◇P<0.001.
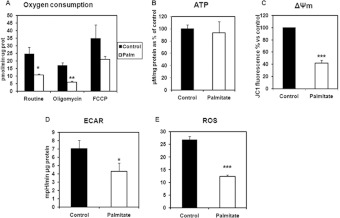
Having established a saturated fatty-acid-induced in vitro model of insulin resistance we next sought to examine the mitochondrial function in these cells. First, we determined the OCR and ATP levels in cells treated with 0.2 mM palmitate and control untreated cells. We observed that the OCR and maximal respiratory capacity (in the presence of FCCP) were decreased in the fatty-acid-treated cells in comparison with controls (Figure 6A). The mitochondrial membrane potential was also significantly reduced in cells treated with palmitate for 24 h (Figure 6C), concomitantly with a reduced formation of ROS (Figure 6E). However, the ATP content in cells was maintained at the same time that glycolytic rate (ECAR) was reduced (Figures 6B and 6D, respectively), suggesting a preference for fatty acid utilization over glucose in the palmitate-exposed cells.
Figure 6. Mitochondrial function in C2C12 myotubes chronically treated with palmitate.
(A) Oxygen consumption. Cells were either left untreated (controls) or treated with 0.2 mM palmitate for 24 h. Oxygen consumption was determined using a Seahorse XF24 Flux analyser and following the manufacturer's instructions. Data were normalized by protein content. As indicated the following mitochondrial inhibitors were used: oligomycin A 10 μg/ml, FCCP 10 μM,). Data are means±S.E.M. for five independent experiments. Statistical analysis: data were analysed by Student's t test (*P<0.05 and **P<0.01). (B) Cellular ATP content. C2C12 myotubes were left untreated or treated with 0.2 mM palmitate for 24 h. ATP content was measured using a luciferin/luciferase kit (Sigma) and following the manufacturer's instructions. The concentrations of ATP were normalized for the total protein concentrations (μM ATP/mg of protein) and the results presented as the percentage respective to the control. Results are means±S.E.M. for three independent experiments, n=6–13 per group. (C) Mitochondrial membrane potential (ΔΨm). C2C12 myotubes were left untreated or treated with 0.2 mM palmitate before they were serum-starved in DMEM without serum for 2 h and stained with JC-1 dye as described in the Materials and methods section. Acute treatment of insulin was for 30 min. Cells were trypsinized and analysed by flow cytometry. Data are presented as the percentage of the control and significance was analysed by Student's t test (***P<0.001). (D) ECAR. Cells were either left untreated (controls) or treated with 0.2 mM palmitate. Proton excretion as a measure of glycolysis was determined using a Seahorse XF24 Flux analyser and following the manufacturer's instructions. Data were normalized by protein content. As indicated the following mitochondrial inhibitors were used: Oligomycin A 10 μg/ml, FCCP 10 μM). Statistical analysis: data were analysed by Student's t test (*P<0.05). (E) ROS production. C2C12 myotubes were either left untreated or treated with 0.2 mM palmitate for 24 h. Cells were washed in PBS and stained with DCF-DA as detailed in the Materials and methods section. Cells were visualized in a Leica DMI6000 microscope under 488 nm excitation filter and fluorescence was quantified over a 5 min time intervals with Leica LAF software. Means±S.E.M. fluorescence for n=48–186 cells are shown. Analysed by one-way ANOVA (***P<0.001).
Saturated fatty acid exposure increases the expression of UCPs
We next set to examine whether fatty acid treatment resulted in changes in the expression of transcripts coding for mitochondrial proteins or proteins associated with regulating mitochondrial biogenesis. Treatment of cells with 0.2 mM palmitate did not change PGC-1α and PGC-1β transcript levels, or indeed the levels of transcripts coding for the transcription factors NRF1 or Tfam (Figure 7). In contrast, fatty acid exposure markedly increased the transcripts coding for UCP2 and UCP3 (Figure 7). In addition, we detected a tendency to increase the transcript coding for ATP6 (ATP synthase subunit 6) (Figure 7).
