Abstract
The propensity of LDLs (low-density lipoproteins) for aggregation and/or oxidation has been linked to their sphingolipid content, specifically the levels of SM (sphingomyelin) and ceramide. To investigate this association in vivo, ldlr (LDL receptor)-null mice (ldlr−/−) were fed on a modified (atherogenic) diet containing saturated fats and cholesterol. The diet led to significantly elevated SM content in all serum lipoproteins. In contrast, ceramide increased only in the LDL particles. MS-based analyses of the lipid acyl chain composition revealed a marked elevation in C16:0 fatty acid in SM and ceramide, consistent with the prevalence of palmitic acid in the modified diet. The diet also led to increased activity of the S-SMase [secretory SMase (sphingomyelinase)], a protein that is generated by ASMase (acid SMase) and acts on serum LDL. An increased macrophage secretion seemed to be responsible for the elevated S-SMase activity. ASMase-deficient mice (asm−/−/ldlr−/−) lacked S-SMase activity and were protected from diet-induced elevation in LDL ceramide. LDL from asm−/−/ldlr−/− mice fed on the modified diet were less aggregated and oxidized than LDL from asm+/+/ldlr−/− mice. When tested in vitro, the propensity for aggregation was dependent on the SM level: only LDL from animals on modified diet that have high SM content aggregated when treated with recombinant S-SMase. In conclusion, LDL-SM content and S-SMase activity are up-regulated in mice fed on an atherogenic diet. S-SMase mediates diet-induced changes in LDL ceramide content and aggregation. S-SMase effectiveness in inducing aggregation is dependent on diet-induced enrichment of LDL with SM, possibly through increased hepatic synthesis.
Keywords: atherosclerosis, ceramide, low-density lipoprotein aggregation, sphingomyelin, secretory sphingomyelinase
Abbreviations: ABV, aorta and blood vessel; apoE, apolipoprotein E; C6-NBD-Cer, 6-[N-(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]hexanoylceramide; C6-NBD-SM, 6-[N-(7-nitro-2,1,3-benzoxadiazol-4-yl) amino]hexanoylsphingosylphosphocholine; ESI, electrospray ionization; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ldlr, LDL receptor; L-SMase, lysosomal ASMase; SM, sphingomyelin; SMase, sphingomyelinase; ASMase, acid SMase; bSMase, bacterial SMase; SPT, serine-palmitoyl transferase; S-SMase, secretory SMase; TBARS, thiobarbituric acid-reactive substances; VLDL, very-low-density lipoprotein
INTRODUCTION
SM (sphingomyelin) is a major lipid component of LDL (low-density lipoprotein) particles and together with phosphatidylcholine forms the polar surface of the lipoproteins. The SM found in the lipoproteins originates from the liver, where it is generated via the de novo pathway of sphingolipid synthesis and then incorporated into VLDL (very-low-density lipoprotein) particles for secretion in the circulation. The availability of SM for lipoprotein formation depends on the rate-limiting step in its de novo synthesis, the SPT (serine-palmitoyl transferase) reaction [1,2]. SPT generates dihydrosphingosine, which is converted consequentially into dihydroceramide, ceramide and SM. S-SMase [secretory SMase (sphingomyelinase)] can convert SM in atherogenic serum lipoprotein particles back into ceramide [3,4]. The S-SMase enzyme, secreted by macrophages and endothelial cells, is an alternatively modified form of the 75 kDa protein product of the asm gene [encoding ASMase (acid SMase)], which also produces the lysosomal form of ASMase [5].
Plasma SM levels have been shown to correlate with the incidence of diet-induced atherosclerosis in primates, inherent atherosclerosis in WHHL (Watanabe hereditable hyperlipidaemic) rabbits and coronary heart disease in humans [6–9]. LDL particles isolated from human arteriosclerotic lesions have high SM levels, implying that increases in serum SM are linked to sub-endothelial retention of atherogenic LDL particles [10]. In contrast, lowering the plasma SM content in apoE (apolipoprotein E)−/− mice through the pharmacological inhibition of SM synthesis in liver delays the development of atherosclerotic lesions by 42% without having any effect on total serum cholesterol or triacylglycerols [9,11,12]. These studies suggest that SM in lipoproteins may influence their atherogenic properties and thus the progression of atherosclerosis. It remains unclear, however, how the elevated SM in the circulation increases the risks for the development of atherosclerosis.
Several studies have shown that the rate of de novo synthesis and incorporation of SM in serum lipoproteins, as well as its degradation by the S-SMase is regulated [2,9,13,14]. Increased consumption of diets enriched in saturated fats and cholesterol leads to stimulation of de novo synthesis of sphingolipids in the liver [13]. The main saturated fat in these diets, palmitic acid, is a substrate for SPT and when provided in excess can stimulate SPT mRNA expression and activity [2,13]. These changes are paralleled by elevation in total plasma ceramide and SM content [9]; however, the effects atherogenic diet have on sphingolipid composition of the individual lipoproteins have not been studied. S-SMase activity, in turn, also seems to be regulated. It increases in response to IL-1β (interleukin-1β) and IFNγ (interferon γ) stimulation of macrophages [14], in patients with CHF (chronic heart failure) [15] and Type 2 diabetes mellitus [16]. Notably, activation of S-SMase may be directly linked to the development of atherosclerosis because elevation of ceramide content in LDL particles has been shown to enhance the activity of secretory PLA2 (phospholipase A2) [17], to promote LDL uptake in macrophages and to facilitate foam cell formation [18]. Ceramide-enriched LDL is isolated from atherosclerotic plaques [19,20] and is associated with accelerated LDL aggregation [21] and microvascular endothelial cell apoptosis [22,23]. Indeed, in vitro treatment of human LDL particles with bSMase (bacterial SMase), which similarly to S-SMase converts LDL-SM into ceramide, induces LDL aggregation [24] and oxidation [25]. It has been suggested that the sub-endothelial retention of LDL is facilitated by S-SMase-induced aggregation and binding of LDL to the matrix proteoglycans [26,27]. In contrast, asm ablation in vivo slows down the sub-endothelial lipoprotein retention and the development of lesions in apoE−/− and ldlr−/− mice [28].
Despite these indications that S-SMase is critical for aggregation of LDL particles, direct evidence for regulation of S-SMase activity during atherosclerosis and its role in LDL modification is still missing. In the present study, we used C57Bl6 and ldlr−/− mice that have ASMase deficiency (asm−/−) to characterize the effects an atherogenic diet has on sphingolipid content of lipoproteins and S-SMase activity and to investigate how S-SMase affects the propensity of LDL particles for aggregation.
EXPERIMENTAL
Materials
C6-NBD-Cer {6-[N-(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]hexanoylceramide} and C6-NBD-SM {6-[N-(7-nitro-2,1,3benzoxadiazol-4-yl)amino]hexanoylsphingosylphosphocholine} were from Molecular Probes, C17:0 ceramide (N-heptadecanoyl-D-erythro-sphingosine), C17:0 SM (N-heptadecanoyl-D-erythro-sphingosylphosphocholine), N-acetyl-C20-sphinganine and bovine brain ceramide were purchased from Avanti Polar Lipids. bSMase was from Sigma–Aldrich. Lowry total protein determination kit (Dc protein Assay) was from Bio-Rad. All other reagents were from Fisher Scientific.
