Abstract
Camalexin (3-thiazol-2′-yl-indole) is the principal phytoalexin that accumulates in Arabidopsis after infection by fungi or bacteria. Camalexin accumulation was detectable in Arabidopsis cell-suspension cultures 3 to 5 h after inoculation with Cochliobolus carbonum (Race 1), and then increased rapidly from 7 to 24 h after inoculation. Levels of radioactivity incorporated into camalexin during a 1.5-h pulse labeling with [14C]anthranilate also increased with time after fungal inoculation. The levels of radioactive incorporation into camalexin increased rapidly between 7 and 18 h after inoculation, and then decreased along with camalexin accumulation. Relatively low levels of radioactivity from [14C]anthranilate incorporated into camalexin in the noninoculated controls. Autoradiographic analysis of the accumulation of chloroform-extractable metabolites labeled with [14C]anthranilate revealed a transient increase in the incorporation of radioactivity into indole in fungus-inoculated Arabidopsis cell cultures. The time-course measurement of radioactive incorporation into camalexin during a 1.5-h pulse labeling with [14C]indole was similar to that with [14C]anthranilate. These data suggest that indole destined for camalexin synthesis is produced by a separate enzymatic reaction that does not involve tryptophan synthase.
One of the most intensively studied disease-resistance mechanisms of plants is the accumulation of phytoalexins. Apart from this function, there is interest in phytoalexins because the induction of synthesis of these secondary metabolites provides unique experimental systems for the study of the coordinate regulation of primary and secondary metabolic pathways. One system that likely involves the coordinate regulation of these pathways is the biosynthesis of phytoalexins in Arabidopsis.
Arabidopsis has several attributes that are ideal for the genetic analysis of disease-resistance mechanisms (Meyerowitz, 1989; Kunkel, 1996). It accumulates the phytoalexin camalexin (3-thiazol-2′-yl-indole) (Tsuji et al., 1992), a compound originally isolated from another crucifer, Camelina sativa (Browne et al., 1991). This metabolite is typical of crucifer phytoalexins in having an indole group substituted at the no. 3 position with a sulfur-containing group (Chapple et al., 1994). In in vitro bioassays, camalexin is inhibitory to bacterial and fungal growth (Jejelowo et al., 1991; Tsuji et al., 1992; Rogers et al., 1996). Mutants of Arabidopsis deficient in camalexin accumulation have been isolated (Glazebrook and Ausubel, 1994). These pad mutants have not yet provided clear evidence for the role of camalexin in the disease resistance of Arabidopsis, but a recently isolated pad4 mutant exhibits an interesting phenotype that may provide insight into differential host plant responses between pathogens and nonpathogens (Glazebrook et al., 1997).
Advances have been made in recent years in the study of Trp synthesis in Arabidopsis (Radwanski and Last, 1995). The study of individual steps in the Trp pathway is important to understand the coordinate regulation of the Trp and camalexin pathways during the induction of camalexin synthesis. Labeling studies have provided evidence that anthranilate, a Trp-pathway intermediate, but not Trp itself, is a precursor of camalexin (Tsuji et al., 1993; Zook and Hammerschmidt, 1997). Furthermore, there is a close correlation between the level of induction of anthranilate synthase and camalexin accumulation in response to different eliciting agents (Zhao and Last, 1996). However, there remains uncertainty about what Trp intermediate is the branch point between the Trp and camalexin biosynthetic pathways. In the current study suspension cultures of Arabidopsis cells inoculated with fungal spores were used to characterize the biosynthetic relationship between Trp-pathway intermediates and the accumulation of the camalexin phytoalexin.
MATERIALS AND METHODS
Cell-Suspension Cultures
Cell-suspension cultures of Arabidopsis (ecotype Columbia) were obtained using a protocol originally devised by Axelos et al. (1992). Cultures were subcultured weekly into 3.2 g/L Gamborg's basal medium with minimal organics (Sigma), 2% (w/v) Suc, and 1.1 mg/L 2,4-D, pH 5.7.
