Abstract
This study was designed to analyze the effect of environmental oxidative stress on human placental monooxygenases, glutathione S-transferase (GST) activity and polycyclic aromatic hydrocarbon (PAH)–DNA adducts in human term placentas from radioactivity-contaminated and chemically-polluted areas of the Ukraine and Belarus, and to compare these biomarkers to the newborn’s general health status. Placental PAH–DNA adduct formation, GST activity, 7-ethoxycoumarin O-deethylase (ECOD) activity, and thiobarbituric reactive substances (TBARS), an index of lipid peroxidation, were measured in groups of women exposed to different levels of radioactivity and PAH pollution. The in vitro metabolism data, obtained from 143 human placental samples at term, were compared to indices of maternal and newborn health. The highest ECOD activity was recorded in placentas obtained from chemically-polluted areas and a radioactivity-contaminated area; the ECOD activity was 7-fold and 2-fold higher compared to the region considered to be “clean”. Newborns with the most compromised health status displayed the greatest down-regulation of GST activity (144–162 mU mg protein−1 vs. 258–395 mU mg protein−1), enhanced ECOD activity and the highest level of PAH–DNA adduct formation. The highest level of TBARS was observed in women exposed to the highest levels of radiation. The efficiency of placental detoxification negatively correlated with maternal age and the health status of the newborn. Environmental oxidative stress was related to an increase in anemia, threatened abortions, toxemia, fetal hypoxia, spontaneous abortions and fetal hypotrophy. Our data suggest that chemically- or radioactivity-induced oxidative stress enhance cytochrome P450-mediated enzymatic activities potentially resulting in increased formation of reactive metabolites. The activity of GSH-transferase is not enhanced. This imbalance in detoxification capacity can be measured as increased production of PAH–DNA adducts, decreased lipid peroxidation and compromised fetal health.
Keywords: Glutathione S-transferase, PAH–DNA adducts, Human term placenta, Biomarker, Monooxygenase, Cytochrome P450
1. Introduction
The high frequency of complications during pregnancy, and the widespread compromised health status of newborns found in the Ukraine after the Chernobyl reactor accident triggered our interest in studying the detoxification efficiency of human placenta in contaminated and polluted areas of the Ukraine and Belarus. The capacity of placenta to detoxify xenobiotics makes it an important determinant of fetal development and well-being. Potential fetal risk is a composite of many properties: xenobiotic physicochemical properties; placental metabolism including oxy- and glutathione-status; the ability of xenobiotic metabolites to interfere with placental macromolecules and cellular structures; the stage of pregnancy controlling blood flow and hormone status; the expression of placental transporters that monitor and control the two-way flux of metabolites and xenobiotics; and finally, individual genotype (Myllynen et al., 2005, 2007; Knapen et al., 1999; Syme et al., 2004). Inadequate excretion of xenobiotics may result in adverse effects on placental vital functions or results in their binding to cellular macromolecules such as DNA, increasing the risk of mutagenesis and potentially tumorigenesis and/or teratogenesis (Lutz, 1979). The human placenta, at term, represents a unique ex vivo/in vitro model for the investigation of detoxification because damage to placental DNA indicates not only maternal exposure and maternal detoxification efficiency, but it also reflects DNA damage occurring in other fetal tissues.
In this study we assessed the relative contribution of several factors – including maternal exposure to environmental oxidative stress, placental metabolic phenotype in vitro and maternal age –on glutathione S-transferase (GST) activity, as well as on the formation of polycyclic aromatic hydrocarbon (PAH)–DNA adducts. Additionally, the occurrence of typical complications of pregnancy (e.g. low Apgar’s index, overall retarded growth of newborn, anemia, threatened abortions, toxemia, fetal hypoxia, spontaneous abortions and fetal hypotrophy) in the whole cohort was analyzed. Here we demonstrate that an imbalance in placental detoxification capacity can be measured as increased production of PAH–DNA adducts, changes in lipid peroxidation status in placenta and as compromised fetal health.
2. Methods
2.1. Human subjects and study design
Human term placentas (143 samples) were obtained in State regional maternity hospitals from November–March of the years 1991–1999. Samples were obtained from mothers living in radioactivity-contaminated and chemically-polluted areas of the Ukraine and Belarus, and in an area of East Poland considered to be uncontaminated. Background information concerning environmental radioactivity or chemical pollution was collected from the local authorities’ data base. The specimens were subdivided into seven groups depending on the type and level of exposure (Ministry of Health of Ukraine, 1993). Radioactive contamination was expressed as Summary Effective Equivalent Annual Exposition Dose (SEEAED, Table 1) (Council of Ministers of Ukraine Republic, 2003). Chemical pollution was expressed as the concentration of B(a)P in the ambient air (Table 1) (Hydrometeoservice of Ukraine 2003). Some of the contamination originates from the industrial pollution and traffic-exhaust in the ambient air of Kiev (groups IV and V). Another group includes specimens from women exposed to high levels of radioactivity for 5–6 years before their pregnancy; these individuals (group I) had been transferred to Kiev and the Kiev district to remove them from radioactive exposure. Group V consists of women from Kiev experiencing typical complications of pregnancy (Department of Obstetrical Pathology, Institute of Pediatrics, Obstetrics and Gynecology, Kiev, Ukraine). Group VI encompasses the samples from Zaporizhzhia, a region known for heavy engineering and metallurgy industry, and severe chemical pollution. The samples in groups II and III originated from women living in small towns with no significant chemical pollution, according to the official data, but with exposure to low levels of radioactivity after the Chernobyl accident. Samples from non-complicated pregnancies taken from an area considered to be unpolluted (East Poland, group VII) were used as controls (Table 1).
