Abstract
A variety of adenosine analogs activate phosphoinositide breakdown in a rat RBL-2H3 mast cell line. It is presumed that an A3-adenosine receptor is involved, since the phosphoinositide response is insensitive to xanthines. However, the very potent A3- receptor agonist 2-chloro-N6-iodobenzyl-N-methylcarboxamidoadenosine (2-CI-IBMECA) with an EC50 of 4.1 µM is about twofold less potent (and less efficacious) than N-ethylcarboxamidoadenosine (NECA) with an EC50 of 2.1 µM. The other agents consisting of N6-p-aminophenylethyladenosine (APNEA), N6-iodobenzylMECA (IB-MECA), N6-R- phenylisopropyladenosine (R-PIA), 2-chloroadenosine, N6-benzyladenosine, N6- cyclohexyladenosine (CHA), N6-cyclohexylNECA (CHNECA), 2-(p- carboxyethylphenyl-ethylaminoNECA (CGS 21680), 1,3-dibutylxanthine 7-riboside-5′-N-methylcarboxamide (DBXRM), adenosine, and 8-bromoadenosine are all nearly equipotent with EC50 values of 5.5-13.9 µM. The rank order of potencies of the analogs in causing an elevation of intracellular calcium is quite different. The potent A3 receptor agonists 2-CI-IBMECA and IB-MECA with EC50 values of 0.07 and 0.11 µM, respectively, are about fourfold more potent than N6-cyclohexylNECA and about 15-fold more potent than NECA. The other analogs are comparable or somewhat less potent than NECA, some are less efficacious, and 8-bromoadenosine is inactive. The results suggest that stimulation of phosphoinositide breakdown by adenosine analogs in RBL-2H3 cells as measured by IP1 accumulation is not predictive of IP3-mediated elevations of intracellular calcium. Rank order of potency for the calcium response is consonant with intermediacy of A3-adenosine receptors, while the former, as measured by [3H]IP1-formation, probably reflects contributions from both an A3-mediated response and some other mechanism. Combinations of subthreshold concentrations of 2-CI-IBMECA with either the A1-selective agonist CHA or the A2A-selective agonist CGS 21680 caused a marked stimulation of phosphoinositide breakdown, providing further evidence for dual mechanisms. The selective A3-adenosine receptor antagonist 3,6-dichloro-2′-(isopropyloxy)-4′-methylflavone (MRS 1067) inhibits 2-CI-IBMECA- and NECA-elicited elevation of calcium levels, and had differential effects on phosphoinositide breakdown, blocking [3H]IP3 accumulation and either blocking (NECA) or having no effect (2-CI-IBMECA) on [3H]IP1 accumulation.
Keywords: adenosine receptors, phosphoinositides, calcium, xanthines
INTRODUCTION
Adenosine receptors are present on mast cells and appear to modulate secretory responses involved in inflammatory reactions [Marquardt, 1988; Beaven et al., 1994]. The effect of adenosine analogs is dependent on the type of cell, but for serosal basophils and for the rat mast cell line RBL-2H3, adenosine and adenosine analogs appear to facilitate antigen-mediated activation. Mast cells from mice have been shown to express A2A- and A2B-adenosine receptors [Marquardt et al., 1994]. NECA, a nonselective adenosine agonist, enhances immunologically stimulated release of hexosaminidase in these cells, but the A2A-selective agonist CGS 21680 does not. Activation of cyclic AMP-dependent protein kinases is not essential to responses in these cells [Marquardt and Walker, 1994]. Instead, protein kinase C (PKC) activation appears to be involved [Marquardt and Walker, 1989]. Thus, in these cells either an A1 and/or an A3-adenosine receptor, stimulatory to phosphoinositide breakdown, would be expected to be involved in the response. NECA also has been shown to activate the rat mast cell line RBL-2H3, leading to increases in phosphoinositide breakdown and intracellular calcium levels, and an enhancement of immunologically activated secretion of serotonin, hexosaminidase, and other allergic mediators [Ali et al., 1990; Ramkumar et al., 1993]. Binding studies have demonstrated the presence of A1-, A2A- and A3-adenosine receptors in RBL-2H3 cells [Ji et al., 1994], although DNA hybridization in another study detected only A3-receptors [Ramkumar et al., 1993]. Based on the lack of antagonism by xanthines, the A3-adenosine receptor, which in rats is relatively insensitive to xanthines, was proposed to be involved in responses to NECA in RBL-2H3 cells [Ramkumar et al., 1993]. A variety of potent and selective agonists for A3-adenosine receptors have been developed [Gallo-Rodriquez et al., 1994; Van Galen et al., 1994]. A series of adenosine analogs have now been probed for effects on phosphoinositide breakdown and intracellular calcium in RBL-2H3 cells and rank order of potencies compared to binding affinities for a rat brain A3-adenosine receptor. A recently developed selective A3-adenosine receptor antagonist, 3,6-dichloro-2′-(isopropyloxy)-4′- methylflavone (MRS 1067) [Karton et al., 1996], was also investigated.