Figure 7. Mitochondrial gene expression in C2C12 myotubes exposed to chronic palmitate.
Cells were treated with fatty-acid-free BSA (4.8% (w/v) in DMEM) or with BSA (4.8%) and palmitate (0.2 mM) for 24 h. The mRNA abundance of mitochondrial genes was determined by quantitative real-time PCR by ΔΔCT method. Data are means±S.E.M. for n=3 experiments, quantified in triplicate. Statistical analysis: two-way ANOVA with Bonferroni post-hoc test (*P<0.05).
Saturated fatty acid exposure increases the expression of some electron transport chain proteins
To further investigate the effects of palmitate on mitochondrial function and dynamics, we examined the expression of the OXPHOS protein complex subunits by Western blot in control (untreated cells) or cells exposed to palmitate. Fatty acid exposure elevated the expression of protein components of Complex IV and V, without affecting the expression levels of the proteins of Complex I–III (Figures 8A and 8C). Interestingly, we found that expression of porin or Mfn-2 were unaltered in fatty-acid-treated cells in comparison with controls (Figure 8B).
Figure 8. Effects of chronic palmitate exposure on mitochondrial protein expression.
(A) and (B) C2C12 myotubes were treated with fatty-acid-free BSA 4.8% in DMEM (lanes 1 and 2) or with 4.8% BSA and palmitate (0.2 mM) for 24 h (lanes 3 and 4). Cell lysates were obtained and separated by SDS/PAGE transferred to nitrocellulose filters and immunoblotted with the specific proteins as indicated. A representative experiment is shown. (C) Quantification of the effects of chronic saturated fatty acid exposure on OXPHOS complex protein expression. Data from three to five experiments were normalized to actin. Non-treated cells were used as reference. Statistical analysis: ANOVA with Bonferroni post-hoc test (*P<0.05).
Expression of reactive oxygen detoxifying enzymes is not altered after chronic insulin or saturated fatty acid exposure
Our in vitro models displayed reduced ROS levels in parallel with lower mitochondrial membrane potentials. However, reduced ROS levels could be due to a lower ROS production or increased ROS clearance. To shed some light into this issue we sought to determine whether chronic insulin or saturated fatty acid exposure altered the expression of several detoxifying enzymes including: SOD (superoxide dismutase) isoforms 1 and 2, Prdx III (peroxiredoxin), GptxI (glutathione peroxidase) and catalase.
As seen in Figure 9, chronic insulin treatment did not modify the mRNA levels of SOD1, SOD2, PrdxIII or catalase. A small but significant decrease was observed for GptxI. Chronic exposure to fatty acid did not modify the mRNA levels of SOD2, GptxI, Prdx III or catalase. A small decrease was observed in the levels of SOD1 after fatty acid treatment (Figure 9). Taken together these findings suggest that the overall expression of ROS detoxifying enzymes are not altered in our insulin-resistance models, and while we were not able to directly measure the specific activities for these enzymes, taken together with the mitochondrial membrane potential data, these results support the hypothesis that lower ROS detected may be due to lower electron transport or increased uncoupling rather than increased ROS clearance.
Figure 9. Expression of enzymes involved in ROS detoxification after exposure to insulin or palmitate.
C2C12 myotubes were either left untreated or treated with either 100 nM insulin or palmitate (0.2 mM, conjugated to BSA 4.8%) for 24 h. The mRNA abundance of mitochondrial genes was determined by quantitative real-time PCR by ΔΔCT method. Data are means±S.E.M. for n=3 experiments, quantified in triplicate. Statistical analysis: ANOVA (*P<0.05).