Table 1.
Contamination with 137Cs isotope and benzo(a)pyrene in specified regions.
| Groups of samples and regions of their collection | Number of samples | Period of samples collection (T) (years) | Soil contamination with 137Cs in 1986 (kBq/m2) | SEEAED (mSv)a | Concentration of B(a)P in ambient air in period T (ng/m3) | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 1986 | Period T | Mean | Maximal | |||||
| I | Radiation zone of exclusion and of obligatory relocation (Ukraine) | 18 | 1992–1993 | ~600 | ~25 | 0.3–1.70b | 1.0–4.1b | 1.0–12.8b |
| II | Zone of the intensive radioactivity monitoring (Ukraine) | 17 | 1991–1993 | 37–185 | 1.0–5.0 | 0.09–0.45 | ~1.0 | ~1.0 |
| III | Zone of the facultative evacuation and periodic monitoring (Belarus) | 26 | 1999 | 17–79 | 0.45–2.10 | 0.02–0.09 | ~1.0 | ~1.0 |
| IV | Radioactivity- and chemically-polluted region (Kiev, Ukraine) | 20 | 1991–1993 | 18–37 | 0.30–0.60 | 0.02–0.05 | 4.1 | 12.8 |
| V | Radioactivity- and chemically-polluted region (Kiev, Ukraine) | 21 | 1995–1996 | 18–37 | 0.30–0.60 | 0.01–0.02 | 4.0 | 12.2 |
| VI | Chemically-polluted region (Zaporizhzhia, Ukraine) | 21 | 1992 | 2–4 | <0.07 | <0.01 | 12.3 | 33.3 |
| VII | Region considered as radioactively and chemically clean (Eastern Carpathian, Poland) | 20 | 2001 | 2–4 | <0.07 | <0.01 | ~1 | ~1 |
Note: all the data about radioactive contamination and chemical pollution are given for specified settlements and are taken and calculated on the basis of official documents [6–10].
SEEAED: summary effective equivalent annual exposition dose.
The range of the data covers the settlements of relocated and non-relocated residence.
Immediately after placental delivery, coagulated blood was removed; the placenta was rinsed with cold 0.9% NaCl solution and gently dried between paper towels. Placental tissue (~50 g), including all layers, was excised from the central part of the organ, frozen in liquid nitrogen and stored at −70 °C before processing. Biochemical assays were conducted within 2 weeks after delivery.
Each sample was accompanied by a personal questionnaire including data such as location and duration of residence in the area, and lifestyle factors such as consumption of alcohol, cigarette smoking, diet, and occupational contamination risks. Maternal clinical background data and newborn health status were taken from medical histories.
This study was carried out according to the principles of the Declaration of Helsinki. The Ethics Committees of the Institute of Molecular Biology and Genetics of National Academy of Sciences, Kiev, Ukraine, approved the study protocol and the use of human tissue. All women who participated in the study signed the informed consent form.
2.2. Biochemical assays
Subcellular fractions were prepared by a standard differential centrifugation technique. Microsomes were isolated in a buffer containing 150 mM KCl and 10 mM EDTA (pH 7.4) and suspended in a buffer containing 100 mM potassium phosphate, 1 mM EDTA and 20% glycerol (pH 7.4) (Honkakoski and Lang, 1989).
GST activity was determined in the cytosolic (100,000 × g) fraction, by monitoring the conjugate formation between glutathione (GSH) and 1-chloro-2,4 dinitrobenzene (CDNB) (Habig et al., 1974). One unit (U) of enzymatic activity was considered as the amount of enzyme catalyzing the formation of 1 μmol of product (GSH-CDNB) per minute under the conditions of the assays. The irreversibility of GST activity down-regulation was verified by its measurement before and after treatment of the cytosolic fraction with 0.01 M dithiothreitol (DTT) (pH 8.0) for 1 h at 37 °C. The fraction was purified from DTT by chromatography on a G-25 Sephadex column eluted with 0.1 M Na-phosphate buffer – 1 mM EDTA, pH 8.0. Lipid peroxidation was estimated in homogenates by reaction with thiobarbituric acid (Gavrilov et al., 1987).
DNA was isolated by standard procedures of lysis, incubation with proteinase K and deproteinization with phenol-chloroform/isoamyl alcohol (Sambrook et al., 1989). PAH–DNA adducts were detected by competitive Chemiluminescence Immunoassay (CIA) using an antiserum elicited against DNA modified with (±)r-7,t-8 dihydroxy-c-9,10 epoxy-7,8,9,10-tetrahydro-benzo(a)pyrene (BPDE) that is also specific for DNA modified with several carcinogenic PAHs including benz(a)anthracene and chrysene (Divi et al., 2002). When assaying human samples, because a mixture of PAH–DNA adducts, including benzo(a)pyrene, will be present and recognized by the antiserum, the data are designated as PAH–DNA adducts. A standard curve consisting of calf thymus DNA modified with a known amount of BPDE was assayed on each plate allowing for quantitation of biological samples and giving an assay detection limit of 2.2 ± 0.4 adducts/109 nucleotides. Biological sample values, the amount of adduct that would cause the same % inhibition as a known amount of standard BPDE–DNA, are expressed as PAH–DNA adducts/109 nucleotides. Samples were assayed in triplicate at 10 μg DNA/well. For samples below the detection limit, a value of half of the detection limit was assigned.