MATERIALS AND METHODS
The adenosine agonists and the xanthine agonist DBXRM were prepared as described [Gallo-Rodriguez et al., 1994; Van Galen et al., 1994, Kim et al., 1994a, b] or were obtained from standard commerical sources. MRS 1067 was prepared as described [Karton et al., 1996]. Dihydrosphingosine, IgE and DNPHSA were provided by Dr. Oksoon Choi (NIH, Bethesda, MD). Other compounds were from standard commercial sources.
Phosphoinositide Breakdown
The RBL-2H3 cells were grown in Eagle’s Minimum Essential Medium with 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 mg/ml). Cells were plated in 12-well plates (5 × 105 cells/well) in the presence of 10 mCi/ml myo-[3H]inositol. In some experiments, 0.5 µg/ml of DNP-specific IgE was included in the media. In others it was not. The following day, the medium was aspirated and cells were washed twice with incubation buffer (108 mM NaC1, 4.7 mM KCl, 10 mM LiCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 0.5 mM EDTA, 10 mM glucose, 20 mM Hepes buffer, pH 7.4). After washing, cells that have been primed by exposure to DNP-specific IGE were preincubated with the antigen DNP-HSA (20 ng/ml) for 10 min at 37°C in incubation buffer containing 3 mM CaCl2. Cells that had not been exposed to DNP-specific IGE were also incubated after washing for 10 min, but without the antigen DNP-HSA. Agents were then added. Incubations were continued for either 1 or 15 min at 37°C in a final volume of 0.5 ml and were stopped by the addition of 12% trichloroacetic acid (TCA). Cell homogenates were scraped and transferred to microfuge tubes for centrifugation at 12,000g for 5 min. Anion-exchange chromatography was performed to elute [3H]inositol phosphates from the supernatants as described [Berridge et al., 1983]. Results are expressed as counts per minute of [3H]inositol phosphate (IP1, IP2 or IP3) per 10,000 cpm of [3H]inositol-labeled membrane lipids.
Intracellular Calcium levels
The RBL-2H3 cells grown in Eagle's Minimum Essential Medium were treated with 0.05% trypsin and 0.53 mM EDTA for 3 min and suspended (106 cells/ml media). Some cells were incubated with 0.5 µg/ml DNP-specific IgE at 37°C for overnight. Cells were then washed and resuspended in Hepes buffer (pH 7.4) containing 2.5 mM probenecid (NaCl, 135 mM; KCl, 5 mM; CaCl2, 1 mM; MgCl2, 1 mM; glucose, 5.6 mM; Hepes, 10 mM; and bovine serum albumin, 0.1%). A stock solution of probenecid (50 mM, pH 11, aq. NaOH) was utilized. Cells were loaded with 2 µM fura 2-acetoxymethyl ester at 37°C for 45 min. Fluorescence changes were then measured in 2-ml samples of continuously stirred cell suspension in polystyrene cuvettes at 37°C in a Deltascan spectrofluorimeter (Photon Technology International, South Brunswick, NJ) with excitation at 340 and 380 nm (emission at 510 nM). [Ca2+]i was calculated from the ratio as described [Grynkiewicz et al., 1985].