Animals and diets
A colony of ASMase-deficient mice (asm−/−) in C56Bl6 background was maintained in the AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care International)-approved animal facility of University of Kentucky Medical Center by breeding heterozygous asm+/− mice. asm−/−/ldlr−/−, a double knockout mouse colony, was generated and maintained in our laboratory as described previously by litter mating of asm+/−/ldlr−/− animals [13]. Litter-matched asm−/−/ldlr−/− and asm−/− offspring and their respective controls – asm+/+/ldlr−/− and asm+/+ were used throughout the experiments. After weaning, all mice were genotyped [29] and placed on a standard chow diet (2918, Teklad-Global; 18% protein rodent diet; Harlan-Teklad), 12 h light/12 h dark cycle in micro-isolation. Eight-week-old litter-matched mice from both genders were randomly placed on either a diet rich in saturated fats and cholesterol (modified diet, TD.88137, adjusted calorie diet, 42% from fat) (Harlan-Teklad) or continued on the standard diet for a period of 10 weeks. At the end of the diet, mice were deeply anaesthetized, blood was collected by heart puncture and the serum was obtained in serum separator tubes. Liver was dissected, flash-frozen in liquid nitrogen and kept at −80°C until further processed. All animal experimentations were included in our animal protocol, approved by IACUC (Institutional Animal Care and Use Committee) and carried out in complete agreement with the recommendations of the AVMA (American Veterinary Medical Association).
Isolation of lipoprotein fractions
Lipoproteins were separated by sequential ultracentrifugation in Beckman Optima tabletop ultracentrifuge (Beckman Instruments) following a previously described procedure [30] with some modifications. Briefly, fresh serum was centrifuged at 540000 g at 4°C for 3.7 h at a density of 1.019 g/ml to obtain VLDL (very-LDL), for 3.4 h at 1.063 g/ml for LDL and for 6.8 h at a density of 1.21 g/ml for HDL (high-density lipoproteins). After isolation, lipoprotein particles were dialysed overnight at 4°C against PBS using 0.5–3 ml Dialysis Cassettes (Pierce). The VLDL, LDL and HDL were stored under argon to prevent oxidation. Quality and physical properties of freshly isolated lipoprotein fractions (10 μg of protein per lane) were monitored by electrophoresis in 1.8% agarose gel and visualized with Coomassie Brilliant Blue staining.
Isolation of peritoneal macrophages and ex vivo tissue cultures
Resident peritoneal macrophages were isolated from asm−/−/ldlr−/− and asm+/+/ldlr −/− on either modified or standard diets. Macrophages collected from three or four animals were pooled together and plated in 35 mm dishes at density ranging from 1.8×106 to 2.6×106 cells/dish. ABVs (aorta and blood vessels) surrounding the heart were dissected, cleared from visible debris of fat, cut longitudinally to expose the endothelial cells and washed twice with PBS. ABVs from mice in the same experimental group were cultured together in a 35 mm dish. For ex vivo assays of the fat tissue, a piece of epididymal fat from several animals (3–5 animals per group) was dissected, washed twice with PBS and placed together in 35 mm dish. Macrophages and ex vivo tissues were cultured in DMEM (Dulbecco's modified Eagle's medium), supplemented with 0.1% FBS and 100 units/ml of penicillin/streptomycin mix (Gibco Laboratories) for 5 h in a 37°C humidified incubator (5% CO2). Conditioned medium was collected, concentrated using 10 K Ultracell centrifugal filters (Amicon, Millipore) following the manufacturer's instructions and frozen for future use.
SMase activity assays
S-SMase and L-SMase (lysosomal SMase) activities were measured using C6-NBD-SM as a substrate. Serum samples from mice and concentrated conditioned medium were used as the source of S-SMase. The standardized assay contained serum (2 μl) or medium (10 μl), 20 μM NBD-SM, 0.1 mM ZnCl2, 0.1 M sodium acetate buffer (pH 5.0) in a final volume of 20 μl. For L-SMase activity, 10 μg of cell homogenates from macrophages were used as the enzyme source. Reactions were allowed to continue for 3 h at 37°C and were stopped by the addition of 0.5 ml of methanol. After further incubation at 37°C for 30 min, the samples were centrifuged at 1000 g and the generation of fluorescent product, NBD-ceramide, was monitored by reverse-phase HPLC using methanol/water/phosphoric acid (850:150:0.15, by vol.) as a mobile phase [31].
Measurements of sphingolipid mass
Mass measurements of SM and Cer by TLC/HPLC
The lipids were extracted by the method of Blight and Dyer [32] modified as described previously [33] and were analysed by TLC on silica gel 60 plates (10 cm×20 cm) using chloroform/methanol/triethylamine/2-propanol/0.25% potassium chloride (30:9:18:25:6, by volume) as the developing solvent. The regions migrating with a standard bovine brain ceramide were scraped from the plate. To quantify the mass of ceramide, the lipids were eluted from the silica with 1 ml of chloroform/methanol (1:1, v/v) followed by 1 ml of methanol. The combined elutes were dried in vacuo, then 0.5–1.0 mol of internal standard, N-acetyl-C20-sphinganine, was added to the unknown ceramide sample and the ceramide mass was quantified by HPLC of the long-chain bases released after an acid hydrolysis in 0.5 M HCl in methanol at 65°C for 15 h. Free long-chain bases were analysed as described by Merrill et al. [34]. SM mass was measured by two approaches with similar results. LDLs were treated with bSMase (0.5 unit/ml) in 0.1 M Tris/HCl buffer, pH 7.4 for 2 h at 37°C to promote hydrolysis of SM to ceramide. The ceramide levels in SMase-treated and not-treated LDL were measured as described above and the SM content was calculated by subtracting ceramide mass measured in non-bSMase-treated samples from those that were treated with bSMase. Hepatic SM content was determined by TLC separation followed by measuring the Pi as described previously [13].
Lipid analysis using tandem MS
Total lipid extracts from 0.5 mg of liver tissue, 30 μl of serum and 50 μg of VLDL and LDL were obtained using acidified organic solvents [35] with the addition of ceramide and SM internal standards containing a C17:0 fatty acid (Avanti Polar Lipids). After the lipid containing lower phase was evaporated to dryness under N2 and reconstituted in methanol, molecular species of SM and ceramide were detected by monitoring species-specific precursor product ion pairs by HPLC–ESI (electrospray ionization) tandem MS using 4000 Q-Trap hybrid linear ion trap triple-quadrupole mass spectrometer as described previously [36] with minor modifications. Recovery was determined by reference to the internal standards and quantification accomplished according to the calibration curves constructed using a set of synthetic standards for each ceramide and SM species obtained from Avanti Polar Lipids which were independently quantified by accurate mass measurements. SM containing cerotic (C26:0), lignoceric (C24:0), nervonic (C24:1), behenic (C22:0), arachidic (C20:0), stearic (C18:0), oleic (C18:0), palmitic (C16:0) and myristic (C14) acids was measured and also ceramides with lignoceric acid (C24:0), nervonic (C24:1), arachidic (C20:0), stearic (C18:0), oleic (C18:1), palmitic (C16:0) and myristic (C14:0) acids.
TBARS (thiobarbituric acid-reactive substances) and turbidity assays
Oxidation in LDL was determined spectrophotometrically by measuring the amount of TBARS as described previously [37]. Briefly, 50 μg of LDL was precipitated with 0.25 ml of 20% trichloroacetic acid. Then, 0.625 ml 0.2% thiobarbituric acid and 0.750 ml of 0.05 M sulfuric acid were added and the samples were boiled for 30 min. After cooling down, addition of 1 ml of n-butyl alcohol, vigorous vortex-mixing and phase separation at 1000 g for 5 min, the absorbance was measured in the upper phase at 530 nm.
