Abstract
Background
The purposes of this study were to (1) describe physical activity of adult manual wheelchair users as measured by wrist actigraphy and two self-report measures, (2) compare exercisers and nonexercisers on measures of physical activity, and (3) examine the relationships between three activity measures.
Methods
Fifty manual wheelchair users wore an activity monitor and completed a physical activity record for 7 days. At the completion of this period, a questionnaire that included the Physical Activity Scale for Individuals with Physical Disabilities, stage of exercise question, and demographic and health questions was completed.
Results
Mean daily hours spent in bed or asleep was 9.1, mean hours of light intensity activity was 12.5, mean hours of moderate intensity activity was 1.3, and mean hours of strenuous activity was 0.33. Thirty-eight percent did not report any strenuous activity, and 56% reported less than the 150 minutes weekly of moderate or strenuous activity required to meet public health guidelines. There was variability in both self-reported and objectively measured physical activity. Regular exercisers were not significantly different from nonexercisers on objective measures of physical activity. Measured physical movement was weakly correlated with recall of physical activity or exercise.
Conclusion
Many wheelchair users do not meet public health guidelines for physical activity, but they are not a homogeneous group in intensity and frequency of physical activity. Multiple measurement methods can provide insights into the nature, intensity, and duration of physical activity that is more complex due to variations in abilities and ways of moving.
Keywords: Physical ability, Wheelchair users, Disability
Promotion of physical activity for people of all ages and abilities is a national public health priority [1]. Since 1995, U.S. public health guidelines for the frequency, intensity, and type of physical activity required to improve and maintain health have targeted “healthy” adults (aged 18-65 years). The most recent recommendations specify moderate-intensity aerobic (cardiovascular endurance) physical activity for a minimum of 30 minutes 5 days each week (150 minutes/week) or 20 minutes of strenuous activity at least 3 days each week [2]. In addition muscle strengthening or muscle endurance activities are recommended at least twice weekly [2]. Recent recommendations for older adults include adults aged 50 to 64 with limitations in function that affect movement ability [3]. These recommendations include modification of the intensity of aerobic and strengthening activity, addition of flexibility activities, performing activities known to be therapeutic for the specific impairment, and a tailored program with goals for frequency and duration similar to those for adults without functional limitations [3].
Healthy People 2010 described disparities in physical activity levels between adults with disabilities (AWD) and adults without disabilities (AWOD) in meeting public health guidelines. A significantly smaller proportion of AWD engaged in moderate activity for at least 30 minutes 5 times/week (23% of AWD, 33% of AWOD), and a smaller proportion engaged in vigorous activity for at least 20 minutes 3 times/week (12% of AWD, 16% of AWOD). There was also a significantly greater proportion of AWD who reported no leisure-time physical activity (56% AWD, 36% AWOD) [4]. The Behavioral Risk Factor Surveillance System (BRFSS) conducted national surveys of adults in 2001 and 2004 and found ongoing physical activity disparities between older AWD and AWOD (60% AWOD not meeting guidelines compared with 70% AWD), and among younger adults, 25.3% of AWD were completely inactive compared with 13.4% of AWOD [5,6].
It is well known that inactivity has adverse health effects. A period of bed rest will set off a cascade of adverse physiological effects including muscle deconditioning and atrophy, slowing of the metabolic rate, loss of bone density, and decreased control over blood pressure [7]. Too little activity over a prolonged period raises risks for heart disease, diabetes, obesity, sleep apnea, chronic pain, depression, and colon cancer [8]. Conversely, there is strong evidence that regular physical activity is associated with decreased risks of cardiovascular disease, thromboembolic stroke, hypertension, type 2 diabetes mellitus, osteoporosis, obesity, colon cancer, breast cancer, anxiety, depression and cognitive decline [2,3].
People with disabilities, especially those with mobility impairments, are at very high risk for the health problems associated with disuse [8,9]. Physical inactivity may also play a major role in contributing to the secondary conditions related to primary impairments and disabilities (depression, anxiety, pain, pressure ulcers, spasticity, urinary tract infections, contractures, fractures, etc.) and reduction in quality of life [10,11]. Demonstrated health and fitness benefits of moderate physical activity for people with disabilities include improvements in strength, stamina, cardiovascular fitness, coordination, posture, weight control, immune function, and circulation [12]. Psychological, functional, and quality of life benefits have also been demonstrated, including reduced depression and anxiety, improved self-esteem and body image, reduced need for medical care, and greater community participation [12].
Mobility-limiting impairments are the most prevalent type of disability reported in the U.S. [13-15]. Approximately 2.2 million people use wheelchairs or other wheeled mobility devices to compensate for mobility limitations, and 90% of these report using manual wheelchairs [15]. People with mobility impairments report a greater number of secondary conditions associated with their primary disability than do ambulatory people with disabilities [15]. It has been suggested that using a wheelchair may not provide enough physical activity to promote health and necessitates a “low exercise” lifestyle due to use of smaller amounts of muscle mass compared with walkers. Although mobility impairments and wheelchair use do change the characteristics of physical activity (types and ways of moving), there is comparative evidence that physically activity wheelchair users are healthier than inactive wheelchair users [16]. There is also epidemiologic evidence that higher levels of activity decrease mortality risks for people with mobility-limiting conditions [17].