RESULTS
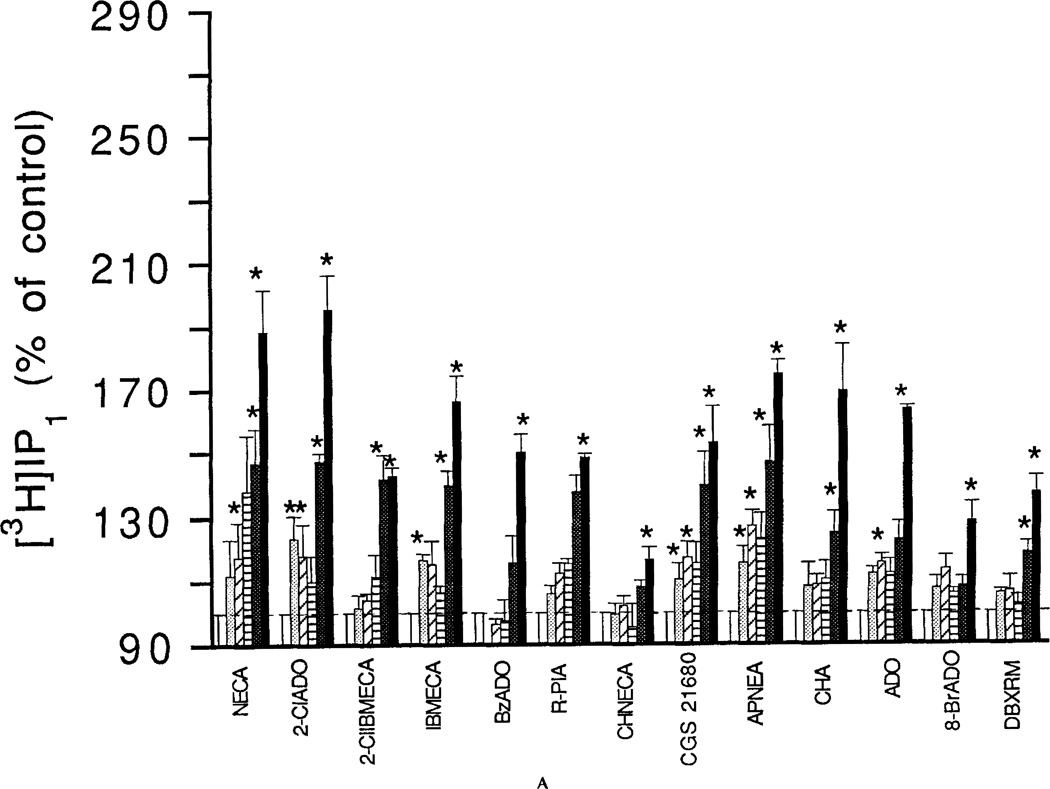
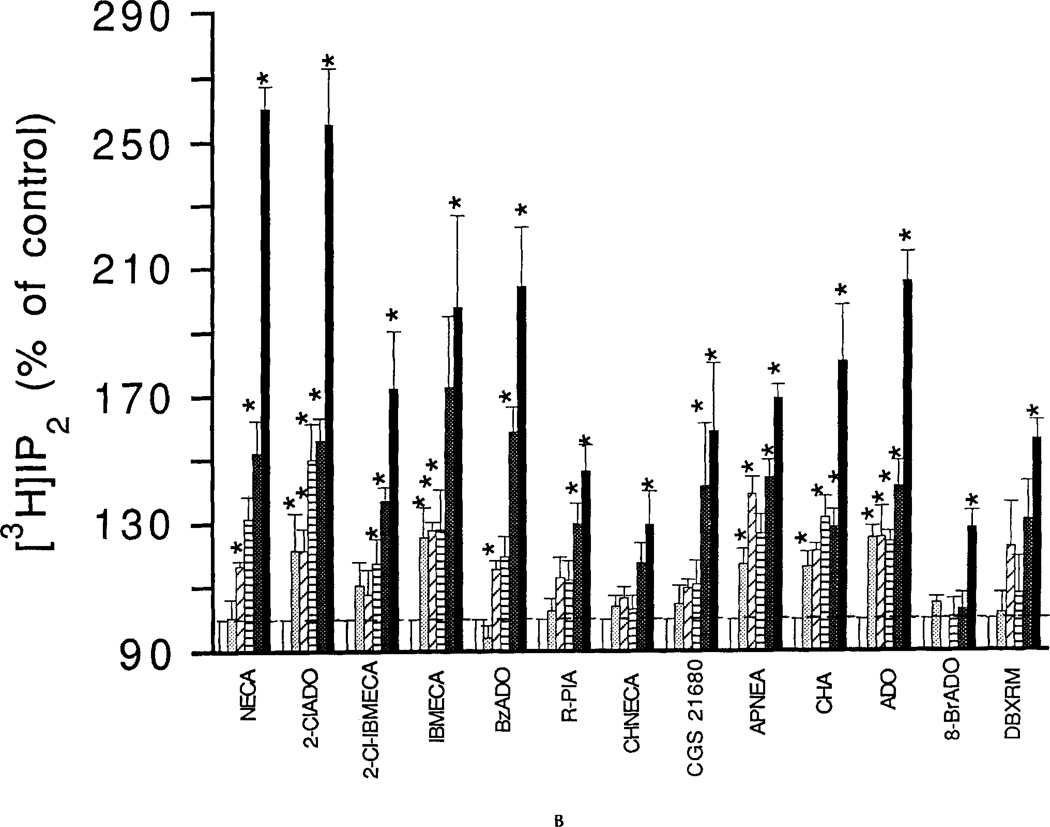
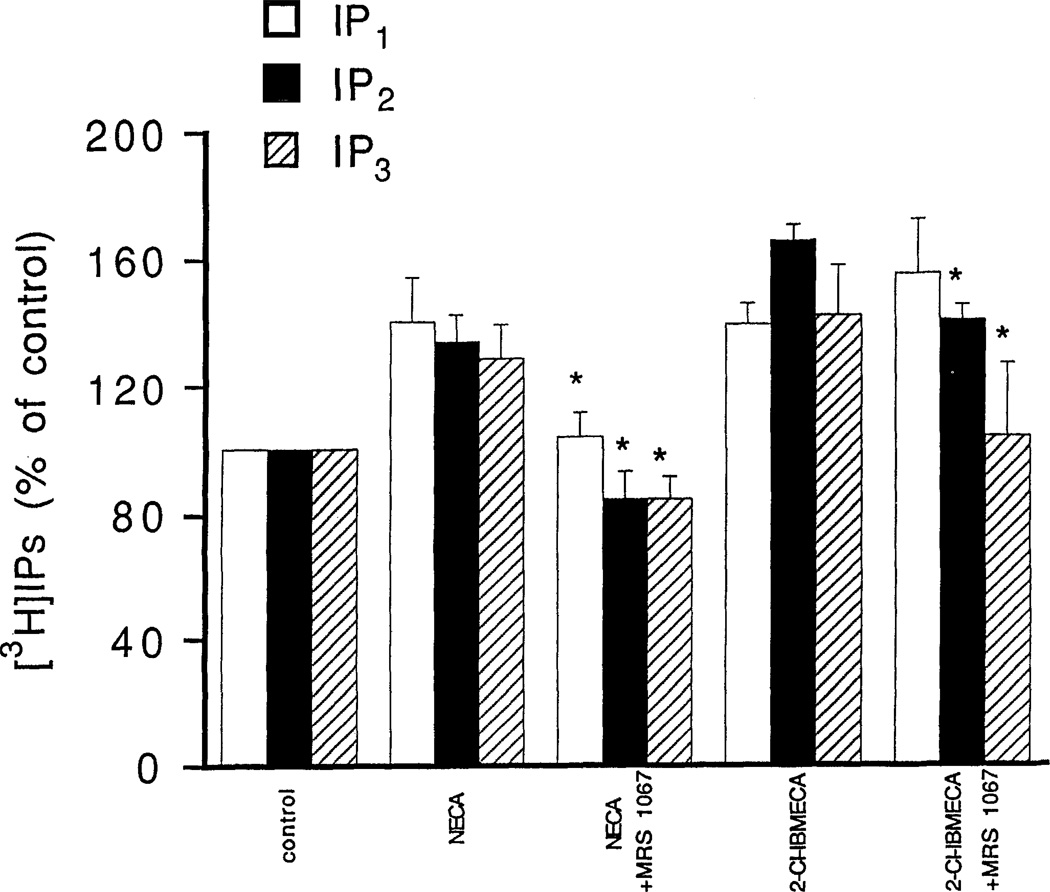
The effects of adenosine analogs on phosphoinositide breakdown in RBL-2H3 mast cells were investigated both with and without a pretreatment with a DNP-specific IgE. Dose-response relationships for adenosine analog-elicited accumulation of [3H]IP1 and [3H]IP2 are shown for 1-min incubations with cells pretreated with the DNP-specific IgE and challenged with DNP-HSA (Fig. 1) and for 15-min incubations with cells not pretreated with the DNP-specific IgE (Fig. 2). Pretreatment with DNP-specific IgE followed by exposure to the antigen DNP-HSA increased phosphoinositide breakdown, but did not markedly affect the percentage stimulation by adenosine analogs (data not shown). The EC50 values for the adenosine analogs and for the xanthine DBXRM are given in Table 1. The mono- and bis-phosphates showed similar dose-response relationships for most agonists. Levels of the trisphosphate [3H]IP3 were much lower and even for the 1-min incubations, there was only a maximal 20–30% increase elicited by any of