The susceptibility of LDL to aggregation was determined as described previously with minor modifications [38]. LDL diluted with PBS (0.25 mg/ml) was continuously vortex-mixed for up to 5 min and the changes in absorbance at 680 nm monitored at the indicated times.
Statistical analyses
After assuming equal variances across groups, the observed differences were tested statistically using Student's t test when two groups with one variable were compared or two-way ANOVA when the effects of the two parameters (diet and genotype) were assessed. Results are means±S.D. and are representative of at least two independent experiments. In the Figures, significance of main diet and genotype effect is indicated by * and ∧ symbols, while # indicates the significance of interaction effects.
RESULTS
Atherogenic diet increases S-SMase activity in serum
The effect atherogenic (modified) diet has on serum S-SMase activity and LDL sphingolipid content and properties was investigated using C57Bl6 and ldlr−/− mice. The latter are the preferred animal model to study atherosclerosis and hypercholesterolaemia, because of their high serum LDL content and the possibility to easily isolate particles for biochemical and biophysical analyses. At the same time, however, our previous studies have shown that endogenous LDL distorts the S-SMase activity assay due to substrate competition [39]. To avoid this, S-Smase activity was also assayed in C57Bl6 mice that have negligible endogenous LDL content.
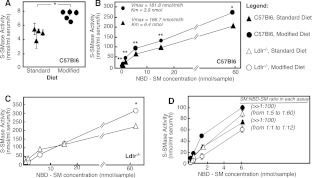
Eight-week-old male mice (C57Bl6 and ldlr−/−) were placed for 10 weeks either on standard chow diet (18% protein diet, containing 1% saturated fats and no cholesterol) or a modified (atherogenic) one (42% fat, calorie-adjusted diet, containing 13.8% saturated fats and 1.5% cholesterol). S-SMase activity was measured in serum using NBD-SM as a substrate. Significant increases in S-SMase activity were observed in C57Bl6 mice fed on the atherogenic diet, as compared with C57Bl6 on standard chow (Figure 1A). Judging by Michaelis–Menten analyses, both the Km and Vmax of the enzyme reaction were affected (Figure 1B). These results indicate that S-SMase activity is elevated in mice fed on a modified diet.
Figure 1. S-SMase activity in serum.
Mice of the indicated genotypes were placed on either a standard or modified diet for 10 weeks. S-SMase activity was measured using C6-NBD-SM as a substrate. (A) S-SMase activity in C57Bl6 mice measured by using 0.4 nmol of NBD-SM per sample. Results are shown as means±S.D. (n=3) for each mouse. (B–D) Michaelis–Menten kinetics of the S-SMase activity in C57Bl6 and ldlr−/− mice on either standard or modified diet. The assays are carried out using serum (4–6 animals per assay) and the indicated substrate concentrations. Mean values of triplicates ±S.D. are shown. *P<0.05 and **P<0.01 according to a Student's t test. SM/NBD-SM ratio in (D) was calculated by dividing the measured endogenous LDL-SM concentration (presented in Table 1) and the amount of NBD-SM in each assay.
In the ldlr−/− strain, however, diet-associated increases in S-SMase activity were only evident when high concentrations of the NBD-SM substrate were used (Figure 1C). Furthermore, the substrate-dependence curves did not follow the classical Michaelis–Menten pattern. One likely explanation is that the serum from ldlr−/− mice on atherogenic diet has very high content of LDL (which carries the endogenous SM substrate of S-SMase), which competes with the exogenous NBD-SM substrate during the assay. Indeed, the calculated ratio between the endogenous SM present in each assay sample and the added NBD-SM was above 1:100 for assays done with C67Bl6 mice on either diets and remained in the 1:5–1:60 range for ldlr−/− mice fed on the standard diet (Figure 1D). Owing to the diet-induced increases in LDL-SM, however, this ratio decreased to between 1:1 and 1:12 in the ldlr−/− mice on modified diet and a competition between the substrates became noticeable. Increasing the exogenous substrate to 60 nmol (when the ratio again became higher than 1:100) overcomes these shortcomings of the assay. Together, these results show conclusively that diet enriched in saturated fats and cholesterol increases the activity of serum S-SMase in both animal models.
Atherogenic diet is associated with parallel elevation of serum and hepatic sphingolipids
Analyses of sphingolipid content in total serum showed that, in ldlr−/− mice on standard chow diet, SM at 64.07 nmol/ml is the main serum sphingolipid. The concentrations of ceramide and dihydroceramide were 9.9 and 1.98 nmol/ml respectively. The diet caused drastic increases in the levels of serum SM to 486 nmol/ml, ceramide to 53.83 nmol/ml and dihydroceramide to 10.42 nmol/ml respectively. The elevation in all serum sphingolipids in mice fed on the atherogenic diet was paralleled by simultaneous accumulation of ceramide, dihydroceramide and SM in the liver (Table 1), suggesting that the changes in serum sphingolipid reflect increased sphingolipid synthesis in the liver [13]. Indeed, previous studies had associated atherogenic diet with stimulation of the de novo sphingolipid synthesis resulting from increased flux of dietary palmitate into the sphingolipid biosynthetic pathway and the induction of hepatic SPT mRNA transcription and activity [13]. In turn, SPT stimulation has been found to elevate hepatic secretion of SM and ceramide in vitro [2]. The results in Table 1 are consistent with these previous findings and illustrate in vivo the paralleled effect of atherogenic diet on hepatic and serum sphingolipids.
Table 1. Effects of atherogenic diet on lipoprotein and liver sphingolipid levels.
ldlr−/− mice were placed on atherogenic diet for 10 weeks and sphingolipid classes were measured by TLC/HPLC. Serum from three or four animals was pooled for the isolation of lipoprotein particles. Results are means±S.D. (n=3). *P<0.05. Cer, ceramide; DH-Cer, dihydroceramide.
| Sphingolipid level (nmol/ml of serum) | Sphingolipid level (nmol/mg of protein) | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Sphingolipid | Diet… | Standard | Modified | Diet… | Standard | Modified |
| VLDL | SM | 14.61±1.35 | 220.10±77.86* | 52.19±4.85 | 120.20±42.54* | ||
| Cer | 4.91±0.06 | 16.27±2.18* | 17.21±0.22 | 8.89±1.19* | |||
| DH-Cer | 1.24±0.11 | 4.15±1.17* | 4.37±0.38 | 2.26±0.64* | |||
| LDL | SM | 48.32±1.64 | 227.64±15.07* | 59.65±2.02 | 115.88±25.03* | ||
| Cer | 2.64±0.02 | 33.24±1.71* | 3.22±0.02 | 15.04±0.77* | |||
| DH-Cer | 0.68±0.21 | 5.43±1.82* | 0.84±0.32 | 2.81±0.82* | |||
| HDL | SM | 0.92±0.64 | 36.41±1.79* | 2.29±1.62 | 20.11±0.98* | ||
| Cer | 0.45±0.06 | 3.48±1.54* | 1.11±0.14 | 1.92±0.85 | |||
| DH-Cer | 0.05±0.05 | 0.47±0.20* | 0.14±0.01 | 0.26±0.11 | |||
| LPDS | SM | 0.22±0.71 | 2.09±0.20* | 0.05±0.01 | 0.06±0.01 | ||
| Cer | 1.90±0.94 | 0.84+0.07 | 0.12±0.06 | 0.03±0.01 | |||
| DH-Cer | 0.01±0.01 | 0.37±0.03* | 0.04±0.07 | 0.60±0.01 | |||
| Liver | SM | N/A | N/A | 0.50±0.02 | 1.90±0.40* | ||
| Cer | N/A | N/A | 0.64±0.04 | 1.09±0.20* | |||
| DH-Cer | N/A | N/A | 0.03±0.02 | 0.09±0.01* | |||
To delineate what role S-SMase has in regulating SM and ceramide content of LDL particles, analyses of individual serum lipoprotein classes were done. In mice on the atherogenic diet, the levels of sphingolipids associated with all three lipoprotein types, VLDL, LDL and HDL, increased substantially (Table 1). After data were normalized to the protein amount in each lipoprotein fraction, the concentration of SM in VLDL and LDL in mice fed on the atherogenic diet remained significantly elevated, indicating that these particles became enriched in SM. Ceramide and dihydroceramide concentrations, on the other hand, increased significantly only in LDL but not VLDL.