DISCUSSION
In the present study, we set out to compare the contribution of insulin resistance induced by chronic hyperinsulinaemia or fatty acid exposure to mitochondrial function and biogenesis in a cultured model of muscle cells. We chose to do our experiments with palmitate exposure at 24 h because this was sufficient time to generate insulin resistance in our cell model. Longer incubations of cells with high concentrations of palmitate result in apoptosis and reduced cell viability. However, for insulin-mediated insulin resistance 24 h treatment was not sufficient, as we saw no decrease in the phosphorylation levels of Akt after 24 h; therefore we evaluated mitochondrial function after 48 h of insulin treatment. Under these experimental conditions we achieved comparable levels of insulin resistance by both treatments, as seen in the similar levels of Akt phosphorylation inhibition after acute insulin exposure. Chronic insulin treatment resulted in the down-regulation of the insulin receptor expression by approx. 60–70% and significantly reduced the subsequent insulin-stimulated phosphorylation of Akt and p44/p42 ERK, whereas activation MAPK p38 or JNK where largely unaffected. Concomitantly with this, chronic insulin treatment increased myocyte differentiation and size (results not shown), consistent with previous observations [16]. Similarly, chronic palmitate treatment reduced PI3K/Akt signalling but increased basal p38 and JNK stress kinase activation. Recently, Yuzefovych et al. [28] have also reported increased JNK kinase activation in a similar muscle cell line. These authors found that palmitate induced DNA damage in L6 muscle cells. Interestingly, however, we found that the mitochondrial readout was different following these two treatments.
Chronically insulin-treated cells displayed a higher OCR than control cells, without any increase in ATP levels, at the same time that mitochondrial membrane potential and ROS production were reduced. This suggests decreased efficiency of mitochondrial energy transduction (mitochondrial uncoupling), as increased oxygen consumption does not correspond with an increase in ATP levels. We believe that ATP levels were maintained in our model through stimulating glycolysis. Similar results were found in myotubes isolated from Type 2 diabetic patients cultured in vitro. These cells exhibited a reduced tricarboxylic acid flux [29]. However, and unlike our model, the diabetic myotubes exhibited lower ATP levels in comparison with controls [13]. A recent study from the same group found reduced levels of ROS (H2O2) in the myotubes obtained from diabetic patients [30], which is also in agreement with our findings. Furthermore, our results are consistent with those obtained in in vivo models of CR (calorie restriction), which are characterized with reduced plasma insulin levels and reduced insulin signalling. Muscle mitochondria from CR mice also showed reduced mitochondrial membrane potential with 21% increase in proton leak respective to control animals [31]. Interestingly, in this model, the proton leak and H2O2 production were reduced with exogenous administration of insulin to these animals. At the moment the precise molecular mechanisms by which chronic insulin caused mitochondrial uncoupling in our cell model is unknown. Given that we found the transcripts for UCP2 and UCP3 proteins to be reduced in cells treated with insulin, it is possible that UCP proteins are not responsible for these actions of insulin. Additional candidate proteins may be the ANT (ATP/ADP translocator), and the aspartate–glutamate carrier. However, recent studies in the CR mice model suggest that neither CR nor insulin treatment alter the expression of ANT in muscle mitochondria [31]. Further experiments are needed to clarify this issue.
In contrast, palmitate-treated cells showed decreased oxygen consumption and reduced ECAR, indicative of a reduced lactate production, which suggests decreased glucose utilization as a consequence of preferred fatty acid utilization. This effect, initially described by Randle and co-workers, and commonly known as the Randle cycle, reflects the competition between the two substrates for oxidation. When fatty acids are in abundant supply, there is an inhibition of glucose oxidation, which is re-routed to be stored as glycogen. Fatty acids have been shown to inhibit several glycolytic enzymes, most notably the PDH (pyruvate dehydrogenase) [32].