the adenosine analogs (data not shown; see Fig. 3). EC50 values could not be reliably determined. Accumulations of [3H]IP1 elicited by NECA or 10 µM 2-CI-IBMECA were not antagonized by the A1/A2 antagonists XAC (5 µM) or 8-p-sulfophenyltheophylline (50 µM) (data not shown). The specific A3 antagonist MRS 1067 at 30 µM caused complete inhibition of the accumulations of [3H]IP1, [3H]IP2, and [3H]IP3 elicited by 10 µM NECA (Fig. 3). By contrast, MRS 1067 had no effect on accumulations of [3H]IP1 elicited by 10 µM 2Cl-IBMECA but did partially inhibit accumulation of [3H]IP2 and completely blocked the accumulation of [3H]IP3.
Figure 1.
Concentration-dependent stimulation of phosphoinositide breakdown by adenosine analogs in RBL-2H3 mast cells. A: [3H]IP1. B: [3H]IP2. Accumulation of [3H]inositol phosphates was measured as described in Methods in the absence or presence of adenosine analogs. Analogs were as follows: NECA, 2-chloroadenosine (2CIADO); 2Cl-IBMECA; N6-3-iodobenzyl-MECA (IBMECA); N6-benzyladenosine (BzADO); N6-R-phenylisopropyladenosine (R-PIA); N6-cyclohexylNECA (CHNECA); CGS 21680; APNEA; N6-cyclohexyladenosine (CHA); adenosine (ADO); 8-bromoadenosine (8-BrADO); 1,3-dibutylxanthine 7-riboside-5′-N-methylcarboxamide (DBXRM). The analogs were tested in the concentration range of 0.3 to 30 µM (open bars control; dotted bars 0.3 µM; hatched bar 1 µM; striped bar 3 µM; heavy dotted bar 10 µM; solid bar 30 µM). Incubations were for 1 min with cells pretreated with the DNP-specific IGE and then challenged with the antigen DNP-HSA. Basal accumulations of [3H]IP1 and [3H]IP2 for the 1-min incubations were 258 ± 21 and 143 ± 10 cpm/10,000 cpm membrane lipids, respectively. Values are means ± s.e.m. (n = 3). *p <0.05.
Figure 2.
Concentration-dependent stimulation of phosphoinositide breakdown by adenosine analogs in RBL-2H3 mast cells. Accumulation of [3H]IP1 was measured as described in Methods in absence or presence of the following adenosine analogs; NECA; IBMECA; 2-CI-IBMECA; N6-benzyladenosine (BzADO). Incubations were for 15 min with cells that were not pretreated with the DNP-specific IgE. Basal accumulation of [3H]IP1 for the 15-min incubations was 326 ± 33 cpm/10,000 cpm membrane lipids. Values are means ± s.e.m. *p < 0.05.