Diet-induced activation of S-SMase is accountable for the ceramide enrichment of LDL
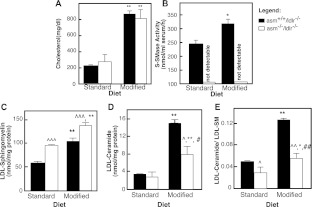
The role S-SMase plays in determining the ceramide enrichment of LDL was investigated in sera from asm−/−/ldlr −/− and asm+/+/ldlr −/− mice placed on either modified or standard diet. In a previous study, we reported the generation of this novel composite knockout mouse strain, in which asm deficiency (asm−/−) was introduced into ldlr−/− background [13]. Feeding asm−/−/ldlr−/− mice and their respective controls, asm+/+/ldlr−/−, with atherogenic diet, revealed that the lack of ASMase had no effect on diet-induced hypercholesterolaemia, and mice from both genotypes exhibited similar increases in serum VLDL and LDL cholesterol and similar lipoprotein profiles [13]. The experiments shown in Figure 2(A) confirm that asm-deficient mice and wild-type control develop comparable hypercholesterolaemia when placed on atherogenic diet. The increases in LDL protein were also similar for both genotypes (results not shown). As anticipated, the asm-null mice have no detectable S-SMase activity thus confirming that S-SMase is the only SM-hydrolysing enzyme in mouse serum (Figure 2B). Mice deficient in S-SMase consequently have significantly higher LDL-SM content than their wild-type counterparts. This LDL-SM increases similarly for both genotypes when placed on an atherogenic diet (Figure 2C). In contrast, diet-induced increases in LDL-ceramide (and in the ceramide to SM ratio) are substantially attenuated in the asm−/−/ldlr −/− mice (Figures 2D and 2E). The statistical significance of the diet–genotype interaction effect based on two-way ANOVA is P=0.016 for LDL-ceramide (Figure 2D) and P=0.01 for the ceramide/SM ratio (Figure 2E) confirming that asm deletion eliminates diet-induced accumulation of ceramide in the LDL particles. This effect is specific for LDL since the corresponding elevation in ceramide observed in the liver is not reversed by the ASMase deletion [13]. Collectively, these results provide evidence that the activation of serum S-SMase facilitates the conversion of LDL-SM into ceramide in mice on atherogenic diet.
Figure 2. Role of S-SMase in diet-induced changes in serum cholesterol levels and LDL-sphingolipid content.
Mice of the indicated genotype were placed on either a standard or modified diet for 10 weeks (3–5 animals per group). Total serum (A and B) or purified LDL particles (C–E) were analysed for total cholesterol (A), S-SMase activity (B), SM, (C), ceramide (D) or the molar ratio of ceramide to SM (E). Values are means±S.D. (n=3 animals per group). Statistical significance was analysed using two-way ANOVA followed by Bonferonni post-hoc test. The results of the Bonferonni post-hoc test with respect to the diet (*P<0.05, **P<0.01 and ***P<0.001), genotype (∧∧P<0.01), as well as the interaction effects from two-way ANOVA (#P<0.05 and ##P<0.01) are shown.
SM and ceramide levels affect the oxidation and aggregation properties of LDL
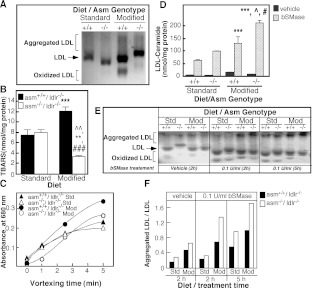
To assess the physiological significance of the observed differences in SM and ceramide content, LDL particles from asm+/+/ldlr −/− and asm−/−/ldlr−/− on either standard or atherogenic diets were analysed for oxidation and aggregation as described previously [22]. When run on agarose gel (Figure 3A), LDL from asm+/+/ldlr −/− and asm−/−/ldlr −/− mice fed on the standard diet run as single bands. LDL particles from asm+/+/ldlr −/− mice fed on the high-fat diet exhibited different electrophoretic mobility, with some migrating very slowly (aggregated LDL) and others migrating at a higher rate and forming a secondary band (oxidized LDL) (Figure 3A). These indications for aggregation and oxidation were less pronounced in the LDL from asm−/−/ldlr−/− mice. LDL oxidation and self-aggregation were further assessed by measuring the levels of TBARS and by assaying changes in turbidity. As shown in Figures 3(B) and 3(C), LDL particles from asm+/+/ldlr −/− mice on the modified diet exhibited higher TBARS values and marked increases in the turbidity of the LDL solution, as compared with LDL from asm+/+/ldlr−/− mice on a standard diet. In contrast, there was little increase in the turbidity and, in fact, a decrease in TBARS levels for LDL from asm−/−/ldlr−/− mice on a high-fat diet, as compared with asm−/−/ldlr−/− mice on a standard diet. Such a decrease is likely to reflect the higher SM content of the former. As mentioned before, SM has been shown to protect against LDL oxidation [25]. These results suggest that S-SMase activity contributes to the increased LDL oxidation and aggregation in mice on an atherogenic diet.
Figure 3. LDL biophysical properties and susceptibility to modifications.
LDL particles were isolated by sequential ultracentrifugation from sera of asm−/−/ldlr−/− [labelled with (−/−)] and asm+/+/ldlr−/− [labelled with (+/+)] mice placed on either standard or modified diet for 10 weeks (3–5 animals per group). (A) Electrophoretic mobility of LDL particles (10 μg of protein per lane) in 1.8% agarose gel visualized by Coomassie Brilliant Blue staining. (B) Oxidation of LDL assessed by measuring TBARS. Results are means±S.D. of triplicate measurements. Statistical significance was analysed by two-way ANOVA followed by Bonferonni post-hoc test. The results of the Bonferonni post-hoc test with respect to the diet (**P<0.01), genotype (∧∧P<0.01) as well as the genotype/diet interaction effect from two-way ANOVA (###P<0.001) are shown. (C) Turbidity assay of LDL aggregation. Changes in absorbance were monitored at 680 nm at the indicated times. (D) TLC/HPLC determination of the increase in LDL ceramide content following treatment with bSMase (0.5 unit/ml). The increases in ceramide content due to bSMase treatment were analysed for statistical significance using two-way ANOVA and Bonferonni post-hoc test with respect to diet and genotype. The results of the Bonferonni test with respect to the diet (**P<0.01 and ***P<0.001) and genotype (∧P<0.05 and ∧∧P<0.01) are shown. (E) Effect of bSMase treatment on the electrophoretic properties of LDL particles (10 μg of protein) in 1.8% agarose gel visualized by Coomassie Brilliant Blue staining. Representative results from at least three independent experiments, including three different isolations of LDL, are shown. Std, Standard; Mod, modified. (F) Quantification of the results shown in (E).