Previous studies describing the physical activity of adults with mobility impairments have mainly relied on self-report measures and epidemiologic surveys. Surveys of adults with disabilities document low levels of self-reported physical activity, including lack of unstructured leisure time physical activity, sports, recreation, or aerobic exercise [5,18-22]. These survey studies have included some wheelchair users, but none have specifically focused on the wheelchair-using population. The survey measures used are mostly validated for an ambulatory population [5,20]. General population instruments can be problematic for wheelchair users because most focus on walking and other ambulatory activities. They may not accurately measure light intensity activity and may underestimate wheelchair activity.
Despite general awareness that most population-based self-report measures are unable to adequately represent the lower end of the continuum of physical activity [23] and that disability-appropriate measures or motion sensors with accelerometer technology may be more appropriate measuring tools to do so, there have been few studies using these methods. Self-report measures of physical activity for people with disabilities have been initially evaluated [24-26] and used to survey adults with disabilities [19] and to measure physical activity changes as the result of intervention programs [27].
Electronic physical activity monitors with accelerometer technology have been shown to be more sensitive than other measures in detecting variability in activity levels, especially when activity levels may be low. Many studies provide evidence that electronic monitors are valid and reliable indicators of physical activity in ambulatory populations with disabling conditions [28-31]. Recent evidence suggests that an electronic monitor can be used to quantify the physical activity of wheelchair users [32-35] and suggests that such measures of physical activity may be a proxy for energy expenditure [30,32].
As a consequence of these measurement limitations and disparity of measures across studies, it is still not clear whether self-report and objective measures of activity in wheelchair users are giving equivalent information and if wheelchair users are meeting public health guidelines for physical activity. The primary aim of this study was to describe community-based free-living physical activity of adult manual wheelchair users as measured by wrist actigraphy and two types of self-report instruments. Secondary aims were (1) to compare exercisers and nonexercisers on measures of physical activity and (2) to examine the relationships between the three activity measures. This report is part of a larger study of physical activity description and correlates in manual wheelchair users. Results on correlates of physical activity in wheelchair users are reported elsewhere [36].
Methods
Study Design
This study used a cross-sectional descriptive design.
Participants
The sample consisted of adults who reported using a manual wheelchair for at least 80% of their mobility. Participants had conditions causing a locomotor impairment including diagnoses such as spinal cord injury (SCI), multiple sclerosis (MS), brain injury, amputation, cerebral palsy (CP), spina bifida, stroke, post-polio, and other neuro-muscular conditions. Inclusion criteria included ability to move independently using one or both arms to propel a manual wheelchair and no current pressure ulcers or hospitalizations within the past month. All participants were able to read or speak English. Participants were recruited through bulletin board postings and newsletter advertisements in community centers, clinics, and disability-focused organizations. Informed consent was obtained from each participant using forms and process approved by the University of Washington Human Subjects Division, the institutional review board providing oversight of the protection of human subjects and research ethics.
Instruments
Actigraphy
Objective measurement of physical activity was done using an accelerometry-based, activity monitor (Actiwatch; Mini Mitter Respironics, Inc., Bend, OR, USA). This small, lightweight, wrist-worn monitor allows unimpeded movement of the wearer during measurement of long-term gross motor activity. The monitor contains an accelerometer that is sensitive to motion in all directions. Information about motion direction and speed are integrated to produce an electrical current with variable magnitude and duration. The electrical current data are stored in the monitor as “activity counts.” A single activity count represents 0.000175 gram force unit, or .0017 N. Time between sampling units (epochs) can be set between 15 and 900 seconds depending on the total length of the monitoring period desired. For this study, epoch length was set at 15 seconds, allowing the greatest sensitivity for low intensity activity. The unit has a 64 K memory and can store data for a recording period of up to 44 days. Data are downloaded to a PC via a reading device connected to a serial port. After downloading, data in the form of activity counts were imported initially into Excel (Microsoft) spreadsheets for data reduction and then into SPSS for analysis. Interinstrument reliability of the six monitors used in this study was high (r = 0.96, p = .000), and there were no significant differences between monitors when data collected for 15-minute periods were compared via one-way analysis of variance. Data from all days on which the activity monitor was worn at least 90% of the day were included, and all 15-minute periods on those days when participants were wearing the device were analyzed. Data were converted to mean activity counts/hour by selecting all periods for which complete data were available, determining the mean counts for each period, and multiplying by 4 to calculate the hourly mean. Average daily activity counts were calculated by multiplying the hourly mean by 24 hours. Activity counts while awake were calculated by removing 15-minute periods when the participants were asleep and averaging activity counts over the hours identified as being awake. In our previous studies of wheelchair users with SCI, actigraphy counts were moderately and significantly associated with self-reported physical activity (r = 0.60, p < .01) [33].
Physical activity record
The physical activity record (PAR) used in this study was based on the instrument created by Bouchard et al. [37]. The form provides a grid with spaces to record average activity intensity for each 15-minute period of the day. Activity intensities are recorded using a single number representing activities of various intensities. For example, moderate activities are recorded as a level 5 with examples provided of “pushing chair on level surface, moderate housework, arm ergometry with light resistance,” etc. This format has been tested with ambulatory adults and children [37], demonstrating moderately strong correlations with physical work capacity (r = 0.70, p < .001 in adults, r = 0.80, p < .001 in children) and percent body fat (r = 0.30, p < .01) in adults, providing indirect validation of the PAR. This instrument was used in our previous study measuring activity in wheelchair users [36]. Test-retest reliability has been reported in previous studies (r = 0.91 in children and r = 0.97 in adults, both p < .05) [7]. The activities listed and grouped by their degree of strenuousness are those known to be common in the daily lives of people who use wheelchairs. Although energy expenditure for wheelchair propulsion is generally considered to be approximately equal to walking at corresponding speeds, actual energy expended by wheelchair users is highly variable depending on wheelchair characteristics, environmental conditions, and efficiency of movement due to the user’s underlying physical condition [38]. Given the lack of a standardized index of metabolic costs of various activities for wheelchair users, the placement of activities in categories was based on categorizations developed for the general population [39]. In addition, the activity intensity groupings were similar to that of Sugimoto et al. [29] to classify low levels of activity in greater detail. Time spent in activities of different intensities was calculated by multiplying each 15-minute segment labeled with a given intensity by 15 minutes to get minutes/week, divided by 60 to get hours/week, then divided by 7 (or number of complete days) to get hours/day.