Our palmitate-treated cells also exhibited a decreased membrane potential and ROS generation. These findings contrast with those found by Yuzefovych et al. [33] in L6 myotubes. In this study, the authors found elevated ROS levels in cells treated with palmitate. Interestingly ROS production was attenuated by the expression of a DNA repair enzyme targeted to the mitochondria [33]. We do not know the causes for this discrepancy; however, while the two cell lines used in these studies are quite similar, they are known to exhibit differences in the degree of responsiveness to insulin and glucose uptake, partially due to the lower expression of glucose transporter Glut4 in the C2C12 cell line. Nevertheless, Yuzefovych et al. used a much higher concentration of palmitate (0.5 and 1 mM) in their studies, which could have had a more potent lipotoxic effect on the cells. In line with this, their study reports a reduction of cell viability to approx. 70 and 50% relative to control untreated cells, respectively at 0.5 and 1 mM palmitate. At the concentrations used in our study (0.2 mM), we did not observe a significant decline in cell viability in the cells treated with palmitate against control (results not shown). In any case, in our study we also detected an increase in the mRNA coding for UCP2 and UCP3, which could increase protein leak and thus reduce ROS generation. Indeed, overexpression of UCP3 was shown to decrease ROS [34]. Other studies performed in isolated muscle mitochondria treated with NEFA (non-esterified fatty acids) also support our findings and suggest that long chain fatty acids (>12 carbons) induce uncoupling and reduce mitochondrial potential and ROS production [31]. However, some in vivo studies done in mice and humans have showed increased ROS production in skeletal muscle after a high-fat diet [35,36]. While we do not know the reason for this discrepancy, it is possible that the levels of ROS generated in vivo may be reflective of a much higher fatty acid availability and fatty acid oxidation than the in vitro studies, which would enhance the supply of reduced equivalents to the respiratory chain and therefore stimulate electron transport and ROS production. Alternatively or additionally, in vivo models could display reduced ROS clearance by altering the expression or activity of ROS detoxifying enzymes. Although we did not measure enzyme activity, we quantified the abundance of mRNA transcripts coding for several detoxifying enzymes in cells treated with insulin or palmitate. Overall these were not different to those observed in control cells. These data, taken together with the reduced membrane potential, strongly suggests that the reduced ROS levels seen in our cells are reflective of either a lower electron transfer or increased uncoupling. In any case our results are consistent with those found in a recent study with myotubes isolated from obese and Type 2 diabetic patients [30].
We also explored whether exposure to chronic insulin or palmitate altered the expression of genes coding for proteins with mitochondrial function and proteins involved in regulating mitochondrial biogenesis and dynamics. In our study there was a tendency for some OXPHOS transcripts to be decreased in the chronic insulin-treated cells after 48 h insulin treatment. However, 48 h was insufficient to detect any changes at the protein level. Our findings contrast those reported by Pagel-Langenickel et al. [37] that observed reduced expression of OXPHOS proteins COX1, COX3 and cytochrome c in cells chronically treated with insulin. These authors also found a decrease in the expression of Tfam, a nuclear-encoded transcription factor that upon activation translocates to the mitochondria to activate transcripton of mitochondrial DNA, which was not evident in our study. Tfam enables PGC-1α to transduce its signal from the nucleus to regulatory events in the mitochondria. As we saw no change in Tfam expression, this may explain why changes in expression of PGC-1α did not manifest into significant changes in the expression of OXPHOS transcripts in our study. Importantly however, in the Pagel-Langenickel et al. study, the cells were cultured at much higher glucose concentrations (40 mM), which could contribute to the differences seen between the two studies. In contrast, the expression of some OXPHOS protein complex subunits (complex I and V) were increased upon exposure of cells to palmitate. This could reflect the greater production of reduced equivalents being supplied to the respiratory chain following fatty acid oxidation.
Mfn2, a protein involved in regulating mitochondrial fusion, is substantially down-regulated in muscle during obesity, both in animal models and in humans [38]. Therefore, we examined whether the expression of this gene was altered in our cellular models. Expression of Mfn2 was not affected by chronic insulin or palmitate treatment. Our previous findings indicate that PGC-1β is implicated in the regulation of Mfn2 gene expression, mediating the co-activation of the nuclear receptor ERRα (oestrogen-related receptor α) [39]. PGC-1β mRNA was not altered by chronic insulin or palmitate exposure. Under chronic insulin we detected a significant decrease in the mRNA content for PGC-1α, which is also known to co-activate ERRα [40]. PGC-1α levels were also not different in palmitate-treated cells in comparison with controls. This is in agreement with a recent study by Bergouignan et al. [41], which showed that the total PGC-1α protein is unaltered in lean or obese humans on a high-fat diet.