To further confirm that the conversion of LDL-SM into ceramide is a key factor determining LDL aggregation and/or oxidation, we incubated LDL particles from all groups with bSMase for 2 h at 37°C to hydrolyse LDL-SM to ceramide. A control group was treated with vehicle under the same conditions. The resulting increases in ceramide content correlated well with the initial SM levels in each type of LDL particles (Figure 3D). bSMase treatment had a pronounced effect on LDL electrophoretic mobility: (i) the proportion of particles that were retained at the start of the gel (indication of aggregation) was elevated and (ii) those particles that migrated into the gel had increased motility (indication of oxidation) (Figure 3E). These effects confirm that conversion of SM into ceramide increases LDL self-aggregation and/or oxidation. Notably, the rate of bSMase-induced self-aggregation correlates well with the initial levels of SM (Figure 3F). These results indicate that the LDL propensity for self-aggregation depends not only on the activity of S-SMase but also on the amount of SM available for hydrolysis. For these reasons, LDL particles from asm+/+/ldlr−/− mice on standard diet show the least susceptibility for aggregation, while those from asm−/−/ldlr−/− fed on a high-fat diet and consequently having highest SM levels were most susceptible to SMase-induced aggregation. Collectively, these findings suggest that, while S-SMase activity is indeed involved in LDL aggregation, unless the LDL particles have high levels of SM (such as those observed in the mice fed on an atherogenic diet), the net impact of S-SMase may be limited.
Atherogenic diet stimulates S-SMase secretion by macrophages, but not by ABVs
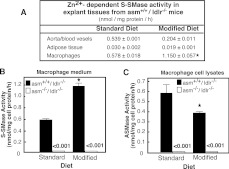
Previous studies have suggested that ABVs and macrophages are the main source of S-SMase activity [14,40]. Therefore, we sought to determine whether these same cell types are responsible for the observed increase in S-SMase activity. Tissues were collected from mice on the respective diets and cultured ex vivo in the presence of defined growth media. After 5 h, the medium was collected, concentrated and S-SMase activity was measured. Only negligible activity was detected in the absence of Zn2+, confirming that the activity measured was not due to enzymes released from dying/lysed cells.
S-SMase activity under standard diet was high for ABVs, as well as for resident peritoneal macrophages, while it was low for epididymal adipose tissue (Figure 4A). The atherogenic diet had seemingly no stimulatory effect on the S-SMase of ABVs and fat tissue. If anything, the activity of ABVs tended to decline. However, macrophages from mice on modified diet exhibited 2-fold higher SMase activity. As expected, the S-SMase-deficient mice had no detectable secretory activity associated with their macrophages on either diet (Figure 4B). This confirmed that the activity measured in our assays came solely from S-SMase. Importantly, the observed diet-induced enzyme stimulation was S-SMase-specific since the activity of the L-SMases, which is produced by the same gene, was not induced (Figure 4C). The ASMase activity in the cells actually declined, which could indicate re-direction of the intracellular trafficking of the ASMase precursor away from the lysosomes and towards the secretory pathways in macrophages of mice on the atherogenic diet. Such a possibility is supported by published studies showing that atherogenic diet interferes with the mannose 6-phosphate receptor system [41]. The latter is known to regulate the processing of the lysosomal and secretory form of ASMase [3]. It is also possible, however, that the decrease in macrophages ASMase results from diet-induced accumulation of cholesterol, which is an ASMase inhibitor [42]. Whatever the case, it is clear that high-fat diet has an impact on macrophage S-SMase activity and/or secretion.
Figure 4. S-SMase and L-SMase activities in peritoneal macrophages, blood vessels and adipose tissue cultured ex vivo.
Mice were placed either on standard or modified diets for 10 weeks (3–5 animals per group). NBD-SM was used as a substrate to measure Zn2+-dependent S-SMase activity in conditioned medium or L-SMase activity in cellular lysates. Results are normalized per mg of cellular protein. Means±S.D. are shown. *P<0.05 compared with mice of same genotype on standard diet. (A) S-SMase activity in various tissues of asm+/+/ldlr−/− mice. (B) S-SMase activity in resident peritoneal macrophages from asm−/−/ldlr−/− and asm+/+/ldlr−/− mice. (C) L-SMase activity in lysates prepared from the same macrophages.
Effects of diet on liver and lipoprotein sphingolipid fatty acid composition
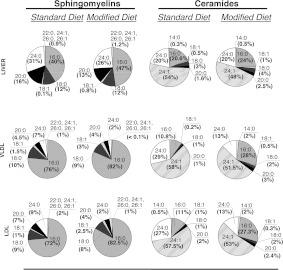
The bioactive properties of sphingolipids seem to depend on the length and saturation of the fatty acid attached to the primary amine group in the sphingoid base [43–45]. Therefore RP-HPLC (reverse-phase HPLC)–ESI–tandem MS analysis was used to determine whether an atherogenic diet affects the levels of sphingolipids with specific fatty-acid content (Figure 5). The major fatty acids found in serum lipoprotein sphingolipids were nervonic (C24:1), lignoceric (C24:0) and palmitic acid (C16:0). The liver sphingolipids exhibited similar fatty-acid distribution, confirming previous studies [46]. The levels of all measured ceramide and SM species were elevated in mice fed on the modified diet. Importantly, the increases in C16:0-containing sphingolipids, however, were larger than those for sphingolipids with unsaturated or very-long-chain saturated fatty acids. As a result, the occurrence of sphingolipids with C16:0 fatty acid in liver and lipoproteins of mice on an atherogenic diet increased, reflecting the high abundance of palmitate in the diet. It should be noted that elevated C16:0 sphingolipid content has been associated with increased rate of apoptosis and fibrosis in liver [46,47]. Thus, the observed increases in the fraction of sphingolipids with C16:0 fatty acid on palmitate-enriched diet could be associated with some of the pathology characteristics of cardiovascular diseases.
Figure 5. Fatty acid composition of the SM and ceramide in liver, VLDL and LDL.
Acidified organic solvents were used to prepare total lipid extracts from liver homogenates, VLDL and LDL from asm+/+/ldlr−/− mice (three to five animals per group) fed either standard or modified diet. Ceramide and SM internal standards containing a C17:0 fatty acid were added at the start to account for any losses during the extraction. Sphingolipid species containing different fatty acids were detected by monitoring species-specific precursor product ion pairs using HPLC–ESI–tandem MS and 4000 Q-Trap hybrid linear ion trap triple-quadrupole mass spectrometer. Levels of the respective dihydro species were sometimes too low for proper quantification and therefore were not included.
Serum ceramide and SM profiles of ASMase-deficient mice
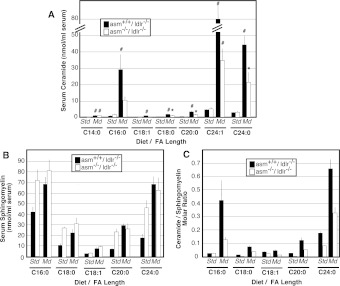
Lastly, we addressed the question whether S-SMase has any preference towards SM with a specific fatty acid length and/or saturation (Figure 6). The major ceramide species found in the serum of asm−/−/ldlr−/− and asm+/+/ldlr−/− mice on both diets were C24:1 ceramide, followed by C24:0 and C16:0 ceramides (Figure 6A), which is similar to what is seen in the serum of healthy humans [23]. The most prominent SM species were C24:0 and C16:0 (Figure 6B). The ceramide/SM ratios for all species were uniformly affected by the diet for both mouse genotypes. This indicates that S-SMase has no apparent specificity for a substrate with a particular fatty acid. Some preference, however, may exist for the very long fatty-acid chains, since the genotype-related changes in C24:0 species appeared to be somewhat larger than those for the C16:0 species.