Physical Activity Scale for Individuals with Physical Disabilities
Recall of physical activity over the past week was measured with the Physical Activity Scale for Individuals with Physical Disabilities (PASIPD) [24]. This 13-item recall questionnaire assesses time spent in physical activity of various intensities over a 7-day period. Possible scores range from 0 to 199.5 with larger numbers representing greater activity. Internal consistency validity Cronbach’s α in this sample was .70. In samples of people with a variety of disabilities PASIPD scores have been shown to discriminate those who were active from those who were not ( p < .05) and those with excellent or good from those with fair or poor self-rated health ( p < .05) [24] and to correlate significantly with physical activity measured by actigraphy (r = 0.30, p < .01) [26].
Stage of exercise behavior
Exercise stage was measured by a single question establishing current stage of readiness for exercise. Definition and description of regular exercise was provided followed by five choices defining current exercise behavior. These choices were (1) “I don’t plan to start exercising” (Pre-Contemplation), (2) “I plan to start within 6 months” (Contemplation), (3) “I am currently exercising occasionally but not regularly” (Preparation), (4) “I have been exercising regularly for less than 6 months” (Action), and (5) “I have been exercising regularly for more than 6 months” (Maintenance). Those selecting option 1, 2, or 3 were considered to be nonexercisers, and those selecting option 4 or 5 were classified as exercisers. This question is consistent with the staging algorithm recommended by Reed and colleagues [40]. Stage as selected in this question has been shown to be significantly associated with a 7-day physical activity recall questionnaire in a general population sample [41] and with exercise self-efficacy and exercise barriers in a large sample of people with disabilities [8].
Demographic and general health information
Additional data about age, sex, employment status, education, marital status, racial/ethnic group, medical diagnoses, and length of time using a wheelchair were collected.
Procedures
People who called to inquire about the study were screened for inclusion criteria. Enrollment was done during a home visit by the study principal investigator or a trained research study assistant. During a 1-hour home visit, study participants were instructed in procedures for wearing the activity monitor and completing the PAR. Participants wore the activity monitor and completed the PAR for 7 days. At the completion of this 7-day period, a questionnaire including the PASIPD, stage of exercise question, and demographic and health questions was completed. Participants were enrolled consecutively between July 2004 and October 2005.
Data analysis
Variables were analyzed with descriptive statistics and tests of skewness and kurtosis. Bivariate zero-order Pearson correlations coefficients were used to examine the relationships between various measures of activities. Comparisons between demographic and diagnostic subgroups and between exercisers and nonexercisers were done using t-tests to compare group means. Exploratory analyses of seasonal activity effects were done using one-way ANOVA. All analyses used two-tailed tests of significance, α = .05. The method of mean substitution was used for missing items.
Results
Demographics
Fifty people agreed to participate in the study. Participants were 27 men and 23 women who were between the ages of 18 and 74 (mean age 46.3 years, SD 13.6 years). Forty-eight of 50 enrolled participants completed all study procedures including demographic variables. Forty-two percent (n = 20) of participants were never married, 25% (n = 12) were married or living with a partner, 29% (n = 14) were separated or divorced, and 4% (n = 2) were widowed. The majority of the sample was Caucasian (79%, n = 38), with 8% (n = 4) Alaskan/American Indian, 6% (n = 3) African American, 2% (n = 1) Asian, 2% (n = 1) Pacific Islander, and 2% (n = 1) Hispanic ethnicity. Almost half of the sample was unemployed (48%, n = 23). Mean years of education were 14.5 years (SD 2.5 years). The length of time using a wheelchair varied from 1 to 690 months (mean 138.5 months, SD 142.5 months). The most common diagnosis leading to the use of a wheelchair was spinal cord injury (50%), although multiple other diagnoses were reported (Table 1).
Table 1.
Sample description by gender and primary diagnosis
| Diagnosis | Female | Male | Total |
|---|---|---|---|
| Spinal cord injury | 9 | 16 | 25 |
| Multiple sclerosis | 3 | 1 | 4 |
| Amputation | 2 | 2 | 4 |
| Stroke | 2 | 1 | 3 |
| Brain injury | 2 | 1 | 3 |
| Spina bifida | 2 | 0 | 2 |
| Post-polio | 1 | 0 | 1 |
| Cerebral palsy | 0 | 1 | 1 |
| Other | 2 | 5 | 7 |
| Total | 23 | 27 | 50 |
| (46%) | 27 | ||
| (54%) | 50 | ||
| (100%) |
Forty of the 50 participants wore the actigraph for the entire 7-day monitoring period, and another 9 people completed at least 4 of the 7 days of actigraphy monitoring and were included in actigraphy analyses. Across the sample, the mean percentage of total time the actiwatch was worn was 94%. One participant was removed from analyses due to activity counts that were more than 3 SDs above the mean and were associated with a diagnosis of Parkinson’s disease with periods of severe involuntary movements and whole body spasms. Forty-nine of 50 participants completed the questionnaire and there were very few missing survey data (<1%).