Microsomal 7-ethoxycoumarin O-deethylase (ECOD) activity was determined using 0.5 mM 7-ethoxycoumarin as the substrate (Greenlee and Poland, 1978). The relative content of GSTP1-specific mRNA was detected by Dot-hybridization with a probe kindly provided by Prof. Kano (Japan). The hybridization signals were normalized by comparison with hybridization signals of the same RNA samples with a probe corresponding to 18S rRNA kindly provided by Dr. V. Nosikov (Russia). The probes were radioactively labeled with 32P by reacting with random primers (Sambrook et al., 1989). Protein concentration was detected by reaction with the method of Bradford (1976).
2.3. Data analyses
All data were tested for normality, and both parametric and non-parametric analyses were undertaken. The non-parametric data are represented as medians (Me) with lower and upper quartiles (Lq–Uq), and parametric values as means with standard deviation (M ± SD). The significance of the differences between the groups and the correlation between the data was evaluated with the help of Kruskal–Wallis, Mann–Whitney, t-test and Spearman’s coefficients (Rebrova, 2003).
3. Results
3.1. Social, economic, demographic and clinical characteristics of the subjects
According to the questionnaires, the women from Ukraine and Belarus whose specimens were taken for investigation were workers with average earnings and similar lifestyles who consumed the traditional national diet. They denied using alcohol, drugs or tobacco products during their pregnancies. Compared to the Ukraine and Belarus, the income of the Polish women was 2–3 times higher and the traditional diet was more enriched with vitamins. None of the women had occupational exposure to xenobiotics, and all of the women, except for those in group I (specimens from women exposed to high levels of radioactivity for 5–6 years before their pregnancy), had been living for at least three years in the indicated territories. In 1986, the women in group I had been re-located to the Kiev district or to the city of Kiev where they lived until delivery.
Demographic data for maternal age, clinical characteristics of pregnancy and general developmental grading of newborns, are presented in Table 2. The median age of the women from the whole cohort is 25 years, this being significantly higher in Kiev (groups IV, V) and lower in the small towns (group II). The ratio between the number of previous pregnancies and deliveries was also higher in the large urban areas (groups IV–VI). The newborn birth weight was higher for groups I and VI, in comparison with the other groups. The difference between the newborn lengths was not significant. Apgar’s indices reflecting the babies’ status, and using five characteristics on a ten-point scale, were highest in the “clean” area (group VII) but lower in the groups with histories of exposures to oxidative stress evoked by chemicals and/or radioactivity (groups I, IV and V) (Table 2).
Table 2.
Some characteristics of mothers and newborns in the different groups.
| Groups | Number of persons | Maternal age in years, median (Lq–Uq) | Number of previous pregnancies (M ± SD) | Number of previous deliveries (M ± SD) | Individuals with obstetrical pathology (%)(absolute values) | Newborns’ body length (cm) median (Lq–Uq) | Newborns’ body weight (g), median (Lq–Uq) | Apgar’s index, median (Lq–Uq) |
|---|---|---|---|---|---|---|---|---|
| I | 18 | 24 (21–7) | 1.80 ± 0.55 | 0.80 ± 0.33 | 33.3 (6/18) | 53 (51–55) | 3700§§ (3300–4100) | 7.2§ (7.0–8.0) |
| II | 17 | 22 (20–24)§ | 0.92 ± 0.29 | 0.42 ± 0.19 | 35.3 (6/17) | 51 (48–52) | 3225§ (3000–3500) | 8.0 (7.5–8.0) |
| III | 26 | 24 (21–27) | 0.85 ± 0.21 | 0.27 ± 0.09 | 100 (26/26) | 52 (50–54) | 3290§ (3080–3450) | 7.8 (7.5–8.5) |
| IV | 20 | 27 (25–36)§§ | 1.20 ± 0.47 | 0.30 ± 0.21 | 65.0 (13/20) | 52 (49–54) | 3050§ (2800–3500) | 7.0§ (6.0–8.0) |
| V | 21 | 27 (25–33)§§ | 1.62 ± 0.38 | 0.38 ± 0.15 | 95.2 (20/21) | 51 (50–52) | 3075§ (2880–3400) | 7.5§ (7.0–8.0) |
| VI | 21 | 25 (24–34) | 1.71 ± 0.52 | 0.43 ± 0.20 | 80.9 (17/21) | 52 (51–53) | 3400§§ (2900–4000) | 8.2 (7.5–8.5) |
| VII | 20 | 26 (19–36) | 1.46 ± 0.77 | 0.2 ± 0.06 | 0 (0/20) | 54 (51–56) | 3500§§ (3000–4000) | 10.0§§ (9.0–10.0) |
symbols mark higher (§§) and lower (§) values with statistically significant differences (Kruskal–Wallis test at p < 0.05).
The frequency of obstetrical pathology in the whole cohort is about 63%. The most common of these conditions are presented in Table 3 (listed in the order of frequency) and include anemia, threatening abortion, toxemia, nephropathy, and fetal hypoxia. None of these conditions was observed in mothers and newborns from the unexposed “clean” area.
Table 3.