Fungal Inoculum
Cochliobolus carbonum (Race 1) was grown on vegetable-juice agar (163 mL/L vegetable juice, 1.0 g/L CaCO3, and 14 g/L agar [Sigma]) for 7 to 10 d. Fifty milliliters of Arabidopsis cell cultures (3–4 d after subculturing) was inoculated under sterile conditions with 1.5 × 106 spores.
Labeling of Cell Cultures with [14C]Anthranilate or [14C]Indole and Quantitation of Camalexin Accumulation
Seven microliters (1.5 × 106 dpm) of [U-14C]anthranilate (60 mCi/mmol) in ethanol or 5 μL (1.1 × 106 dpm) of [2-14C]indole (25 mCi/mmol) in ethanol was added to 50 mL of Arabidopsis cell-suspension culture 1.5 h before harvesting of the cell culture for determination of levels of radioactivity incorporated into camalexin. At the time of harvest, the cell-suspension cultures were filtered through a single layer of premoistened Miracloth (Calbiochem). The filtrate was then extracted twice with an equal volume of CHCl3. The pooled CHCl3 extracts were evaporated under reduced pressure, and the remaining residue was redissolved in CHCl3 and applied to a TLC plate. The TLC plate was developed with CHCl3:methanol (9:1, v/v) and analyzed by autoradiography, or camalexin was visualized with short-wave UV light and scraped from the TLC plate for determination of radioactivity. For quantitation of camalexin, the TLC plate scrapings were treated with ethyl acetate and filtered through a sintered-glass funnel. The ethyl acetate solution was evaporated under a stream of nitrogen, and the remaining residue was redissolved in 100 μL of 7% isopropanol in hexane (HPLC mobile phase) before injection onto a 5-μm × 150-mm × 4.6-mm silica HPLC column (Econosphere, Alltech, Deerfield, IL) developed with a 1.0 mL/min flow rate. The eluent from the column was monitored at A210 using a variable-wavelength detector. The amount of camalexin detected at A210 was determined by comparison of known standards of camalexin.
RESULTS
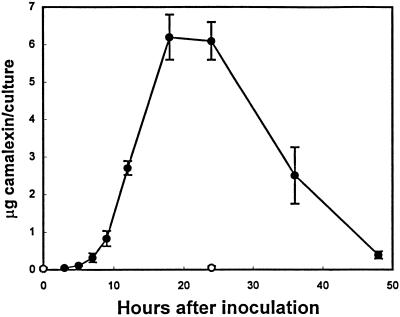
To be useful as an experimental system for the study of camalexin biosynthesis, a suitable eliciting agent had to be found that could generate a sufficient amount of camalexin after treatment or inoculation of cell-suspension cultures. Several elicitors that have proven to be effective in other plant cell-suspension cultures were applied. A “void-glucan elicitor” prepared by partial acid hydrolysis of mycelial walls of Phytophthora sojae (provided by Dr. Michael Hahn, University of Georgia, Athens) and cellulase R10 (Karlan Research Products, Santa Rosa, CA), which elicit phytoalexin accumulation in parsley and tobacco cell-suspension cultures, respectively (Lois et al., 1989; Vögeli et al., 1990), were ineffective. A variety of commercially available cell wall-degrading enzymes were either ineffective or elicited only a relatively small amount of camalexin. Inoculation of cell-suspension cultures with the bacterial pathogen Pseudomonas syringae pv maculicola strain ES4326 elicited only small amounts of camalexin. The only treatment that proved effective in eliciting a significant accumulation of camalexin was viable spores of C. carbonum (Fig. 1). Camalexin accumulation began to increase 5 to 7 h after inoculation, peaked between 18 and 24 h after inoculation, and then decreased. Very low levels of camalexin were present in untreated cell-suspension cultures.
Figure 1.