Physical activity description
Mean daily activity counts across the sample were 323,000 (SD 119,000) with a wide range of individual daily counts (111,000 to 615,000). Activity counts per hour when participants were not in bed or asleep ranged from 6,264 to 38,312 (mean 19,797; SD 7,434).
Scores on the PASIPD varied from 0.17 to 93.3 (mean 22.5; SD 20.4), again demonstrating wide variability. Mean daily activity scores from the activity record were 216 (SD 31), ranging from 135 to 278 (Table 2).
Table 2.
Summary physical activity data
| Variable | N | Mean (SD) | Range |
|---|---|---|---|
| Mean daily activity counts | 48 | 323,204 (119,017) | 111,269-615,362 |
| Mean activity counts/hour while awake |
48 | 19,797 (7,434) | 6,264-38,312 |
| Mean activity scale score (PASIPD) |
48 | 22.5 (20.4) | 0.17-93.3 |
| Mean daily activity record score |
48 | 216 (31) | 135-278 |
Activity record scores indicating the average activity intensity for each 15-minute period of the day were used to examine the average amount (and %) of time spent in different activity intensities on a daily basis. Mean daily hours spent in bed or asleep was 9.1 (SD 1.96) (38.5%); mean hours of light intensity activity was 12.5 (SD 2.71) (52.5%); mean hours of moderate intensity activity was 1.3 (SD 1.31) (5.5%); and mean hours of strenuous activity was 0.33 (SD 0.45) (1%). Across the sample, data on 2.5% of daily activity were not recorded (Fig. 1). Nineteen participants (38%) did not report any strenuous activity and 28 (56%) reported less than the 150 minutes weekly of moderate or strenuous activity required to meet public health guidelines.
Figure 1.
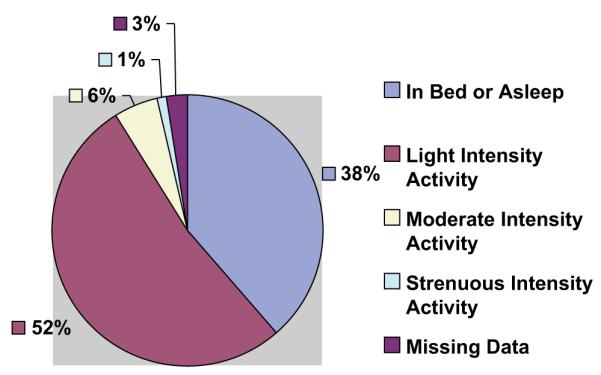
Average daily time spent in activity of various intensities. Mean time spent in bed or asleep = 9.1 hrs. (38.5%). Mean time spent in light intensity activity = 12.5 hrs. (52.5%). Mean time spent in moderate activity = 1.3 (5.5%). Mean time spent in strenuous activity = .3 (1%).
Seasonal variation in activity
Of the 48 participants, 10 were measured during the winter months, 6 were measured during spring, 18 were measured during summer, and 14 were measured during autumn. One-way ANOVA on all measures of activity showed no significant seasonal variation.
Comparison of subgroups
Males were compared with females, people aged 46 or older were compared with those aged 45 or younger, and people with SCI, who comprised half the sample, were compared with those with other diagnoses on activity measures (Table 3). There were no significant differences between males and females or those with SCI versus other diagnoses. There was a significant difference between the older and younger groups on the PASIPD score (t = 2.63, p = .012). Differences on the other measures did not reach the level of significance; however, the data did consistently demonstrate that younger people were more active than older people.
Table 3.
Comparison of subgroups on activity measures
| Measure | PAS (total), mean (SD) | Daily activity counts (total), mean (SD) |
Daily activity score (total), mean (SD) |
|---|---|---|---|
| Males (n=26) | 22.2 (19.6) | 325,860 (120,045) | 218.7 (32.7) |
| Females (n=22) | 22.9 (21.8) | 320,064 (120,533) | 212.9 (29.8) |
| t-test, df, p | 0.136, 46, .893 | −0.166, 46, .869 | −0.645, 46, .944 |
| Age ≤45 (n=23) | 30.1 (22.9) | 346,911 (129,244) | 217.7 (29.2) |
| Age ≥46 (n=25) | 15.5 (14.9) | 301,394 (106,743) | 214.6 (33.5) |
| t-test, df, p | 2.63, 46, 012a | 1.34, 46, .176 | 1.39, 46, .176 |
| SCI (n=24) | 26.8 (18.7) | 348,791 (104,448) | 224.1 (32.2) |
| Other diagnoses (n=24) | 18.3 (21.5) | 259,008 (119,309) | 207.9 (28.6) |
| t-test, df, p | 1.45, 46, .153 | 1.51, 46, .138 | 1.84, 46, .073 |
p < .05.
Comparison of exercisers and nonexercisers
Regular exercisers (participants who endorsed either the Action or Maintenance stages of exercise behavior, n = 24) were compared with nonexercisers or irregular exercisers (endorsing either Precontemplation, Contemplation, or Preparation stages, n = 24) (Table 4). Regular exercisers had significantly greater scores on the total PASIPD (t = −2.49, p = .016) and the moderate and vigorous sports subscales (t = −3.14, p = .003 and t = −2.67, p = .011, respectively) but were not significantly different on the PASIPD housework subscale or activity counts or daily activity scores.
Table 4.