Lastly, we found that chronic insulin and palmitate differentially affect the expression of UCPs. UCP protein expression is tightly regulated in skeletal muscle. For instance, physical activity and muscle wasting regulate UCP2 and UCP3 expression. In our study, chronic insulin alone decreased UCP2 and UCP3 expressions, whereas palmitate exposure significantly increased the expression of UCP2. It has been reported that acute insulin administration up-regulates the expression of UCP2 in rat skeletal muscle [42]. Furthermore, expression of UCP2 and UCP3 is compromised in muscle of insulin-resistant animal models [43] and in diabetic subjects [44]. Our study supports these findings and demonstrates that impaired insulin signalling can result in the reduced expression of UCPs in vitro. We hypothesize that these effects may be mediated at least in part by the reduced PGC-1α expression levels seen in the C2C12 insulin-resistant cells. Although we did not directly test this hypothesis in our experiments, however, existing literature supports this. First, Pagel-Langenickel et al. [37] showed that knock-down of the insulin receptor in C2C12 myotubes reduced PGC1α expression. Secondly, insulin up-regulates UCP1 expression in brown adipocytes via a PI3K/Akt mechanism [45]. However, in the presence of the saturated fatty acid, UCP expression was increased. This is consistent with findings of elevated levels of UCP2 and UCP3 transcripts found in physiological conditions with elevated plasma levels of fatty acids such as fasting and high-fat feeding, in both animals and humans (for recent reviews see [46,47]). At present, the specific molecular mechanisms that regulate the expression of UCP2 and UCP3 by insulin or fatty acids are not fully understood. Some evidence suggests that SREBPs (sterol-regulatory-element-binding proteins) and PGC1β can collaborate to up-regulate UCP2 [48]. However, in our study, we did not see any significant changes in the expression level of PGC1β in palmitate-treated cells, where UCP expression was increased. Taken together, our results suggest a greater role for PGC1α in the regulation of UCP2 and UCP3 proteins in muscle. Given the complex regulation of PGC1α function by post-translational modifications and interaction with other binding proteins, further experiments are needed to confirm this hypothesis. In addition, PPARs have been reported to bind fatty acids, particularly those of a polyunsaturated nature [49], and synthetic PPAR ligands can up-regulate UCP2 [50]. Recent work by Bugge et al. [51] demonstrates that PPARγ can activate both UCP2 and UCP3 expression via an enhancer located within the first intron of the UCP3 gene.
In conclusion, our findings demonstrate that exposure to chronic insulin or saturated fatty acid differentially has an impact on mitochondrial function and mitochondrial gene expression.
Online data
AUTHOR CONTRIBUTION
Chenjing Yang, Cho Cho Aye, Xiaoxin Li and Angels Diaz Ramos acquired the experimental data and analysed the data; Antonio Zorzano interpretated the data and revised the paper; Silvia Mora designed the study, interpreted the data and wrote the paper.
FUNDING
This work was supported by the European Commission [grant number HEALTH-F4-2008-223450].