Accumulation of camalexin in Arabidopsis cell-suspension cultures after inoculation with a C. carbonum spore suspension (•) or no treatment (○). The fungal inoculum and determination of the levels of camalexin accumulation in each 50-mL culture are described in Methods. All data points represent means of three determinations ± se (except the 0- and 3-h time points, which represent means of two determinations ± se).
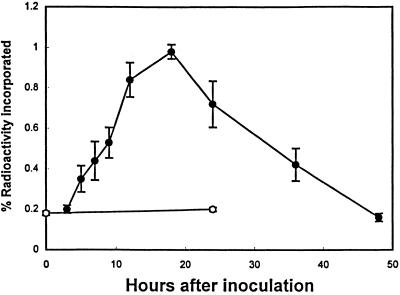
One of the main advantages of using a cell-suspension system for the study of phytoalexin synthesis is that radiolabeled compounds can be easily applied to cell-suspension cultures. Arabidopsis cultures were pulse labeled with [14C]anthranilate for 1.5 h before determining the incorporation of radioactivity into camalexin as a function of time after inoculation with C. carbonum (Fig. 2). The level of incorporation of radioactivity that occurs during a relatively short period of labeling (1.5 h) is expected to be a measure of the rate of synthesis or substrate flow into the final product (camalexin). Therefore, as the level of camalexin accumulation increases, the level of incorporation of biosynthetic intermediates would be expected to increase because of the increased rate of synthesis or flow of substrate through the pathway.
Figure 2.
Incorporation of radioactivity from [14C]anthranilate into camalexin in Arabidopsis cell-suspension cultures after inoculation with a C. carbonum spore suspension (•) or no treatment (○). Radioactivity was allowed to incorporate into camalexin for 1.5 h before each time point, as described in Methods. All data points represent means of three determinations ± se (except the 0- and 3-h time points, which represent means of two determinations ± se).
Data from previous studies (Tsuji et al., 1993; Zook and Hammerschmidt, 1997) suggest that anthranilate is an intermediate of camalexin synthesis. Consistent with this hypothesis, the level of radioactivity incorporated into camalexin from [14C]anthranilate increased as the rate of camalexin accumulation increased. The greatest increase in camalexin accumulation occurred between 12 and 18 h after inoculation with C. carbonum; likewise, the highest level of incorporation of radioactivity into camalexin occurred 18 h after inoculation. The levels of radioactivity incorporated into camalexin also decreased in a manner similar to the levels of camalexin between 24 and 48 h after inoculation.
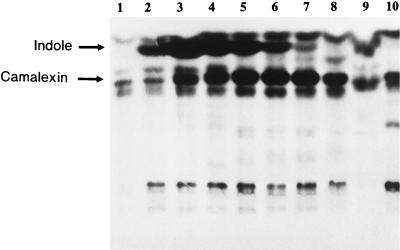
An autoradiographic analysis of chloroform extracts from each time point of the [14C]anthranilate pulse-labeling experiments (Fig. 2) revealed the transient accumulation of a compound with a higher RF than camalexin (Fig. 3). The compound was eluted from the TLC plate with ethyl acetate, and after careful evaporation of the solvent under a stream of nitrogen, the residue had the very distinctive aroma of indole. HPLC analysis of the residue revealed that more than 95% of the radioactivity in a single peak comigrated with a pure indole standard. Pulse labeling of C. carbonum with [14C]anthranilate grown in plant cell culture medium failed to reveal the presence of compounds that comigrated with indole or camalexin by TLC analysis (data not shown).
Figure 3.
Autoradiogram of a TLC separation of chloroform extracts of Arabidopsis cell-suspension culture filtrates after inoculation with a C. carbonum spore suspension (lanes 1–9) or no treatment (lane 10). [14C]Anthranilate was applied 1.5 h before each of the following times after fungal inoculation: 3 h (lane 1), 5 h (lane 2), 7 h (lane 3), 9 h (lane 4), 12 h (lane 5), 18 h (lane 6), 24 h (lane 7), 36 h (lane 8), 48 h (lane 9), and 24 h (lane 10). Preparation of chloroform extracts and TLC/autoradiography is described in Methods.