Figure 6. Ceramide and SM species in the serum of mice on atherogenic or control diet.
Total serum lipid extracts were prepared from asm−/−/ldlr−/− and asm+/+/ldlr−/− mice placed on either standard (Std) or modified (Md) diet for 10 weeks. Ceramide (A) and SM (B) species were quantified by monitoring precursor product ion pairs using HPLC–ESI/tandem MS. The ceramide/SM molar ratio was calculated in each sample (C). Means±S.D. are shown (n=3 animals per group). Bonferonni post-hoc test analyses comparing the effect of the diet for mice from the same genotype are shown (*P<0.05 and #P<0.001). Std, standard diet; Md, modified diet.
DISCUSSION
In the present study, we describe how a diet rich in saturated fats and cholesterol affects the regulation of serum S-SMase activity and, consequently, LDL aggregation in mice. The results presented here indicate that S-SMase activity and the pool of its substrate in the form of LDL-SM are increased simultaneously and that these are two key factors that influence LDL aggregation. We also show that S-SMase activity is required for LDL aggregation; however, unless the LDL particles have sufficiently high SM levels (such as those observed during consumption of an atherogenic diet), the net impact of S-SMase may be limited.
SM is the second most abundant phospholipid class in LDL particles and together with phosphatidylcholine makes up the surface of the lipoproteins [48]. The main determinant of serum SM levels is the rate of its synthesis and secretion by the liver in the form of VLDL. This VLDL is later modified to LDL via the action of various lipases [49]. In the liver, the synthesis of SM is controlled by the rate of the SPT reaction, which is a rate-limiting step of sphingolipid biosynthesis [1]. Palmitic acid, the most abundant fatty acid in many pro-atherogenic diets is also the sole fatty acid used as a substrate of SPT. Numerous studies have reported that an increased supply of palmitate stimulates SPT activity and leads to the accumulation of dihydroceramide, ceramide, SM and other complex sphingolipids in liver, muscle and fat tissue [50]. The experiments described in the present study provide the first evidence that diet-induced changes in sphingolipid content are not limited to these tissues but are passed on to the serum lipoproteins. Elevation of serum VLDL and LDL cholesterol is the hallmark of atherosclerosis and is a well-established consequence of extended consumption of diets that are rich in saturated fats and cholesterol. Our studies provide evidence that the elevated serum cholesterol is paralleled by elevation in serum sphingolipids. In magnitude, the changes in SM synthesis seemingly surpass the stimulation of VLDL and LDL secretion, causing the production of particles that are enriched in SM.
The experiments shown in this study also reveal that LDL-SM influences the propensity of LDL for aggregation. The underlying mechanism apparently involves two independent steps. First, diet-induced stimulation of de novo SM synthesis in the liver results in the secretion of LDL particles that are enriched in SM, providing excess substrate for S-SMase. Secondly, simultaneous stimulation of S-SMase activity causes the increased conversion of LDL-SM into ceramide, leading to aggregation. Previous studies from Ira Tabas's group, which first characterized S-SMase activity, have shown that recombinant bacterial SMase can hydrolyse SM in human LDL, inducing aggregation [51]. Our work builds upon these findings to further show that LDL isolated from animals deficient in S-SMase that are fed on an atherogenic diet are in fact less aggregated and oxidized than LDL from animals with a functional S-SMase. These observations are direct indications for the key role S-SMase plays in LDL aggregation and are also consistent with the previously reported decrease in diet-induced plaque formation in asm−/−/ldlr−/− mice [28].
The LDL susceptibility for aggregation depends on the levels of S-SMase substrated LDL-SM. In the present study, LDL from asm−/−/ldlr−/− animals fed on an atherogenic diet that had the highest SM content, were most susceptible to aggregation upon treatment with bSMase. In contrast, particles from asm+/+/ldlr−/− mice on the standard diet had the lowest SM content and aggregated the least when similarly treated. These observations suggest that LDL-SM in animals on a standard diet is so low that even its complete conversion into ceramide is not sufficient to induce aggregation without diet-induced SM enrichment of LDL particles. In support of this notion, it has been reported that LDL particles isolated from SM synthase 2 transgenic mice aggregate more when treated with recombinant SMase than LDL from wild-type mice [52].
Another important finding of our studies is that palmitate-rich diet not only elevates SM and ceramide content but also modifies the fatty acid profile of these sphingolipids by increasing the proportion of C16:0 species. The bioactive properties of ceramide are known to depend on the length and saturation of the fatty acid attached to the primary amine group in the sphingoid base [43–45]. An elevated proportion of C16-sphingolipids in the liver is associated with increased apoptosis, even in the absence of net accumulation of ceramide [46,47]. In our study, the atherogenic diet led to the hepatic accumulation of sphingolipid species that are potentially detrimental to cellular functions and could be involved in some of the pathologies and complications typical of atherosclerosis. Whether or not the specific increases in LDL C16:0 ceramide and SM affect LDL properties is not clear. However, uptake of LDL with elevated ceramide content by vascular endothelial cells has been linked to increased apoptosis [22]; therefore the enrichment with C16:0 and C18:0 species may have indirect effects in such cases of LDL particle uptake.
In conclusion, S-SMase is emerging as a key regulator of LDL aggregation, based on direct evidence from knockout animal models of atherosclerosis presented in this work as well as in previous reports by other investigators. Importantly, the effects of S-SMase are dependent on the SM content of LDL, which is substantially increased by the atherogenic diet. Furthermore, the fatty-acid composition of sphingolipids is seemingly sensitive to changes in dietary fat that might be vital for understanding the effects saturated fatty acids have on cell functions.
AUTHOR CONTRIBUTION
Gergana Deevska was responsible for carrying out all the experiments described in the study except for MS. She also participated in experimental design, data interpretation and manuscript preparation. Manjula Sunkara performed the MS analysis. Andrew Morris developed the methods for lipid analyses by MS, supervised Manjula Sunkara, helped with interpretation of the results from MS and critically evaluated the paper. Mariana Nikolova-Karakashian participated in experimental design, troubleshooting, data interpretation and preparation of the paper.
ACKNOWLEDGEMENTS
We thank A.A. Karakashian for critical reading and editing of the manuscript prior to submission, and two former members of the laboratory, Dr Boyanovsky and Dr Giltiay for preliminary studies with asm−/−/ldlr−/− mice.
FUNDING
This work was supported by the National Institutes of Health [grant numbers AG019223 and AG026711 (to M.N.K.) and GM50388 and P20RR021954 for LC-MS/MS analyses (to A.J.M.)].