References
- 1.Petersen K. F., Dufour S., Befroy D., Garcia R., Shulman G. I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Short K. R., Nair K. S., Stump C. S. Impaired mitochondrial activity and insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 2004;350:2419–2421. doi: 10.1056/NEJM200406033502320. [DOI] [PubMed] [Google Scholar]
- 3.Ritov V., Gnaiger E., Schjerling P., Skovbro M., Kraunsoe R., Dela F. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Patti M. E., Butte A. J., Crunkhorn S., Cusi K., Berria R., Kashyap S., Miyazaki Y., Kohane I., Costello M., Saccone R., et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heilbronn L. K., Gan S. K., Turner N., Campbell L. V., Chisholm D. J. Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J. Clin. Endocrinol. Metab. 2007;92:1467–1473. doi: 10.1210/jc.2006-2210. [DOI] [PubMed] [Google Scholar]
- 6.Mootha V. K., Lindgren C. M., Eriksson K. F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstrale M., Laurila E., et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 7.Morino K., Petersen K. F., Dufour S., Befroy D., Frattini J., Shatzkes N., Neschen S., White M. F., Bilz S., Sono S., et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J. Clin. Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang H., Bowen B., Lefort N., Flynn C., De Filippis E., Roberts C., Smoke C., Meyer C., Højlund K., Yi Z., Mandarino L. Proteomics analysis of human skeletal muscle reveals novel abnormalities in obesity and type 2 diabetes. Diabetes. 2010;59:33–42. doi: 10.2337/db09-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley D. E., He J., Menshikova E. V., Ritov V. B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 10.Befroy D., Petersen K., Dufour S., Mason G., de Graaf R., Rothman D., Shulman G. I. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–1381. doi: 10.2337/db06-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrauwen-Hinderling V. B., Kooi M. E., Hesselink M. K., Jeneson J. A., Backes W. H., van Echteld C. J., van Engelshoven J. M., Mensink M., Schrauwen P. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007;50:113–120. doi: 10.1007/s00125-006-0475-1. [DOI] [PubMed] [Google Scholar]
- 12.Phielix E., Schrauwen-Hinderling V., Mensink M., Lenaers E., Meex R., Hoeks J., Eline Kooi M., Moonen-Kornips E., Sels J. P., Hesselink M. K., Schrauwen P. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57:2943–2949. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minet A., Gaster M. ATP synthesis is impaired in isolated mitochondria from myotubes established from type 2 diabetic subjects. Biochem. Biophys. Res. Commun. 2010;402:70–74. doi: 10.1016/j.bbrc.2010.09.115. [DOI] [PubMed] [Google Scholar]
- 14.Boushel R., Gnaiger E., Schjerling P., Skovbro M., Kraunsøe R., Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle function in skeletal muscle. Diabetologia. 2007;50:790–796. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frederiksen C., Højlund K., Hansen L., Oakeley E., Hemmings B., Abdallah B., Brusgaard K., Beck-Nielsen H., Gaster M. Transcriptional profiling of myotubes from patients with type 2 diabetes: no evidence for a primary defect in oxidative phosphorylation genes. Diabetologia. 2008;51:2068–2077. doi: 10.1007/s00125-008-1122-9. [DOI] [PubMed] [Google Scholar]
- 16.Pawlikowska P., Gawjkowska B., Hocquette J., Orzechowski A. Not only insulin stimulates mitochondriogenesis in muscle cells, but mitochondria are also essential for insulin-mediated myogenesis. Cell Prolif. 2006;39:127–145. doi: 10.1111/j.1365-2184.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikula M., Dzwonek A., Hennig E., Ostrowski J. Increased mitochondrial gene expression during L6 cell myogenesis is accelerated by insulin. Int. J. Biochem. Cell. Biol. 2005;37:1815–1828. doi: 10.1016/j.biocel.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Gershman B., Puig O., Hang L., Peitzsch R. M., Tatar M., Garofalo R. S. High-resolution dynamics of the transcriptional response to nutrition in Drosophila: a key role for dFOXO. Physiol. Genomics. 2007;29:24–34. doi: 10.1152/physiolgenomics.00061.2006. [DOI] [PubMed] [Google Scholar]