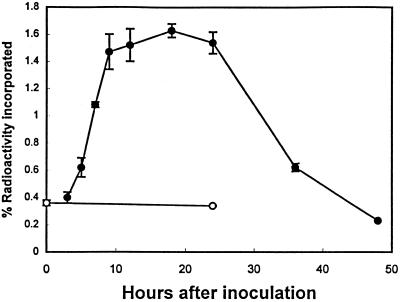
To determine if indole is a precursor of camalexin, Arabidopsis cell cultures were pulse labeled with [14C]indole (Fig. 4) in the same manner as shown in Figure 2 for [14C]anthranilate. Similar to [14C]anthranilate incorporation, the levels of radioactivity incorporated into camalexin increased as the rate of camalexin accumulation increased.
Figure 4.
Incorporation of radioactivity from [14C]indole into camalexin in Arabidopsis cell-suspension cultures after inoculation with a C. carbonum spore suspension (•) or no treatment (○). Radioactivity was allowed to incorporate into camalexin for 1.5 h before each time point, as described in Methods.
DISCUSSION
Because previous studies of camalexin biosynthesis using detached leaves of Arabidopsis have provided evidence that anthranilate is a biosynthetic precursor of camalexin (Tsuji et al., 1993; Zook and Hammerschmidt, 1997), similar results were expected from these experiments with cell-suspension cultures of Arabidopsis. The level of radioactivity incorporated into camalexin from [14C]anthranilate increased as the rate of camalexin accumulation increased (Figs. 1 and 2). An unexpected result of the pulse-labeling experiments with [14C]anthranilate, however, was the transient accumulation of label from anthranilate into indole (Fig. 3). Indole is the immediate precursor of Trp in the Trp synthase complex, but there is evidence that indole is not a “free intermediate” in Trp synthesis (Creighton, 1970). Indole is thought to pass directly from the α-subunit to the β-subunit though a tunnel in the Trp synthase complex (Hyde et al., 1988). Therefore, indole is not expected to accumulate, even if there is an increase in the flux through the pathway.
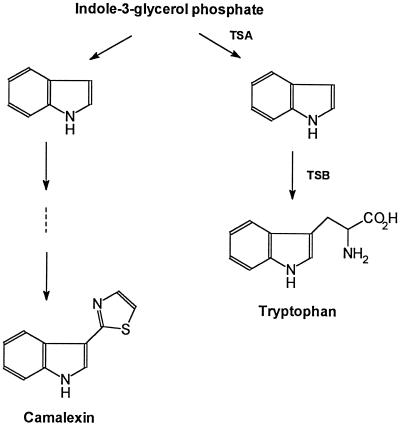
A recent study by Frey et al. (1997) may offer an explanation for the apparent accumulation of free indole in camalexin-synthesizing Arabidopsis cell cultures. This study identified a gene (BX1) that encodes a Trp synthase α-subunit homolog. The encoded enzyme catalyzes the formation of a free indole destined for 2,4-dihydroxy-1,4-benzoxazin-3-one synthesis rather than for Trp synthesis. Evidence for the formation of free indole during the elicitation of camalexin synthesis in the present study (Fig. 3) supports the hypothesis originally proposed by Frey et al. (1997) that there may be a homolog of BX1 in Arabidopsis. This homolog would encode an enzyme that catalyzes the formation of indole from indole-3-glycerol phosphate for camalexin synthesis (Fig. 5).
Figure 5.
Abbreviated Trp biosynthetic pathway showing hypothetical branch point for camalexin biosynthesis. TSA, Trp synthase α-subunit; TSB, Trp synthase β-subunit.