TABLE 1.
Adenosine Analogs: Affinity for Rat Brain A3-Adenosine Receptors and EC50 Values for Stimulation of [3H]IP1 Formation and Elevation of Intracellular Calcium in a Rat RBL-2H3 Mast Cell Line
| RBL-2H3 cells |
||||
|---|---|---|---|---|
| Rat brain | Stimulation | Elevation of [Ca2+]i | ||
| Analoga | A3-receptors Ki (nM)b |
[3H]IP1 EC50c (µM) |
EC50d (µM) |
Relative efficacye |
| 2-CI-IBMECA | 0.33 | 4.1 ± 1 | 0.07 ± 0.04 | 60 |
| IBMECA | 1.1 | 8.5 ± 1.7 | 0.11 ± 0.00 | 55 |
| CHNECA | 16 | 12.2 ± 2.1 | 0.4 | 54 |
| NECA | 113 | 2.1 ± 0.3 | 1.37 ± 0.18 | 100 |
| APNEA | 116 | 7.5 ± 1.5 | 0.9 | 38 |
| BZADO | 120 | 12.7 ± 2.6 | 2.7 | 60 |
| R-PIA | 158 | 5.5 ± 0.9 | 2.4 | 38 |
| CHA | 167 | 11.6 ± 1.1 | 0.88 ± 0.00 | 30 |
| CGS 21680 | 584 | 5.8 ± 0.6 | 0.79 ± 0.02 | 28 |
| ADO | ~1,000 | 11.8 ± 1.1 | 1.45 ± 0.25 | 72 |
| 2-CIADO | 1890 | 8.1 ± 1.5 | 1.7 | 25 |
| 8-BrADO | >100,000 | 13.9 ± 1.0 | Inactive | |
Abbreviations in legend to Fig. 1.
Data from Gallo-Rodriguez et al. (1994), Van Galen et al. (1994), and Jacobson et al. (1995).
EC50 values determined from data of Fig. 1A.
EC50 values determined from data such as that presented in Fig. 4. Values are means ± s.e.m. (n = 3) or are from single determinations.
Efficacies relative to NECA set equal to 100 estimated from data such as those presented in Fig. 4.
Figure 3.
Inhibition by MRS 1067 of phosphoinositide breakdown elicited by NECA and 2-CI-IBMECA in RBL-2H3 mast cells. Accumulation of [3H]inositol phosphates was measured as described in METHODS with NECA (10 µM) or 2-CI-IBMECA (10 µM) either alone or with MRS 1067 (30 µM). Incubations were for 1 min in cells pretreated overnight with DNP-specific IGE and then challenged with the antigen DNP-HSA. Values are means ± s.e.m. (n = 3). *p <0.05.