The occurrence of obstetrical pathological cases in the whole cohort and in the separate groups.
| Type of pathology | Percentage of pathology (%) (pathological cases/all cases)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| In the whole cohort | In the groups
|
|||||||
| I | II | III | IV | V | VI | VII | ||
| Anemia | 27.9 (40/143) | 16.7 (3/18) | 17.6 (3/17) | 46.1 (12/26) | 40.0 (8/20) | 57.1 (12/21) | 9.5 (2/21) | 0.0 (0/20) |
| Threatened abortion | 21.7 (32/143) | 5.6 (1/18) | 5.9 (1/17) | 42.3 (11/26) | 35.0 (7/20) | 38.1 (8/21) | 19.0 (4/21) | 0.0 (0/20) |
| Toxemia | 16.6 (26/143) | 0.0 (0/18) | 17.6 (3/17) | 23.0 (6/26) | 15.0 (3/20) | 47.0 (10/21) | 9.5 (2/21) | 0.0 (0/20) |
| Fetal hypoxia | 16.0 (24/143) | 11.1 (2/18) | 0.0 (0/17) | 19.2 (5/26) | 15.0 (3/20) | 33.3 (7/21) | 28.5 (6/21) | 0.0 (0/20) |
| Nephropathy | 13.3 (19/143) | 0.0 (0/18) | 11.8 (2/17) | 7.7 (2/26) | 15.0 (3/20) | 17.6 (10/21) | 9.5 (2/21) | 0.0 (0/20) |
| Labor weakness | 11.9 (17/143) | 22.2 (4/18) | 0.0 (0/17) | 11.5 (3/26) | 15.0 (3/20) | 14.3 (3/21) | 19.0 (4/21) | 0.0 (0/20) |
| Spontaneous abortion | 10.5 (15/143) | 0.0 (0/18) | 0.0 (0/17) | 7.7 (2/26) | 25.0 (5/20) | 19.0 (4/21) | 19.0 (4/21) | 0.0 (0/20) |
| Pyelonephritis | 9.7 (14/143) | 0.0 (0/18) | 5.9 (1/17) | 38.2 (10/26) | 10.0 (2/20) | 4.8 (1/21) | 0.0 (0/21) | 0.0 (0/20) |
| Placental insufficiency | 9.7 (14/143) | 22.2 (4/18) | 0.0 (0/17) | 3.8 (1/26) | 0.0 (0/20) | 9.5 (2/21) | 19.0 (4/21) | 0.0 (0/20) |
| Fetal hypotrophy | 6.7 (10/143) | 0.0 (0/18) | 0.0 (0/17) | 0.0 (0/26) | 38.5 (8/20) | 9.5 (2/21) | 0.0 (0/21) | 0.0 (0/20) |
| Hydropsy | 5.6 (8/143) | 0.0 (0/18) | 5.8 (1/17) | 0.0 (0/26) | 0.0 (0/20) | 23.8 (5/21) | 9.5 (2/21) | 0.0 (0/20) |
| Total types of pathology (%) (absolute values) | 100 (11) | 45.4 (5/11) | 45.4 (5/11) | 81.8 (9/11) | 81.8 (9/11) | 83.3 (11/12) | 100.0 (11/11) | 0.0 (0/11) |
3.2. GST and ECOD activity
The GST activity in samples from the seven groups revealed a “bimodality” (Table 4). The lowest median GST range, 144.1–162.0 mU mg protein−1 was found in groups with high levels of exposure, including the specimens obtained from women living in large chemically-polluted towns (Zaporizhzhia, group VI; and, Kiev, groups IV and V), and those exposed to the highest levels of radioactivity prior to their pregnancy (group I). The women from groups with the lowest levels of exposure had GST levels of 258.1–395.3 mU mg protein−1 and included women living in small towns with minimal chemical and radioactive pollution (groups II and III), and women from the “clean” area (group VII). The differences in median GST values between the highly-exposed and lesser-exposed groups were statistically significant (Kruskal–Wallis test, p < 0.035, Table 4). The level of GSTP1 mRNA was analyzed only in the samples from groups I, VI, and VII (Table 5). Relative amounts of GSTP1-specific mRNA in total placental RNA were nearly 5 times lower in the specimens from group I but 3.5 times higher in the specimens from group VI compared to the specimens from the “clean” area (group VII).
Table 4.
Glutathione S-transferase (GST) activity, amount of polycyclic aromatic hydrocarbon DNA (PAH–DNA) adducts and the level of thiobarbituric reactive substances (TBARS) in placental samples.
| Groups | No | GST activity (mU mg protein−1)
|
PAH–DNA adducts/109 nucleotides♣
|
TBARS, (nmoles g tissue−1)
|
|||
|---|---|---|---|---|---|---|---|
| Median | Lq–Uq | Median (positive/total) | Lq–Uq | Median | Lq–Uq | ||
| I | 17 | 144.1§ | 128.6–154.8 | 8.4** (12/17) | 7.8–9.0 | 129.75♠ | 73.2–198.7 |
| II | 17 | 258.1§§ | 198.5–296.2 | 5.1* (13/17) | 3.8–6.5 | 50.65 | 45.6–58.4 |
| III | 26 | 289.7§§ | 275.5–301.0 | 6.1 (14/26) | 4.7–9.4 | 60.48 | 58.36–66.8 |
| IV | 20 | 160.0§ | 139.7–213.7 | 9.3 (13/20) | 7.5–11.1 | 62.70 | 32.9–93.4 |
| V | 21 | 162.0§ | 124.1–223.9 | 7.1 (11/21) | 3.4–8.8 | 28.68♥ | 13.7–28.7 |
| VI | 21 | 149.3§ | 139.3–180.4 | 4.0 (14/21) | 3.1–10.7 | 62.61 | 45.6–92.3 |
| VII | 20 | 395.3§§ | 289.3–580.2 | 3.1* (10/20) | 3.1–3.4 | 81.7 | 74.3–94.4 |
symbols denote correspondingly higher and lower values with statistically significant differences (Kruskal–Wallis test at p < 0.035).
symbols denote the values with statistically significant difference from other values in the column (Kruskal–Wallis tests, p < 0.02 and p < 0.01, respectively).
denotes the median and lower and upper quartiles of the values exceeding the limit of detection. In the brackets is the number of the samples with the values exceeding the limit of detection (positive) to the total number of samples.
Table 5.