There is a gene in Arabidopsis that may be the homolog of BX1 (accession no. F20005 for the 5′ end of the expressed sequence tag and accession no. F20004 for the 3′ end of the expressed sequence tag). This gene is also found in a 100-kb bacterial artificial chromosome clone (T10P11, Cold Spring Harbor Laboratory; accession no. AC002354) located in the upper portion of chromosome 4. The putative coding region of the Arabidopsis gene encodes a 275-amino acid protein (Mr = 30,000) with approximately 80% sequence identity to the α-subunit of the Arabidopsis Trp synthase (data not shown). The presence of this homolog may account for one or more of the additional bands that appeared under low-stringency genomic DNA gel-blot hybridization using TSA1 cDNA as a probe (Radwanski et al., 1995).
The relative efficiency of incorporation of radioactivity from [14C]indole into camalexin that accompanied the increase in the accumulation rate of camalexin (Fig. 4) is consistent with the hypothesis that indole is a biosynthetic intermediate of camalexin. The overall efficiency of incorporation of radioactivity into camalexin from [14C]indole was approximately twice that for [14C]anthranilate. If indole is the more immediate precursor of camalexin compared with anthranilate, then the incorporation efficiency of anthranilate may be less because of diversion of this intermediate toward the synthesis of other secondary metabolites that do not include indole as a biosynthetic precursor. Differences in incorporation efficiencies between indole and anthranilate may also result from differences in chemical properties of these two compounds.
The results of the current study and those of previous studies (Tsuji et al., 1993; Zhao and Last, 1996; Zook and Hammerschmidt, 1997) are consistent with the hypothesis that the synthesis of camalexin involves steps of the Trp pathway. Also, there is good evidence for the induction of an increase in transcript and protein levels of Trp-pathway enzymes after microbial infection (Niyogi and Fink, 1992; Niyogi et al., 1993; Zhao and Last, 1996). This induction of an apparent increase in substrate flow through the Trp pathway during camalexin synthesis is expected if biosynthetic precursors of camalexin originate from the Trp pathway. In addition to camalexin, other defense-related secondary metabolites derived from the Trp pathway may be synthesized after microbial infection.
There are also results from investigations of biosynthetic pathways that do not offer obvious explanations. Zhao and Last (1996) reported that a phosphoribosyl-anthranilate transferase (PAT1) mutant, trp1-100, and an anthranilate synthase/PAT1 double mutant, trp4/trp1-100, accumulated to near wild-type levels of camalexin after infection with P. syringae pv maculicola ES4326. A large reduction in the activity of enzymes early in the Trp pathway is expected to reduce the flow of substrate into camalexin. There was some reduction in the levels of camalexin accumulation in the infected Trp mutants compared with infected wild-type plants, but the percentage level of reduction was not as large as that previously reported (Tsuji et al., 1993) using AgNO3 as an elicitor of camalexin accumulation. P. syringae pv maculicola ES4326 elicited about a 20-fold greater amount of camalexin compared with AgNO3, and this greater flux of substrate through the pathway after infection may overcome constraints caused by enzyme-activity deficiencies in the mutants.
Analysis of an allelic series of trp1 mutants revealed that the prototrophic mutants, including trp1-100, grew normally with less than 1% of normal PAT1 activity (Rose et al., 1997). If the single-copy PAT1 gene were completely knocked out, the Trp pathway would be blocked, and the mutation would be lethal because of auxin and Trp starvation (Rose et al., 1997). Therefore, the amount of PAT1 activity required for normal growth and camalexin accumulation is much less than that present in wild-type plants. Characterization of the anthranilate synthase β-subunit gene (Niyogi et al., 1993), the defective gene in trp4 mutants, revealed three very similar genes in the Arabidopsis genome. This gene duplication apparently accounts for the normal growth of trp4-1 mutants (Niyogi et al., 1993). Despite the fact that the trp4/trp1-100 double mutant is an auxotroph, there is apparently enough flow through the Trp pathway to support near wild-type levels of camalexin accumulation.