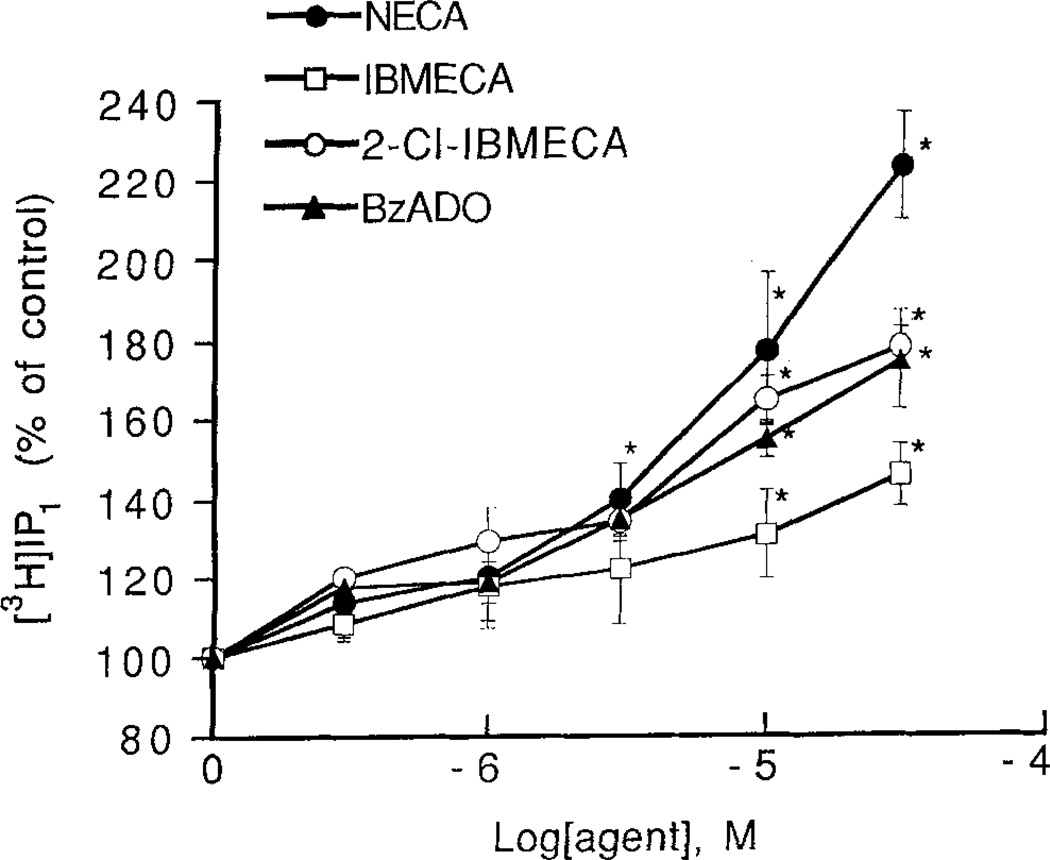
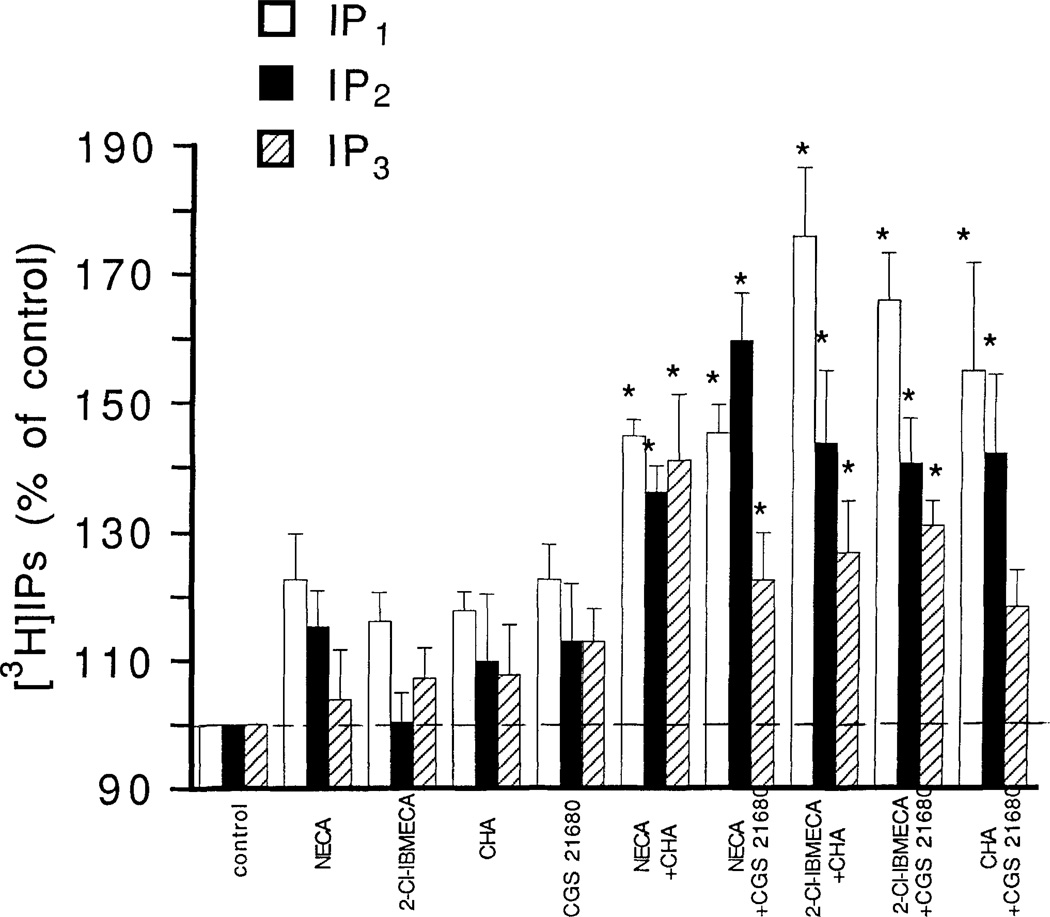
Combinations of subthreshold concentrations of 2-CI-IBMECA, CHA and CGS 21680 were investigated with respect to phosphoinositide breakdown. A combination of 1 µM 2-CI-IBMECA with either 1 µM CHA or 1 µM CGS 21680 caused a significant accumulation of [3H]IP1 (Fig. 4). At a concentration of 1 µM these agents had marginal but not significant effects on accumulation of [3H]IP1. The responses to the combinations were not blocked by the potent A1/A2 antagonist XAC (5 µM) (data not shown). Combinations of a subthreshold 1 µM concentration