Comparative characteristics of human placental relative amounts of GSTP1-specific mRNA, glutathione S- transferase (GST) activity and 7-ethoxycoumarin O-deethylase (ECOD) determinations in radioactivity- and chemically-exposed pregnancies.
| Groups | Level of GSTP1-specific mRNA in relative units | GST activity (nmoles mg protein−1 min−1)
|
ECOD activity (pmol mg protein−1 min−1) (M ± SD) | |
|---|---|---|---|---|
| Before DTT treatment (M ± SD) | After DTT treatment (M ± SD) | |||
| I | 0.2 (n = 3) | 125.3 ± 17.4* (n = 10) | 134.1 ± 30.9** (n = 10) | 24.9 ± 3.5** (n = 9) |
| VI | 3.5 (n = 4) | 128.9 ± 20.8* (n = 9) | 152.9 ± 25.5** (n = 9) | 70.3 ± 13.7*** (n = 8) |
| VII | 1.0 (n = 4) | N.D. | N.D. | 9.9 ± 1.9* (n = 10) |
Note: similar symbols denote higher (**, ***) and lower (*) values with statistically significant differences between them. Comparisons were made between GST activity determinations before and after DTT treatment. Statistical significance of ECOD activity was done between the groups using group VII as a reference (t-test, p < 0.05). N.D., not determined.
Treatment of cytosol with reducing agent (Table 5) dithiothreitol (DTT) resulted in no significant changes in GST activity in group I (134.1 ± 30.9 mU mg protein−1 after treatment vs. 125.3 ± 17.4 mU mg protein−1 before DTT treatment; t-test, n = 10, p = 0.2). The same treatment procedure with specimens from group VI increased the GST activity from 128.9 ± 20.8 nmoles mg protein−1 min−1 up to 152.9 ± 25.5 nmoles mg protein−1 min−1 (t-test, n = 9, p = 0.003).
The pattern for placental ECOD activity in these samples exhibited the opposite results (Table 5). ECOD activity in the “clean” region (group VII) was 9.89 ± 1.94 pmol mg protein−1 min−1 (n = 10), while in the specimens from groups I and VI it was higher; nearly 2.5 times higher (24.9 ± 3.5 pmol mg protein−1 min−1, n = 9) in group I and 7 times higher (70.3 ± 13.7 pmol mg protein−1 min−1, n = 8) in group VI. In the specimens from the area with the lower SEEAED and chemical exposure (group IV, Kiev), ECOD activities were at an intermediate level, 39.0 ± 8.3 pmol mg protein−1 min−1 (n = 4).
3.3. PAH–DNA adduct formation
Detectable levels of PAH–DNA adducts were found in more than 50% of all placenta samples and varied from 50% in the “clean” area to 70–75% in groups I and II, and 65% in the chemically-polluted towns (groups IV and VI). The absolute values of PAH–DNA adducts varied from non-detectable (see Section 2.2) to about 24 adducts per 109 nucleotides. The PAH–DNA adduct levels were significantly higher in group I, compared to groups II and VII (Kruskal–Wallis test) (Table 4). Differences between the other groups were not statistically significant.
3.4. TBARS levels
The level of thiobarbituric reactive substances (TBARS) was used as an indicator of lipid peroxidation (Table 4). The highest TBARS values were detected in Group I samples. In groups II, III, IV and VI, which had similar values, the TBARS values were about 50% of the value obtained in groups I and VII, whereas in group V the TBARS value was significantly lower (Kruskal–Wallis tests, p < 0.02 and p < 0.01, Table 4).
3.5. GST activity, maternal age and newborns’ health status: comparative and correlation analysis of the data
The results reveal (Fig. 1, Tables 1–5) that GST activities in human placentas from pregnancies that took place in industrial areas with high chemical exposures, and areas with high radioactivity exposure, were significantly lower than the corresponding enzyme activity in human placentas from women living in small towns with less pollution. The highest placental DNA adducts were found in placentas with the lowest GST activity in placental cytosol (in the whole cohort rs = −0.27, p = 0.05 and even more prominently in groups I–V (rs = −0.47, p = 0.0034).
Fig. 1.
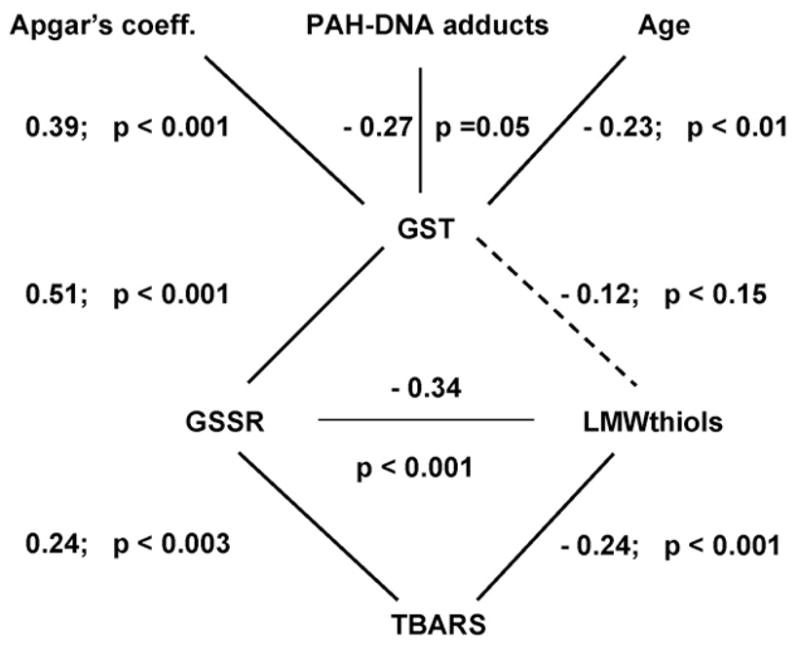
The inter-relation of maternal age, newborn health and placental PAH–DNA adducts with placental cytosolic GST activity and other indices of glutathione and redox status measured in human placenta. The solid lines show statistically significant correlations between biomarkers and clinical indices of fetal integrity and maternal age. The dashed line shows a non-significant relationship between GST and LMWthiols. The numerals nearby each line denote the Spearman’s coefficients with their levels of statistical significance.