References
- 1.Merrill A. H., Jr, Wang E., Mullins R. E. Kinetics of long-chain (sphingoid) base biosynthesis in intact LM cells: effects of varying the extracellular concentrations of serine and fatty acid precursors of this pathway. Biochemistry. 1988;27:340–345. doi: 10.1021/bi00401a051. [DOI] [PubMed] [Google Scholar]
- 2.Merrill A. H., Jr, Lingrell S., Wang E., Nikolova-Karakashian M., Vales T. R., Vance D. E. Sphingolipid biosynthesis de novo by rat hepatocytes in culture. Ceramide and sphingomyelin are associated with, but not required for, very low density lipoprotein secretion. J. Biol. Chem. 1995;270:13834–13841. doi: 10.1074/jbc.270.23.13834. [DOI] [PubMed] [Google Scholar]
- 3.Tabas I. Secretory sphingomyelinase. Chem. Phys. Lipids. 1999;102:123–130. doi: 10.1016/s0009-3084(99)00080-8. [DOI] [PubMed] [Google Scholar]
- 4.Oorni K., Posio P., Ala-Korpela M., Jauhiainen M., Kovanen P. T. Sphingomyelinase induces aggregation and fusion of small very low-density lipoprotein and intermediate-density lipoprotein particles and increases their retention to human arterial proteoglycans. Arterioscler. Thromb. Vasc. Biol. 2005;25:1678–1683. doi: 10.1161/01.ATV.0000168912.42941.60. [DOI] [PubMed] [Google Scholar]
- 5.Schissel S. L., Jiang X., Tweedie-Hardman J., Jeong T., Camejo E. H., Najib J., Rapp J. H., Williams K. J., Tabas I. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J. Biol. Chem. 1998;273:2738–2746. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- 6.Benade A. J., Fincham J. E., Smuts C. M., Tung M. T., Chalton D., Kruger M., Weight M. J., Daubitzer A. K., Tichelaar H. Y. Plasma low density lipoprotein composition in relation to atherosclerosis in nutritionally defined Vervet monkeys. Atherosclerosis. 1988;74:157–168. doi: 10.1016/0021-9150(88)90202-x. [DOI] [PubMed] [Google Scholar]
- 7.Chao F. F., Blanchette-Mackie E. J., Chen Y. J., Dickens B. F., Berlin E., Amende L. M., Skarlatos S. I., Gamble W., Resau J. H., Mergner W. T., et al. Characterization of two unique cholesterol-rich lipid particles isolated from human atherosclerotic lesions. Am. J. Pathol. 1990;136:169–179. [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang X. C., Paultre F., Pearson T. A., Reed R. G., Francis C. K., Lin M., Berglund L., Tall A. R. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 9.Hojjati M. R., Li Z., Zhou H., Tang S., Huan C., Ooi E., Lu S., Jiang X. C. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J. Biol. Chem. 2005;280:10284–10289. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- 10.Hara A., Taketomi T. Characterization and change of phospholipids in the aorta of Watanabe hereditable hyperlipidemic rabbit. Japan. J. Exp. Med. 1990;60:311–318. [PubMed] [Google Scholar]
- 11.Park T. S., Panek R. L., Rekhter M. D., Mueller S. B., Rosebury W. S., Robertson A., Hanselman J. C., Kindt E., Homan R., Karathanasis S. K. Modulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in ApoE knockout mice. Atherosclerosis. 2006;189:264–272. doi: 10.1016/j.atherosclerosis.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Glaros E. N., Kim W. S., Quinn C. M., Jessup W., Rye K. A., Garner B. Myriocin slows the progression of established atherosclerotic lesions in apolipoprotein E gene knockout mice. J. Lipid Res. 2008;49:324–331. doi: 10.1194/jlr.M700261-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Deevska G. M., Rozenova K. A., Giltiay N. V., Chambers M. A., White J., Boyanovsky B. B., Wei J., Daugherty A., Smart E. J., Reid M. B., et al. Acid sphingomyelinase deficiency prevents diet-induced hepatic triacylglycerol accumulation and hyperglycemia in mice. J. Biol. Chem. 2009;284:8359–83568. doi: 10.1074/jbc.M807800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marathe S., Schissel S. L., Yellin M. J., Beatini N., Mintzer R., Williams K. J., Tabas I. Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide-mediated cell signaling. J. Biol. Chem. 1998;273:4081–4088. doi: 10.1074/jbc.273.7.4081. [DOI] [PubMed] [Google Scholar]
- 15.Doehner W., Bunck A. C., Rauchhaus M., von Haehling S., Brunkhorst F. M., Cicoira M., Tschope C., Ponikowski P., Claus R. A., Anker S. D. Secretory sphingomyelinase is upregulated in chronic heart failure: a second messenger system of immune activation relates to body composition, muscular functional capacity, and peripheral blood flow. Eur. Heart J. 2007;28:821–828. doi: 10.1093/eurheartj/ehl541. [DOI] [PubMed] [Google Scholar]
- 16.Gorska M., Baranczuk E., Dobrzyn A. Secretory Zn2+-dependent sphingomyelinase activity in the serum of patients with type 2 diabetes is elevated. Horm. Metab. Res. 2003;35:506–507. doi: 10.1055/s-2003-41810. [DOI] [PubMed] [Google Scholar]
- 17.Koumanov K. S., Momchilova A. B., Quinn P. J., Wolf C. Ceramides increase the activity of the secretory phospholipase A2 and alter its fatty acid specificity. Biochem. J. 2002;363:45–51. doi: 10.1042/0264-6021:3630045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morita S. Y., Kawabe M., Sakurai A., Okuhira K., Vertut-Doi A., Nakano M., Handa T. Ceramide in lipid particles enhances heparan sulfate proteoglycan and low density lipoprotein receptor-related protein-mediated uptake by macrophages. J. Biol. Chem. 2004;279:24355–24361. doi: 10.1074/jbc.M402035200. [DOI] [PubMed] [Google Scholar]
- 19.Ichi I., Nakahara K., Kiso K., Kojo S. Effect of dietary cholesterol and high fat on ceramide concentration in rat tissues. Nutrition. 2007;23:570–574. doi: 10.1016/j.nut.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Schissel S. L., Tweedie-Hardman J., Rapp J. H., Graham G., Williams K. J., Tabas I. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J. Clin. Invest. 1996;98:1455–1464. doi: 10.1172/JCI118934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walters M. J., Wrenn S. P. Effect of sphingomyelinase-mediated generation of ceramide on aggregation of low-density lipoprotein. Langmuir. 2008;24:9642–9647. doi: 10.1021/la800714w. [DOI] [PubMed] [Google Scholar]
- 22.Boyanovsky B., Karakashian A., King K., Giltiay N., Nikolova-Karakashian M. Uptake and metabolism of low density lipoproteins with elevated ceramide content by human microvascular endothelial cells: implications for the regulation of apoptosis. J. Biol. Chem. 2003;278:26992–26999. doi: 10.1074/jbc.M301536200. [DOI] [PubMed] [Google Scholar]
- 23.Ichi I., Nakahara K., Miyashita Y., Hidaka A., Kutsukake S., Inoue K., Maruyama T., Miwa Y., Harada-Shiba M., Tsushima M., Kojo S. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids. 2006;41:859–863. doi: 10.1007/s11745-006-5041-6. [DOI] [PubMed] [Google Scholar]
- 24.Pentikainen M. O., Lehtonen E. M., Kovanen P. T. Aggregation and fusion of modified low density lipoprotein. J. Lipid Res. 1996;37:2638–2649. [PubMed] [Google Scholar]
- 25.Subbaiah P. V., Subramanian V. S., Wang K. Novel physiological function of sphingomyelin in plasma. Inhibition of lipid peroxidation in low density lipoproteins. J. Biol. Chem. 1999;274:36409–36414. doi: 10.1074/jbc.274.51.36409. [DOI] [PubMed] [Google Scholar]