Comparison of exercisers and nonexercisers on activity measures
| Exercisers (n=24) | Nonexercisers (n=24) | ||
|---|---|---|---|
| Measure | Mean (SD) | Mean (SD) | t-test, df, p |
| PAS (total) | 29.5 (22.8) | 15.6 (15.1) | −2.49, 46, .016a |
| PAS Moderate Sport | 7.3 (8.6) | 1.5 (2.13) | −3.14, 45, .003b |
| PAS Vigorous Sport | 9.2 (9.2) | 3.0 (6.5) | −2.67, 46, .011a |
| PAS Housework | 3.2 (3.3) | 4.5 (7.0) | 0.853, 47, .121 |
| PAS Garden | 3.6 (3.6) | −3.3 (20.5) | −1.58, 47, .121 |
| PAS Occupation/Transportation | 6.4 (4.8) | 5.8 (5.9) | −0.420, 47, .677 |
| Daily counts total | 327,918 (160,954) | 339,967 (115,582) | 0.300, 47, .771 |
| Daily counts without sleep | 274,887 (121,258) | 306,423 (108,323) | 0.950, 46, .347 |
| Counts/hour without sleep | 20,230 (7,151) | 19,364 (7,837) | 0.400, 46, .691 |
| Daily score total | 218 (31) | 216 (33) | −0.166, 47, .869 |
| Daily score without sleep | 181 (41) | 181 (33) | −0.024, 46, .981 |
p < .05.
p < .01.
Comparison of measurement methods
Activity record scores for each 15-minute time period compared to actigraphy counts for corresponding periods were moderately correlated. For individuals, correlations ranged from r = 0.404 to 0.829, with a mean of 0.675. Across the sample, mean daily activity counts during periods of activity correlated moderately with mean daily scores during periods of activity (r = .506, p = .000). Mean activity counts for each corresponding recorded activity record score (i.e., each 15-minute time period) increased incrementally with each higher score and differences between counts/score were significant ( p = .000) (Figure 2).
Figure 2.
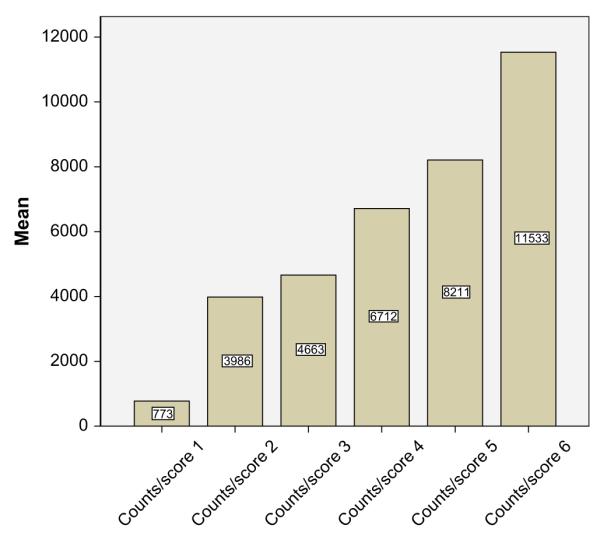
Mean counts per activity intensity score. Score 1 = Sleeping, lying down. Score 2 = Sitting and resting, reading, watching tv. Score 3 = Very light activities (desk work, eating, brushing teeth). Score 4 = Light activities (driving, light housework, showering). Score 5 = Moderate activities (pushing chair on level, moderate housework, arm ergometry). Score 6 = Strenuous activities (wheeling on hills, sports, rowing, wheeling on uneven ground).
Total scores on the PASIPD were weakly correlated with daily activity counts (r = 0.193, p = .188) and total daily activity record scores (r = 0.267, p = 0.67). The housework subscale scores on the PASIPD were significantly correlated with daily activity record scores (r = 0.358, p = .013).
Discussion
Results of this study support the findings of previous studies documenting low levels of self-reported physical activity in adults with disabilities. The majority of this sample of adult manual wheelchair users (56%) reported not meeting the public health guidelines for 150 minutes of moderate physical activity weekly. However, the percentage not meeting guidelines is much smaller than the 70% previously reported by AWD who were 50 years of age or older [5]. This may be because this sample was on average younger than age 50 (mean 46.3 years) and included individuals as young as 18. It is also possible that using a disability-specific measure of physical activity provides a more accurate indication of how much physical activity a wheelchair user actually does. However, the results may also provide a hopeful indication that more wheelchair users are finding ways to be physically active and incorporating regular physical activity into their daily lives. Without a comparison group of ambulatory individuals, it is not possible to confirm if this represents progress toward decreasing the disparity in physical activity between AWD and AWOD or if it simply represents progress across the entire population in reducing the problem of inactivity.
An important finding is that the number of hours spent in bed and doing light intensity activity is, on average, greater than those reported in the general population. Given the nature of many of the medical diagnoses that limit mobility and necessitate the use of a wheelchair, health care providers and people with these conditions who may not be aware of the benefits of activity may expect that greater time spent resting and/or sleeping is necessary for energy conservation and prevention of pain and fatigue. But, given the known deleterious effects of bed rest (muscle deconditioning and atrophy, decreased metabolic rate, decreased bone density, blood pressure instability, etc.), it is quite possible that this increased amount of time in bed is actually detrimental to health. Health care providers and physical activity intervention planners might want to target the amount of time spent in bed as a logical first step toward encouraging greater physical activity in people with mobility impairments.