Maternal age and parity did have some contribution to the GST activity; there was a negative association of GST activity with maternal age (rs = −0.23, p = 0.011) and parity (rs = −0.33, p = 0.0003, Fig. 1).
It seems that higher GST activity in placenta is more beneficial for the health of the newborn (Fig. 1). This is demonstrated by the moderate positive correlation between GST activity and Apgar’s index (rs = 0.39, p = 0.00003) and by the comparative analysis of GST activity in those samples with and without obstetrical pathology. In the whole cohort (categorized as present/absent pathology) placental GST activity was lower in cases of fetal hypoxia, hypotrophy and stillbirth in anamnesis (Mann–Whitney U-test for each comparison, 0.035 ≤ p ≤ 0.05). The placental TBARS level was statistically significantly lower in the pregnancies in which there was anemia, threatening abortion, toxemia, fetal hypoxia or nephropathy (Mann–Whitney U-test for each type of pathology, 0.0068 ≤ p ≤ 0.0085).
A more detailed comparative analysis was undertaken with samples from groups I and VI. Group I included women who were highly exposed to radioactivity for 5–6 years prior to this pregnancy. These samples contained elevated TBARS and, as assessed in a different study, radionuclides (137Cs, 1.2–1.8 Bq/kg placental weight, Zadorozhna et al., 1993). The higher level of TBARS is a typical indicator of irradiation exposure (Vladimirov and Archakov, 1971) and supports the endogenous irradiation detected in these placentas. The level of GST activity in these samples was low, and similar to that found in samples from the chemically-polluted area (group VI). Levels of PAH–DNA adducts did not differ significantly in samples from groups I and IV, suggesting that ambient air may not be the only source of PAH exposure.
4. Discussion
In the present study, we examined the usefulness of three potential human placental biomarkers (ECOD and GST activities and PHA–DNA adducts) in detecting effects of environmental chemical stress on the newborns’ general health status. Based on our data, overall well-being of newborn seems to be associated with the GSH supply. Moreover, it seems that there is an imbalance between inducible monooxygenase reactions and the production of glutathione.
As reviewed by Hayes and McLellan (1999), glutathione associated metabolism is a major contribution to cellular defense against reactive oxygen species (ROS), since it can inactivate products of oxidant damage to DNA and lipids and monooxygenized xenobiotics. Placental GST detoxifies the noxious compounds crossing the placenta in both directions. Our data demonstrate that the products of incomplete combustion of organic substances (e.g. PAH) in conjunction with the down-regulated placental GST activity promote accumulation of PAH–DNA adducts with a subsequent risk of fetal exposure.
To further elucidate the role of glutathione and redox status on placental GST activity, we have correlated the data presented here to other biochemical end points obtained from the same samples (Obolenskaya et al., 1997). Our data points to inter-relationships between glutathione reductase activity (GSSR, EC 1.8.1.7), reduced low molecular weight thiols (rLMWT), and TBARS activity, and shows a clear association with GST activity. Therefore, GST activity may well be a common denominator in the regulation of glutathione levels and redox status.
The association of other biochemical indices, PAH–DNA adducts and TBARS levels, is less clear. The high TBARS values correlated with the highest exposure to radioactivity (group I), and the lowest TBARS value was found in the group with low radioactivity- and chemically-exposed samples (group V). The lowest level of PAH–DNA adducts was observed in placentas from non-complicated pregnancies in the “clean” area (group VII), while higher DNA adduct levels and maximal TBARS content were seen in placentas from pregnancies with high radioactive exposure. We do not have any clear explanation for this result but one possibility could be the malnutrition-related low content of Fe2+ ions causing anemia and poor oxygen consumption resulting in hypoxia which may depress the peroxidation processes (Vladimirov and Archakov, 1971). Anemia was also recorded in group V.
An important comparison concerns two groups of samples (I and VI), which are similar in terms of GST activity but different in several other characteristics: the type of predominant exposure, presence/absence of radionuclides in placental tissue, the level of lipid peroxidation, ECOD activity, GSTP1 expression and sensitivity to reducing agents. On the basis of the above-mentioned biomarker pattern differences, we propose that different mechanisms may be responsible for the down-regulation of GST activity in each of these exposure situations. The pronounced shift in redox balance (group I) may affect various processes potentially responsible for GSTP1 activity down-regulation. For example, the aryl hydrocarbon receptor (AhR) that binds to the xenobiotic responsive element in the CYP1A1 promoter is extremely sensitive to the redox potential, and when oxidized, its inducing activity becomes impaired (Masten and Shiverick, 1996). This in turn may lead to a less intensive induction of the CYP1A1 gene, and consequently GSTP1, by metabolites of Phase I detoxification. This would provide a partial explanation for the decrease in the relative amount of GSTP1-specific mRNA. Our previous data demonstrate that hypermethylation of the CpG-rich GSTP1 gene promoter, a typical “silencer” of this gene, is not responsible for GST expression/activity down-regulation in any of the investigated samples (Slonchak et al., 2007)). Changes in the expression of other transcription factors regulating GSTP1 transcription may contribute to this down-regulation. As we reported previously, the lower content of rLMWT in the specimens from group I (Obolenskaya et al., 1997) may be an additional factor that decrease the GSTP1 enzymatic activity due to the presence of SH-groups (Cys47) which are extremely sensitive to oxidation (Ricci et al., 1991).