- 19.Stump C., Short K., Bigelow M., Schimke J., Nair K. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc. Natl. Acad. Sci. U.S.A. 2003;100:7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boirie Y. Insulin regulation of mitochondrial proteins and oxidative phosphorylation in human muscle. Trends Endocrinol. Metab. 2003;14:393–394. doi: 10.1016/j.tem.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Halvatsiotis P., Short K. R., Bigelow M., Nair K. S. Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes. 2002;51:2395–2404. doi: 10.2337/diabetes.51.8.2395. [DOI] [PubMed] [Google Scholar]
- 22.Bijur G., Jope R. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J. Neurochem. 2003;87:1427–1435. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J.-Y., Yeh H., Lin K. J., Wang P. Insulin stimulates Akt translocation to mitochondria: implications on dysregulation of mitochondrial oxidative phosphorylation in diabetic myocardium. J. Mol. Cell. Cardiol. 2009;46:919–926. doi: 10.1016/j.yjmcc.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Didier L., Yerby B., Deacon R., Gao J. Diet-induced modulation of mitochondrial activity in rat muscle. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1169–E1177. doi: 10.1152/ajpendo.00263.2007. [DOI] [PubMed] [Google Scholar]
- 25.Sparks L., Xie H., Koza R., Mynatt R., Hulver M., Bray G., Smith S. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 26.Erion D., Schulman G. Diacylglycerol-mediated insulin resistance. Nat. Med. 2010;16:400–402. doi: 10.1038/nm0410-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupte A., Mora S. Activation of the Cbl insulin signaling pathway in cardiac muscle; dysregulation in obesity and diabetes. Biochem. Biophys. Res. Commun. 2006;342:751–757. doi: 10.1016/j.bbrc.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Yuzefovych L., Wilson G., Rachel L. Different effects of oleate vs palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells, role of oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2010;299:E1096–E1105. doi: 10.1152/ajpendo.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaster M. Reduced TCA flux in diabetic myotubes: a governing influence on the diabetic phenotype? Biochem. Biophys. Res. Commun. 2009;387:651–655. doi: 10.1016/j.bbrc.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 30.Minet A., Gaster M. Hydrogen peroxide production is not primarily increased in human myotubes established from type 2 diabetic subjects. J. Clin. Endocrinol. Metab. 2011;96:E1486–E1490. doi: 10.1210/jc.2011-1384. [DOI] [PubMed] [Google Scholar]
- 31.Ash C. E., Merry B. The molecular basis by which dietary restricted feeding reduces mitochondrial reactive oxygen species generation. Mech. Ageing Dev. 2011;132:43–54. doi: 10.1016/j.mad.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Hue L., Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am. J. Physiol. Endocrinol. Metab. 2009;297:E578–E591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuzefovych L., Solodushko V. A., Wilson G., Rachek L. Protection from palmitate-induced mitochondrial DNA damage prevents from mitochondrial oxidative stress, mitochondrial dysfunction, apoptosis and impaired insulin signalling in rat L6 skeletal muscle cells. Endocrinology. 2012;153:92–100. doi: 10.1210/en.2011-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacLellan J., Gerrits M., Gowing A., Smith P., Wheeler M., Harper M. Physiological increases in uncoupling protein 3 augment fatty acid oxidation and decrease reactive oxygen species production without uncoupling respiration in muscle cells. Diabetes. 2005;54:2343–2350. doi: 10.2337/diabetes.54.8.2343. [DOI] [PubMed] [Google Scholar]