We could locate only one other published study that measured physical activity over a 7-day period in ambulatory adults using wrist actigraphy [42]. This study described self-reported and actigraphically measured activity of 27 women with heart transplants. Methods were similar to this study, and the same type and model of actigraph was used. Surprisingly, mean daily activity counts in these ambulatory women were 280,000 (SD 52,000), less than that in these manual wheelchair users. It might be that these women were more inactive than healthy adults due to their physical limitations as a result of having had a heart transplant. However, it is possible that ambulatory activity requires less effort than wheelchair activity and that this sample of wheelchair users may actually be moving more frequently and intensively than ambulatory individuals and that their reports of low activity levels may not be as low as is commonly thought when compared to activity of other populations. The greater time spent in bed may be compensation for the extra effort required to accomplish daily activities or due to advice from health professionals and beliefs that rest will improve pain and fatigue.
A small previous study using actigraphy to measure physical activity in four ambulatory individuals with rheumatoid arthritis showed very little variation between individuals in levels of physical activity over 4 days [43]. In contrast, this larger sample of wheelchair users with mixed diagnoses had wide variability in both self-reported and objectively measured physical activity. This wide variability was demonstrated across all three measures. Also, in our sample, people who exercised differed significantly from people who did not exercise in self-reported activity, specifically in self-reported moderate and vigorous sports. But, there were no significant differences between exercisers and non-exercisers in objectively measured activity. These two findings together illustrate that, despite similarities in the mode of ambulation, wheelchair users are actually widely variable in physical activity frequency, intensity, and duration. In this sample, all participants were able to self-propel a manual wheelchair, but some people required assistance for other activities of daily living such as transfers, dressing, and bathing and some could stand for brief periods whereas others could not. The fact that objectively measured activity was not greater in individuals who exercised or participated in sports suggests that there may be types of activity other than exercise that are of equal intensity or duration as a regular program of exercise that require equal or greater amounts of energy expenditure. There is no way to determine what those activities are from the data in this study because specific activities being done were not recorded.
In this study, measured physical movement was only weakly correlated with the recall of physical activity or exercise as measured by the PASIPD. Studies in various ambulatory populations have also found low correlations of accelerometry-based monitor outputs with recall measures [43,44]. One past study with a similar size sample of individuals with physical disabilities who were not wheelchair users showed a somewhat stronger correlation between the PASIPD and an accelerometer (r = 0.30) [6]. Possible explanations for the lower correlation in this study include limitations of the PASIPD and variability in ways of moving among the participants. The PASIPDs Cronbach’s α in this sample was only .70, adequate but not strong. The PASIPD seems to be a better measure of exercise and sports participation than a measure of household and self-care activities. This is supported by the significant difference between self-identified exercisers and nonexercisers on the PASIPD total score and moderate and vigorous sports subscales in this sample. Actigraphy is more accurate in measuring all activity, including low intensity activities and involuntary movements that are more commonly reported by wheelchair users. Thus, the poor correlation of the two measures may be due to lack of sensitivity of the PASIPD to capture the full range of wheelchair users’ physical activity. It might also explain a stronger correlation in an ambulatory population with possibly less variability in ways of moving.
What continues to be missing from all studies measuring physical activity in wheelchair users is a reliable method to determine the amount of energy consumed during various activities and activity intensities. Energy expenditure equations can be derived for ambulatory individuals based on body weight and gender, but the ability to derive such equations for people with mobility limitations is difficult due to the unlimited variability of active body muscle mass, ways of moving, and potential for involuntary movements such as muscle spasms, myoclonus, and tremors. Individuals can be measured using calorimetry, but generalizing from individuals to a larger population is not possible.
Study strengths and limitations
To our knowledge, this is the first study to describe physical activity in wheelchair users using both objective and subjective measures of activity. Participants were quite adherent to the 7-day study protocol, and most of them were able to wear the activity monitor 24 hours/day for all 7 days. However, the findings should be interpreted cautiously due to the small sample. These participants who volunteered to be in the study may not be representative of the larger population of wheelchair users. They also may have changed their usual behavior in response to being in the study. Measuring physical activity history would have improved the study. Given that some people had used a wheelchair for years and others were relatively new to wheelchair use, assessing previous levels of activity would have added important information. Another limitation is that this study did not examine characteristics of the manual wheelchairs used by study participants or the appropriateness and fit of the wheelchair for the individual. Wheelchair fit and “fine-tuning” of the wheelchair and person interface are likely to be important determinants of physical activity participation for wheelchair users [45].
Conclusion
Manual wheelchair users continue to report levels of physical activity that do not meet public health guidelines of 150 minutes per week required to improve and maintain health. They also report spending more time in bed and in light intensity activity than do ambulatory individuals. But this study suggests that a greater percentage of wheelchair users may be meeting guidelines now than previously reported. Measuring physical activity in this population continues to be problematic, and despite the use of multiple measures, measurement instruments <comment > OK as edited?</comment>can only provide a partial picture of actual physical activity. However, using multiple measurement methods can provide insights into the nature, intensity, and duration of physical activity, which are complicated by variations in abilities and ways of moving. Future research employing multiple measurement methods is needed. However, although physical activity is more complex, it should not be overlooked by health care providers in counseling patients or by planners of targeted physical activity interventions. A logical place to begin intervention in this population would be to reduce the time spent in bed and doing light intensity activity. Reducing inactivity is a good first step for promoting greater physical activity for wheelchair users.
Acknowledgments
We acknowledge Sarah Matthies, BSN, RN, and Diana Cantrell, RN, who assisted with data collection for this study.
Footnotes
This project was supported by NIH Research Grants 5 P20 NR008351-04 and NR008360-04 funded by the National Institute of Nursing Research and the National Center for Minority Health and Health Disparities (NCMHD).