The duplication of genes of the Trp pathway (Radwanski and Last, 1995), with the exception of PAT1 (Rose et al., 1992), has been proposed as one of the reasons for the failure to isolate Trp auxotrophs (Radwanski et al., 1996). The existence of multiple isozymes of Trp-pathway genes also may allow for independent regulation of substrate flow through the Trp and camalexin pathways. In the case of Ruta graveolens, there are two isozymes of the α-subunit of anthranilate synthase (Bohlmann et al., 1995) that appear differentially with respect to the synthesis of alkaloids and Trp (Bohlmann et al., 1996). A similar method of regulation may exist for camalexin synthesis (Fig. 5). The synthesis of free indole destined for camalexin synthesis may provide a means to independently regulate the flow of substrate between the camalexin and Trp pathways.
Work is currently in progress to determine the enzymatic reactions that might involve indole in the synthesis of camalexin. One possible reaction is the carboxylation of indole, and it has been proposed that indole-3-carboxaldehyde condenses with Cys to form camalexin (Browne et al., 1991). There is evidence that Cys is involved in camalexin biosynthesis (Zook and Hammerschmidt, 1997). Elucidation of the steps in the camalexin biosynthetic pathway is important for gaining additional insight into the regulation of the biosynthesis of secondary metabolites used in plant defense mechanisms.
Footnotes
This research was supported in part by the Michigan State University Agricultural Experiment Station and by grant no. IBN-9220912 from the National Science Foundation.
LITERATURE CITED
- Axelos M, Curie C, Mazzolini L, Bardet C, Lescure B. A protocol for transient gene expression in Arabidopsis thaliana protoplasts isolated from cell suspension cultures. Plant Physiol Biochem. 1992;30:123–128. [Google Scholar]
- Bohlmann J, DeLuca V, Eilert U, Martin W. Purification and cDNA cloning of anthranilate synthase from Ruta graveolens: modes of expression and properties of native and recombinant enzymes. Plant J. 1995;7:491–501. doi: 10.1046/j.1365-313x.1995.7030491.x. [DOI] [PubMed] [Google Scholar]
- Bohlmann J, Lins T, Martin W. Anthranilate synthase from Ruta graveolens. Plant Physiol. 1996;111:507–514. doi: 10.1104/pp.111.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne LM, Conn KL, Ayer WA, Tewari JP. The camalexins: new phytoalexins produced in the leaves of Camelina sativa (Cruciferae) Tetrahedron. 1991;47:3909–3914. [Google Scholar]
- Chapple CCS, Shirley BW, Zook M, Hammerschmidt R, Somerville S. Secondary metabolism in Arabidopsis. In: Meyerowitz EM, Somerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 989–1030. [Google Scholar]
- Creighton TE. A steady-state kinetics investigation of the reaction mechanisms of the tryptophan synthetase of Escherichia coli. Eur J Biochem. 1970;13:1–10. doi: 10.1111/j.1432-1033.1970.tb00892.x. [DOI] [PubMed] [Google Scholar]
- Frey M, Chomet P, Glawischnig E, Stettner C, Grün S, Winklmair A, Eisenreich W, Bacher A, Meeley RB, Briggs SP and others. Analysis of a chemical plant defense mechanism in grasses. Science. 1997;277:696–699. doi: 10.1126/science.277.5326.696. [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Ausubel FM. Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc Natl Acad Sci USA. 1994;91:8955–8959. doi: 10.1073/pnas.91.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J, Zook M, Mert F, Kagan I, Rogers EE, Crute IR, Holub EB, Hammerschmidt R, Ausubel F. Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics. 1997;146:381–392. doi: 10.1093/genetics/146.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde CC, Ahmed SA, Padlan EA, Miles EW, Davies DR. Three-dimensional structure of the tryptophan synthase α2β2 multienzyme complex from Salmonella typhimurium. J Biol Chem. 1988;263:17857–17871. [PubMed] [Google Scholar]