In the chemically-polluted area (group VI), the scenario of GST down-regulation and the accumulation of PAH–DNA adducts may be traced to a different mechanism. The significant increase of CYP1A1 and related ECOD activity, a typical marker induced by PAH, may evoke to an increase in the oxidized end products of the monooxygenases, potential activators of GSTP1 transcription. This putative sequence of events could account for the increase in the relative amount of GSTP1-specific mRNA in representative samples of group VI and the discrepancy between GSTP1 protein detected by Western-blot analysis and GST enzymatic activity (unpublished data). The significant restoration of GST activity after DTT treatment indicates that enzymatic activity in these samples had been partly inhibited. Thus the imbalance between CYP1A1 and GSTP1 enzymatic activities detected in placentas from the area with high PAH pollution in the ambient air resembles that described in the placentas from smoking and drug-abusing mothers (Pasanen and Pelkonen, 1990; Paakki et al., 2000; Huuskonen et al., 2008); maternal cigarette smoking does not enhance the expression of GSTP1 gene (Huuskonen et al., 2008). Elevated ROS production and ECOD activity seem to act in both groups of samples mainly at different levels of the general adaptive antioxidant response pathway, favoring down-regulation of GST activity and genotoxic damage in human placenta. The data suggest that the determination of placental ECOD activity could also serve as a biomarker of oxidative stress, with higher levels indicating greater radioactive and/or chemical exposure during the pregnancy.
The presence of PAH–DNA adducts in human placenta is a surrogate marker of fetal exposure to carcinogenic substances. Several lines of evidence point to fetal susceptibility to carcinogens, and in utero exposure to carcinogens can manifest itself as an increased risk of childhood cancer (Alexander et al., 2001). An analysis undertaken in New York City has revealed a significant association between individual prenatal exposure to the airborne carcinogen B(a)P {0.44 (0.02–6.44) ng/m3 B(a)P in the air} and the frequencies of stable chromosomal aberrations in cord blood (Bocskay et al., 2005). In another study, maternal exposure to ambient air pollution, especially black smoke and sulphur dioxide, was observed to be a risk for anomalies (Rankin et al., 2008). However in these studies the authors did not carry out any biochemical assays with fetoplacental tissues. In our study, the level of airborne PAH, though not measured for each individual, was higher (for details see Table 1) and the association between placental detoxification activity, genotoxic damage and the health status of the newborns was demonstrated. Further investigations will be needed to confirm whether placental PAH–DNA adducts could be used as prognostic biomarkers for later development and well-being of newborns.
In conclusion, this study reveals a complex inter-relationship between GST activity, glutathione redox status and genotoxic damage in human term placenta. Under oxidative stress, the expression of xenobiotic-metabolizing human placental monooxygenases is enhanced as demonstrated by an increase in ECOD activity. However, GSH status does not follow the same pattern and this can potentially result in an imbalance in reactive intermediates generated by phase I monooxygenases but these intermediates cannot be broken down by detoxificating phase II reactions (e.g. GST). This is the first study to demonstrate that an imbalance in detoxification capacity can be measured as increased production of PAH–DNA adducts, changes in lipid peroxidation status and as compromised fetal health. This area merits further investigations, especially since there is a clear need to define which intracellular factors can affect the expression and catalytic activity of glutathione S-transferase as a major component of cellular protection against oxidative stress and environmental carcinogens.
Acknowledgments
We thank the personnel of Third Zaporizhzhia Clinic (Ukraine), Institute of Pediatrics, Obstetrics and Gynecology (Kiev, Ukraine), Gomel District Clinic (Belarus) and of the Jagiellonian University School of Medicine (Krakow, Poland) for supplying placental specimens and the data from medical histories. This work was supported by UICC fellowship (ICRETT 580, 2001 to MYuO) and in part by the intramural research program of the Center for Cancer Research, National Cancer Institute, USA; The Academy of Finland No. 122859/2007 to MP, The Finnish Funding Agency for Technology and Innovation No. 40225/2008 to MP.
Footnotes
Conflict of interest
None declared.