- 35.Bonnard C., Durand A., Peyrol S., Chanseaume E., Chauvin M., Morio B., Vidal H., Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Invest. 2008;118:789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lefort N., Glancy B., Bowen B., Willis W., Bailowitz Z., De Filippis E., Brophy C., Meyer C., Hojlund K., Yi Z., Mandarino L. Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1b protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle. Diabetes. 2010;59:2444–2452. doi: 10.2337/db10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pagel-Langenickel I., Bao J., Joseph J., Schwartz D., Mantell B., Xu X., Raghvachari N., Sack M. PGC1-a Integrates insulin signaling, mitochondrial regulation and bioenergetic function in skeletal muscle. J. Biol. Chem. 2008;283:22464–22472. doi: 10.1074/jbc.M800842200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bach D., Naon D., Pich S., Soriano F. X., Vega N., Rieusset J., Laville M., Guillet C., Boirie Y., Wallberg-Henriksson H., et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–2693. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 39.Liesa M., Borda-d'Agua B., Medina-Gomez G., Lelliott C. J., Paz J. C., Rojo M., Palacin M., Vidal-Puig A., Zorzano A. Mitochondrial fusion is increased by the nuclear coactivator PGC-1β. PLoS ONE. 2008;3:e3613. doi: 10.1371/journal.pone.0003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soriano F. X., Liesa M., Bach D., Chan D. C., Palacin M., Zorzano A. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1α, estrogen-related receptor-α and mitofusin 2. Diabetes. 2006;55:1783–1791. doi: 10.2337/db05-0509. [DOI] [PubMed] [Google Scholar]
- 41.Bergouignan A., Gozansky W., Barry D., Leitner W., McLean P., Hill J., Draznin B., Melanson E. Increasing dietary fat elicits similar changes in fat oxidation and markers of muscle oxidative capacity in lean and obese humans. Plos ONE. 2012;7:e30164. doi: 10.1371/journal.pone.0030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen S., Lund S., Buhl E., Richelsen B. Insulin and contraction directly stimulate UCP2 and UCP3 mRNA rat skeletal muscle in vitro. Biochem. Biophys. Res. Commun. 2001;283:19–25. doi: 10.1006/bbrc.2001.4736. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C., Zhang M., Zhu J.-G., Ji C.-B., C, Z., Kou C.-Z., D-N, Q., Tong M.-L., Guo X.-P. Tissue-specific expression profiles of the uncoupling protein family in normal control mice and genetically ob/ob mice. J. Bioenerg. Biomembr. 2010;42:255–259. doi: 10.1007/s10863-010-9292-9. [DOI] [PubMed] [Google Scholar]
- 44.Krook A., Digby J., O'Rahilly S., Zierath J., Wallberg-Henriksson H. Uncoupling protein 3 is reduced in skeletal muscle of NIDDM patients. Diabetes. 1998;47:1528–1531. doi: 10.2337/diabetes.47.9.1528. [DOI] [PubMed] [Google Scholar]
- 45.Valverde A., Arribas M., Mur C., Navarro P., Pons S., Cassard-Doulcier A., Kahn C., Benito M. Insulin-induced upregulated uncoupling protein-1 expression is mediated by insulin receptor substrate 1 through the phosphatydilinositol 3-kinase/Akt signaling pathway in fetal brown adipocytes. J. Biol. Chem. 2003;278:10221–10231. doi: 10.1074/jbc.M209363200. [DOI] [PubMed] [Google Scholar]
- 46.Yonezawa T., Kurata R., Hosomichi K., Kono A., Kimura M., Inoko H. Nutritional and hormonal regulation of uncoupling protein 2. IUBMB Life. 2009;61:1123–1131. doi: 10.1002/iub.264. [DOI] [PubMed] [Google Scholar]
- 47.Thompson M., Kim D. H. Links between fatty acids and expression of UCP2 and UCP3 mRNAs. FEBS Lett. 2004;568:4–9. doi: 10.1016/j.febslet.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 48.Oberkofer H., Klein K., Felder T. K., Krempler F., Patsch W. Role of peroxisome-proliferator activated receptor (PPAR)-γ co-activator-1beta is involved in the regulation of glucose-stimulated insulin secretion in INS-1E cells. J. Mol. Med. 2006;87:299–306. doi: 10.1007/s00109-008-0425-0. [DOI] [PubMed] [Google Scholar]
- 49.Forman B., Chen J., Evans R. Hypolipidemic drugs, polyunsaturated fatty acids and eicosanoids are ligands for peroxisome-proliferator activated receptors α and δ. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aubert J., Champigny O., Saint-Marc P., Negrel R., Collins S., Ricquier D., Aihaud G. Upregulation of UCP2 gene expression by PPAR agonists in preadipose and adipose cells. Biochem. Biophys. Res. Commun. 1997;238:606–611. doi: 10.1006/bbrc.1997.7348. [DOI] [PubMed] [Google Scholar]
- 51.Bugge A., Siersbæk M., Madsen M., Göndör A., Rougier C., Mandrup S. A novel intronic peroxisome-proliferator activated receptor γ enhancer in the uncoupling protein (UCP) 3 gene as a regulator of both UCP2 and 3 expression in adipocytes. J. Biol. Chem. 2010;285:17310–17317. doi: 10.1074/jbc.M110.120584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.