References
- [1].Centers for Disease Control and Prevention. United States Department of Health and Human Services [December 7, 2007];Physical activity and good nutrition: Essential elements to prevent chronic diseases and obesity. http://www.cdc.gov/nccdphp/dnpa.
- [2].Haskell WL, Lee I, Pate RR, et al. Physical activity and public health: updated recommendations for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- [3].Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendations from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;116:1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- [4].United States Department of Health and Human Services . Healthy People 2010: Understanding and Improving Health. 2nd ed U.S. Government Printing Office; Washington, DC: 2000. [Google Scholar]
- [5].Brown DR, Yore MM, Ham SA, Macera CA. Physical activity among adults ≥ 50 yrs with and without disabilities, BRFSS 2001. Med Sci Sports Exerc. 2005;37(4):620–629. doi: 10.1249/01.mss.0000158189.17546.ed. [DOI] [PubMed] [Google Scholar]
- [6].United States Department of Health and Human Services . Office of the Surgeon General. US Dept of Health and Human Services; Washington, DC: 2005. The Surgeon General’s Call to Action to Improve the Health and Wellness of Persons With Disabilities. [PubMed] [Google Scholar]
- [7].Greenleaf JF. Physiological responses to prolonged bedrest and fluid immersion in humans. J Appl Physiol. 1984;57(3):619–633. doi: 10.1152/jappl.1984.57.3.619. [DOI] [PubMed] [Google Scholar]
- [8].Cardinal BJ, Kosma M, McCubbin JA. Factors influencing the exercise behavior of adults with physical disabilities. Med Sci Sports Exerc. 2004;36(5):868–875. doi: 10.1249/01.mss.0000126568.63402.22. [DOI] [PubMed] [Google Scholar]
- [9].Washburn RA, Figoni SF. High-density lipoprotein cholesterol in individuals with spinal cord injury: the potential role of physical activity. Spinal Cord. 1999;37:685–695. doi: 10.1038/sj.sc.3100917. [DOI] [PubMed] [Google Scholar]
- [10].Kinne S, Patrick D, Doyle L. Prevalence of secondary conditions among people with disabilities. Am J Public Health. 2004;94(3):443–445. doi: 10.2105/ajph.94.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Santiago MC, Coyle CP. Leisure-time physical activity and secondary conditions in women with physical disabilities. Disabil Rehabil. 2004;26(8):485–494. doi: 10.1080/09638280410001663139. [DOI] [PubMed] [Google Scholar]
- [12].Crawford A, Hollingsworth HH, Morgan K, Gray DB. People with mobility impairments: physical activity and quality of participation. Disabil Health J. 2008;1:7–13. doi: 10.1016/j.dhjo.2007.11.004. [DOI] [PubMed] [Google Scholar]
- [13].Iezzoni LI, McCarty EP, Davis RB, Siebens H. Mobility difficulties are not only a problem of old age. J Gen Intern Med. 2001;16:235–243. doi: 10.1046/j.1525-1497.2001.016004235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McNeil JM. Prevalence of disabilities and associated health conditions among adults—United States, 1999. MMWR. 2001;50(7):120–125. [PubMed] [Google Scholar]
- [15].Kaye HS, Kang T, LaPlante MP. National Institute on Disability and Rehabilitation Research. US Dept of Education; Washington, DC: 2000. Mobility Device Use in the United States. Disability Statistics Report 14. [Google Scholar]
- [16].Stotts KM. Health maintenance: paraplegic athletes and nonathletes. Arch Phys Med Rehabil. 1986;67:109–114. doi: 10.1016/0003-9993(86)90116-4. [DOI] [PubMed] [Google Scholar]
- [17].Heath GW, Fentem PH. Physical activity among persons with disabilities: a public health perspective. Exerc Sport Sci Rev. 1997;25:195–234. [PubMed] [Google Scholar]
- [18].Washburn R, Hedrick BN. Descriptive epidemiology of physical activity in university graduates with locomotor disabilities. Int J Rehabil Res. 1997;20(3):275–287. doi: 10.1097/00004356-199709000-00004. [DOI] [PubMed] [Google Scholar]
- [19].Rimmer JH, Rubin SS, Braddock D, Hedman G. Physical activity patterns of African-American women with physical disabilities. Med Sci Sports Exerc. 1999;31(4):613–618. doi: 10.1097/00005768-199904000-00020. [DOI] [PubMed] [Google Scholar]
- [20].Stuifbergen AK. Physical activity and perceived health status in persons with multiple sclerosis. J Neurosci Nurs. 1997;29(4):238–243. doi: 10.1097/01376517-199708000-00004. [DOI] [PubMed] [Google Scholar]
- [21].Hogan A, McLellan L, Bauman A. Health promotion needs of young people with disabilities: a population study. Disabil Rehabil. 2000;22(8):352–357. doi: 10.1080/096382800296593. [DOI] [PubMed] [Google Scholar]
- [22].Heller T, Ying G, Rimmer JH, Marks BA. Determinants of exercise in adults with cerebral palsy. Public Health Nurs. 2002;19(3):223–231. doi: 10.1046/j.0737-1209.2002.19311.x. [DOI] [PubMed] [Google Scholar]
- [23].Tudor-Locke CE, Myers AM. Challenges and opportunities for measuring physical activity in sedentary adults. Sports Med. 2001;3(2):91–100. doi: 10.2165/00007256-200131020-00002. [DOI] [PubMed] [Google Scholar]