References
- Alexander FE, Patheal SL, Biondi A, et al. Transplacental chemical exposure and risk of infant leukemia with MLL gene fusion. Cancer Res. 2001;61:2541–2546. [PubMed] [Google Scholar]
- Bocskay K, Tang D, Orjuela MA, Liu X, Warburton DP, Perera FP. Chromosomal aberrations in cord blood are associated with prenatal exposure to carcinogenic polycyclic aromatic hydrocarbons. Cancer Epidemiol Biomarkers Prev. 2005;142:506–511. doi: 10.1158/1055-9965.EPI-04-0566. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Council of Ministers of Ukraine Republic. Data from Hydrometeoservice of Ukraine. 2003. The list of settlements and the objects in the radioactively contaminated territories. Minsk 2003. [Google Scholar]
- Divi RL, Beland FA, Fu PP, Von Tungeln LS, Schoket B, Camara JE, Ghei M, Rothman N, Sinha R, Poirier MC. Highly sensitive chemiluminescence immunoassay for benzo[a]pyrene-DNA adducts: validation by comparison with other methods, and use in human biomonitoring. Carcinogenesis. 2002;23:2043–2049. doi: 10.1093/carcin/23.12.2043. [DOI] [PubMed] [Google Scholar]
- Gavrilov VB, Gavrilova AP, Mazhul LM. Comparative analysis of tests for lipid peroxidation products in serum with the help of thiobarbituric acid. Vopr Med Chim. 1987;33:118–122. (in Russian) [PubMed] [Google Scholar]
- Greenlee WF, Poland A. An improved assay of 7-ethoxycoumarin O-deethylase activity: induction of hepatic enzyme activity in C57BL/6J and DBA/2J mice by phenobarbital, 3-methylcholanthrene and 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Pharm Exp Ther. 1978;205:596–605. [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Lang MA. Mouse liver phenobarbital-inducible P450 system: purification, characterization and differential inducibility of four cytochrome P450 isozymes from D2 mouse. Arch Biochem Biophys. 1989;273:42–57. doi: 10.1016/0003-9861(89)90160-4. [DOI] [PubMed] [Google Scholar]
- Huuskonen P, Storvik M, Reinisalo M, Honkakoski P, Rysä J, Hakkola J, Pasanen M. Microarray analysis of the global alterations in the gene expression in the placentas from tobacco-smoking mothers. Clin Parmacol Ther. 2008;83:542–550. doi: 10.1038/sj.clpt.6100376. [DOI] [PubMed] [Google Scholar]
- Knapen MFCM, Zusterzeel PLM, Peters WHM, Steegers EAP. Glutathione and glutathione-related enzymes in reproduction. Eur J Obstet Gynecol Reprod Biol. 1999;82:171–184. doi: 10.1016/s0301-2115(98)00242-5. [DOI] [PubMed] [Google Scholar]
- Lutz WK. In vivo covalent binding of organic chemicals to DNA as a quantitative indicator in the process of chemical carcinogenesis. Mutat Res. 1979;65:289–356. doi: 10.1016/0165-1110(79)90006-x. [DOI] [PubMed] [Google Scholar]
- Masten SA, Shiverick KT. Characterization of the aryl hydrocarbon receptor complex in human B lymphocytes: evidence for a distinct nuclear DNA-binding form. Arch Biochem Biophys. 1996;336:297–308. doi: 10.1006/abbi.1996.0561. [DOI] [PubMed] [Google Scholar]
- Ministry of Health of Ukraine. Dosimetric Passportisation of the Settlements of Ukraine Exposed to Radioactive Contamination in Result of Chernobyl Accident. 1993. p. 178. (in Ukrainian) [Google Scholar]
- Myllynen P, Pasanen M, Pelkonen O. Human placenta: a human organ for developmental toxicology research and biomonitoring. Placenta. 2005;26:361–371. doi: 10.1016/j.placenta.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Myllynen P, Pasanen M, Vähäkangas K. The fate and effects of xenobiotics in human placenta. Expert Opin Drug Metab Toxicol. 2007;3:331–346. doi: 10.1517/17425255.3.3.331. [DOI] [PubMed] [Google Scholar]
- Obolenskaya M Yu, Tschaikovskaya TL, Lebedeva LM, Macewicz LL, Didenko LV, Decker K. Glutathione status of placentae from differently polluted regions of Ukraine. Eur J Obstet Gynecol Reprod Biol. 1997;71:23–30. doi: 10.1016/s0301-2115(96)02611-5. [DOI] [PubMed] [Google Scholar]
- Paakki P, Stockmann H, Kantola M, Wagner P, Lauper U, Huch R, Elovaara E, Kirkinen P, Pasanen M. The effect of drug abuse on term human placental xenobiotic and steroid metabolising enzymes in vitro. Environ Health Perspect. 2000;108:141–146. doi: 10.1289/ehp.00108141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasanen M, Pelkonen O. Xenobiotic and steroid-metabolizing monooxygenases catalyzed by cytochrome P450 and glutathione S-transferase conjugations in the human placenta and their relationships to maternal cigarette smoking. Placenta. 1990;11:75–85. doi: 10.1016/s0143-4004(05)80445-x. [DOI] [PubMed] [Google Scholar]
- Rankin J, Chadwick T, Natarajan M, Howel D, Pearce MS, Pless-Mulloli T. Maternal exposure to ambient air pollutants and risk of congenital anomalies. Environ Res. 2008;109:181–187. doi: 10.1016/j.envres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Yu Rebrova O. Application of Statistica Software. MediaSphera; Moscow: 2003. Statistical Analysis of Medical Data; p. 312. (in Russian) [Google Scholar]
- Ricci G, Del Boccio G, Pennelli A, Lo Bello M, Petruzelli R, Caccuri AM, Barra D, Federici G. Redox forms of human placenta glutathione transferase. J Biol Chem. 1991;266:21409–21415. [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T, editors. Molecular Cloning: A Laboratory Manual. 2. Cold Spring Harbor Laboratory Press; 1989. Section V1. [Google Scholar]
- Slonchak A, Martseniuk O, Rzeszowska-Wolny J, Widlak P, Obolenska M. Some aspects of glutathione S-transferase P1-1 gene transcription regulation in human placenta. Ukr Biochem J. 2007:79–88. [PubMed] [Google Scholar]
- Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43:487–514. doi: 10.2165/00003088-200443080-00001. [DOI] [PubMed] [Google Scholar]
- Yu Vladimirov A, Archakov AI. Lipid Peroxidation in Biological Membranes. Nauka; Moscow: 1971. p. 252. (in Russian) [Google Scholar]
- Zadorozhna TD, Lukianova OM, Chenzhow D, Antipkiin YG, Didienko LV, Yetschenko O, Yakovlev OO, Bomko OI, Zhuravel AO. Morphological changes in placenta and the children’s health under the influence of low dose irradiation. J Pediatr Obstet Gynecol. 1993;2:8–11. (in Ukrainian) [Google Scholar]