- 26.Williams K. J., Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oorni K., Hakala J. K., Annila A., Ala-Korpela M., Kovanen P. T. Sphingomyelinase induces aggregation and fusion, but phospholipase A2 only aggregation, of low density lipoprotein (LDL) particles. Two distinct mechanisms leading to increased binding strength of LDL to human aortic proteoglycans. J. Biol. Chem. 1998;273:29127–29134. doi: 10.1074/jbc.273.44.29127. [DOI] [PubMed] [Google Scholar]
- 28.Devlin C. M., Leventhal A. R., Kuriakose G., Schuchman E. H., Williams K. J., Tabas I. Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arterioscler. Thromb. Vasc. Biol. 2008;28:1723–1730. doi: 10.1161/ATVBAHA.108.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Z. F., Nikolova-Karakashian M., Zhou D., Cheng G., Schuchman E. H., Mattson M. P. Pivotal role for acidic sphingomyelinase in cerebral ischemia-induced ceramide and cytokine production, and neuronal apoptosis. J. Mol. Neurosci. 2000;15:85–97. doi: 10.1385/JMN:15:2:85. [DOI] [PubMed] [Google Scholar]
- 30.Tong H., Knapp H. R., Van Rollins M. A low temperature flotation method to rapidly isolate lipoproteins from plasma. J. Lipid Res. 1998;39:1696–1704. [PubMed] [Google Scholar]
- 31.Nikolova-Karakashian M., Morgan E. T., Alexander C., Liotta D. C., Merrill A. H., Jr Bimodal regulation of ceramidase by interleukin-1β. Implications for the regulation of cytochrome P450 2C11. J. Biol. Chem. 1997;272:18718–18724. doi: 10.1074/jbc.272.30.18718. [DOI] [PubMed] [Google Scholar]
- 32.Bligh E. G., Dyer W. J. A rapid method of total lipid extraction and purification. Can. J. Med. Sci. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 33.Williams R. D., Wang E., Merrill A. H., Jr Enzymology of long-chain base synthesis by liver: characterization of serine palmitoyltransferase in rat liver microsomes. Arch. Biochem. Biophys. 1984;228:282–291. doi: 10.1016/0003-9861(84)90069-9. [DOI] [PubMed] [Google Scholar]
- 34.Merrill A. H., Jr, Wang E., Mullins R. E., Jamison W. C., Nimkar S., Liotta D. C. Quantitation of free sphingosine in liver by high-performance liquid chromatography. Anal. Biochem. 1988;171:373–381. doi: 10.1016/0003-2697(88)90500-3. [DOI] [PubMed] [Google Scholar]
- 35.Pamuklar Z., Federico L., Liu S., Umezu-Goto M., Dong A., Panchatcharam M., Fulkerson Z., Berdyshev E., Natarajan V., Fang X., et al. Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis. J. Biol. Chem. 2009;284:7385–7394. doi: 10.1074/jbc.M807820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullards M. C., Allegood J. C., Kelly S., Wang E., Haynes C. A., Park H., Chen Y., Merrill A. H., Jr Structure-specific, quantitative methods for analysis of sphingolipids by liquid chromatography-tandem mass spectrometry: ‘inside-out’ sphingolipidomics. Methods Enzymol. 2007;432:83–115. doi: 10.1016/S0076-6879(07)32004-1. [DOI] [PubMed] [Google Scholar]
- 37.Ordovas J. M. Lipoprotein Protocols. Totowa, NJ: Humana Press; 1998. [Google Scholar]
- 38.Guyton J. R., Klemp K. F., Mims M. P. Altered ultrastructural morphology of self-aggregated low density lipoproteins: coalescence of lipid domains forming droplets and vesicles. J. Lipid Res. 1991;32:953–962. [PubMed] [Google Scholar]
- 39.Sathishkumar S., Boyanovsky B., Karakashian A. A., Rozenova K., Giltiay N. V., Kudrimoti M., Mohiuddin M., Ahmed M. M., Nikolova-Karakashian M. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: implications for endothelial apoptosis. Cancer Biol. Ther. 2005;4:979–986. doi: 10.4161/cbt.4.9.1915. [DOI] [PubMed] [Google Scholar]
- 40.Schissel S. L., Schuchman E. H., Williams K. J., Tabas I. Zn2+-stimulated sphingomyelinase is secreted by many cell types and is a product of the acid sphingomyelinase gene. J. Biol. Chem. 1996;271:18431–18436. doi: 10.1074/jbc.271.31.18431. [DOI] [PubMed] [Google Scholar]
- 41.Wu D., Sharan C., Yang H., Goodwin J. S., Zhou L., Grabowski G. A., Du H., Guo Z. Apolipoprotein E-deficient lipoproteins induce foam cell formation by downregulation of lysosomal hydrolases in macrophages. J. Lipid Res. 2007;48:2571–2578. doi: 10.1194/jlr.M700217-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Reagan J. W., Jr, Hubbert M. L., Shelness G. S. Posttranslational regulation of acid sphingomyelinase in Niemann-pick type C1 fibroblasts and free cholesterol-enriched Chinese hamster ovary cells. J. Biol. Chem. 2000;275:38104–38110. doi: 10.1074/jbc.M005296200. [DOI] [PubMed] [Google Scholar]
- 43.Renert A. F., Leprince P., Dieu M., Renaut J., Raes M., Bours V., Chapelle J. P., Piette J., Merville M. P., Fillet M. The proapoptotic C16-ceramide-dependent pathway requires the death-promoting factor Btf in colon adenocarcinoma cells. J. Proteome Res. 2009;8:4810–4822. doi: 10.1021/pr9005316. [DOI] [PubMed] [Google Scholar]
- 44.Senkal C. E., Ponnusamy S., Bielawski J., Hannun Y. A., Ogretmen B. Antiapoptotic roles of ceramidesynthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010;24:296–308. doi: 10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seumois G., Fillet M., Gillet L., Faccinetto C., Desmet C., Francois C., Dewals B., Oury C., Vanderplasschen A., Lekeux P., Bureau F. De novo C16- and C24-ceramide generation contributes to spontaneous neutrophil apoptosis. J. Leukocyte Biol. 2007;81:1477–14786. doi: 10.1189/jlb.0806529. [DOI] [PubMed] [Google Scholar]
- 46.Pewzner-Jung Y., Park H., Laviad E. L., Silva L. C., Lahiri S., Stiban J., Erez-Roman R., Brugger B., Sachsenheimer T., Wieland F., et al. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. J. Biol. Chem. 2010;285:10902–10910. doi: 10.1074/jbc.M109.077594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laviad E. L., Albee L., Pankova-Kholmyansky I., Epstein S., Park H., Merrill A. H., Jr, Futerman A. H. Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J. Biol. Chem. 2008;283:5677–5684. doi: 10.1074/jbc.M707386200. [DOI] [PubMed] [Google Scholar]
- 48.Kuksis A., Myher J. J., Geher K., Shaikh N. A., Breckenridge W. C., Jones G. J., Little J. A. Comparative determination of plasma phospholipids by automated gas–liquid chromatographic and manual colorimetric phosphorus methods. J. Chromatogr. 1980;182:1–26. doi: 10.1016/s0378-4347(00)81646-1. [DOI] [PubMed] [Google Scholar]
- 49.Nilsson A., Duan R. D. Absorption and lipoprotein transport of sphingomyelin. J. Lipid Res. 2006;47:154–171. doi: 10.1194/jlr.M500357-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Deevska G. M., Nikolova-Karakashian M. N. The twists and turns of sphingolipid pathway in glucose regulation. Biochimie. 2011;93:32–38. doi: 10.1016/j.biochi.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu X. X., Tabas I. Sphingomyelinase enhances low density lipoprotein uptake and ability to induce cholesteryl ester accumulation in macrophages. J. Biol. Chem. 1991;266:24849–24858. [PubMed] [Google Scholar]
- 52.Liu J., Zhang H., Li Z., Hailemariam T. K., Chakraborty M., Jiang K., Qiu D., Bui H. H., Peake D. A., Kuo M. S., et al. Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arterioscler. Thromb. Vasc. Biol. 2009;29:850–856. doi: 10.1161/ATVBAHA.109.185223. [DOI] [PMC free article] [PubMed] [Google Scholar]