- [24].Washburn RA, Zhu W, McAuley E, et al. The Physical Activity Scale for Individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil. 2002;83:193–200. doi: 10.1053/apmr.2002.27467. [DOI] [PubMed] [Google Scholar]
- [25].Rimmer JH, Riley BB, Rubin SS. A new measure for assessing the physical activity behaviors of persons with disabilities and chronic health conditions: the Physical Activity and Disability Survey. Am J Health Promot. 2001;16(1):34–45. doi: 10.4278/0890-1171-16.1.34. [DOI] [PubMed] [Google Scholar]
- [26].Van der Ploeg HP, Streppel KRM, Van der Beek AJ, et al. The Physical Activity Scale for individuals with physical disabilities: test-retest reliability and comparison with an accelerometer. J Phys Act Health. 2007;4(1):96–100. doi: 10.1123/jpah.4.1.96. [DOI] [PubMed] [Google Scholar]
- [27].Van der Ploeg HP, Streppel KRM, Van der Beek AJ, et al. Counseling increases physical activity behaviour nine weeks after rehabilitation. Br J Sports Med. 2006;40:223–229. doi: 10.1136/bjsm.2005.021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ng AV, Kent-Braun JA. Quantitation of lower physical activity in persons with multiple sclerosis. Med Sci Sport Exerc. 1997;29(4):517–523. doi: 10.1097/00005768-199704000-00014. [DOI] [PubMed] [Google Scholar]
- [29].Sugimoto A, Hara Y, Findley TW, Yonemoto K. A useful method for measuring daily activity by a three-direction monitor. Scand J Rehabil Med. 1997;29(1):37–42. [PubMed] [Google Scholar]
- [30].Westerterp KR. Physical activity assessment with accelerometers. Int J Obes Relat Metab Disord. 1999;23(Suppl 3):S45–S49. doi: 10.1038/sj.ijo.0800883. [DOI] [PubMed] [Google Scholar]
- [31].Khemthong S, Packer TL, Dhaliwal SS. Using the actigraph to measure physical activity of people with disabilities: an investigation into measurement issues. Int J Rehabil Res. 2006;29:315–318. doi: 10.1097/MRR.0b013e328010c592. [DOI] [PubMed] [Google Scholar]
- [32].Washburn RA, Copay AG. Assessing physical activity during wheelchair pushing: validity of a portable accelerometer. Adapted Phys Act Q. 1999;16:290–299. [Google Scholar]
- [33].Warms CA, Belza BL. Actigraphy as a measure of physical activity for wheelchair users with spinal cord injury. Nurs Res. 2004;53(2):136–143. doi: 10.1097/00006199-200403000-00010. [DOI] [PubMed] [Google Scholar]
- [34].Postma K, van den Berg-Emons HJG, Bussman JBJ, et al. Validity of the detection of wheelchair propulsion as measured with an activity monitor in patients with spinal cord injury. Spinal Cord. 2005;43:550–557. doi: 10.1038/sj.sc.3101759. [DOI] [PubMed] [Google Scholar]
- [35].Nunn A, McLeod J, Brown L, et al. Monitoring spinal cord injured patients during activity using a datalogger: preliminary results. Technol Disabil. 2005;17:77–83. [Google Scholar]
- [36].Warms CA, Belza BL, Whitney JD. Correlates of physical activity in adults with mobility limitations. Fam Commun Health. 2007;30(2S):S5–S16. doi: 10.1097/01.FCH.0000264876.42945.e4. [DOI] [PubMed] [Google Scholar]
- [37].Bouchard C, Tremblay A, Leblanc C, et al. A method to assess energy expenditure in children and adults. Am J Clin Nutr. 1983;37:461–467. doi: 10.1093/ajcn/37.3.461. [DOI] [PubMed] [Google Scholar]
- [38].Fisher SV, Gulickson G. Energy cost of ambulation in health and disability: a literature review. Arch Phys Med Rehabil. 1978;59:124–132. [PubMed] [Google Scholar]
- [39].Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9):S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- [40].Reed GR, Velicer WF, Prochaska JO, et al. What makes a good staging algorithm? Examples from regular exercise. Am J Health Promot. 1997;12:57–66. doi: 10.4278/0890-1171-12.1.57. [DOI] [PubMed] [Google Scholar]
- [41].Marcus BH, Rakowski W, Rossi JS. Assessing motivational readiness and decision making for exercise. Health Psych. 1992;11(4):257–262. doi: 10.1037//0278-6133.11.4.257. [DOI] [PubMed] [Google Scholar]
- [42].Evangelista LS, Dracup K, Doering L, et al. Physical activity patterns in heart transplant women. J Cardiovasc Nurs. 2005;20(5):334–339. doi: 10.1097/00005082-200509000-00007. [DOI] [PubMed] [Google Scholar]
- [43].Belza B, Steele BG, Hunziker J, et al. Correlates of physical activity in COPD. Nurs Res. 2001;50(4):195–202. doi: 10.1097/00006199-200107000-00003. [DOI] [PubMed] [Google Scholar]
- [44].Dishman RK, Darracott CR, Lambert LT. Failure to generalize determinants of self-reported physical activity to a motion sensor. Med Sci Sports Exerc. 1992;24(8):904–910. [PubMed] [Google Scholar]
- [45].Van der Woude LHV, deGroot S, Janssen TWJ. Manual wheelchairs: research and innovation in rehabilitation, sports, daily life and health. Med Eng Phys. 2006;28:905–915. doi: 10.1016/j.medengphy.2005.12.001. [DOI] [PubMed] [Google Scholar]