- Jejelowo OA, Conn KL, Tewari JP. Relationship between conidial concentration, germling growth and phytoalexin production by Camelina sativa leaves inoculated with Alternaria brassicae. Mycol Res. 1991;95:928–934. [Google Scholar]
- Kunkel BN. A useful weed put to work: genetic analysis of disease resistance in Arabidopsis thaliana. Trends Genet. 1996;12:63–69. doi: 10.1016/0168-9525(96)81402-8. [DOI] [PubMed] [Google Scholar]
- Lois R, Dietrich A, Hahlbrock K, Schulz W. A phenylalanine ammonia-lyase gene from parsley: structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO J. 1989;8:1641–1648. doi: 10.1002/j.1460-2075.1989.tb03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz EM. Arabidopsis, a useful weed. Cell. 1989;56:263–269. doi: 10.1016/0092-8674(89)90900-8. [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Fink GR. Two anthranilate synthase genes in Arabidopsis: defense-related regulation of the tryptophan pathway. Plant Cell. 1992;4:721–733. doi: 10.1105/tpc.4.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Last RL, Fink GR, Keith B. Suppressors of trp1 fluorescence identify a new Arabidopsis gene, TRP4, encoding the anthranilate synthase β subunit. Plant Cell. 1993;5:1011–1027. doi: 10.1105/tpc.5.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanski ER, Barczak AJ, Last RL. Characterization of tryptophan synthase alpha subunit mutants of Arabidopsis thaliana. Mol Gen Genet. 1996;253:353–361. doi: 10.1007/pl00008602. [DOI] [PubMed] [Google Scholar]
- Radwanski ER, Last RL. Tryptophan biosynthesis and metabolism: biochemical and molecular genetics. Plant Cell. 1995;7:921–934. doi: 10.1105/tpc.7.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanski ER, Zhao J, Last RL. Arabidopsis thaliana tryptophan synthase alpha: gene cloning, expression, and subunit interaction. Mol Gen Genet. 1995;248:657–667. doi: 10.1007/BF02191705. [DOI] [PubMed] [Google Scholar]
- Rogers EE, Glazebrook J, Ausubel FM. Mode of action of the Arabidopsis thaliana phytoalexin camalexin and its role in Arabidopsis-pathogen interactions. Mol Plant Microbe Interact. 1996;9:748–757. doi: 10.1094/mpmi-9-0748. [DOI] [PubMed] [Google Scholar]
- Rose AB, Casselman AL, Last RL. A phosphoribosylanthranilate transferase gene is defective in blue fluorescent Arabidopsis thaliana tryptophan mutants. Plant Physiol. 1992;100:582–592. doi: 10.1104/pp.100.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AB, Li J, Last RL. An allelic series of blue fluorescent trp1 mutants of Arabidopsis thaliana. Genetics. 1997;145:197–205. doi: 10.1093/genetics/145.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji J, Jackson EP, Gage DA, Hammerschmidt R, Somerville SC. Phytoalexin accumulation in Arabidopsis thaliana during the hypersensitive reaction to Pseudomonas syringae pv. syringae. Plant Physiol. 1992;98:1304–1309. doi: 10.1104/pp.98.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji J, Zook M, Hammerschmidt R, Somerville S, Last R. Evidence that tryptophan is not a direct biosynthetic intermediate of camalexin in Arabidopsis thaliana. Physiol Mol Plant Pathol. 1993;43:221–229. [Google Scholar]
- Vögeli U, Freeman JW, Chappell J. Purification and characterization of an inducible sesquiterpene cyclase from elicitor-treated tobacco cell suspension cultures. Plant Physiol. 1990;93:182–187. doi: 10.1104/pp.93.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Last RL. Coordinate regulation of the tryptophan biosynthetic pathway and indolic phytoalexin accumulation in Arabidopsis. Plant Cell. 1996;8:2235–2244. doi: 10.1105/tpc.8.12.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zook M, Hammerschmidt R. Origin of the thiazole ring of camalexin, a phytoalexin from Arabidopsis thaliana. Plant Physiol. 1997;113:463–468. doi: 10.1104/pp.113.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]