of NECA with either or CGS 21680 also caused a significant accumulation of [3H]IP3 (Fig. 4).
Figure 4.
Effect of combinations of adenosine analogs on phosphoinositide breakdown in RBL-2H3 mast cells. Accumulation of [3H]inositol phosphates was measured as described in Methods with the following adenosine analogs, either alone or in combination: NECA, 1 µM, 2-CI-IBMECA, 1 µM; CHA, 1 µM; CGS 21680, 1 µM. Incubations were for 1 min in cells pretreated overnight with DNP-specific IgE and then challenged with the antigen DNP-HSA. Values are means ± s.e.m. (n = 3). *p <0.05.
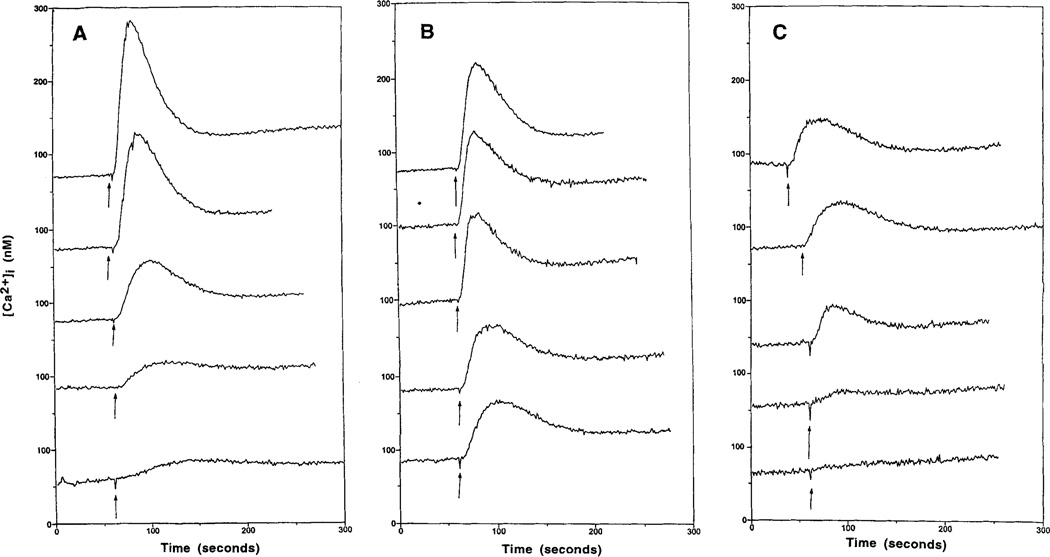
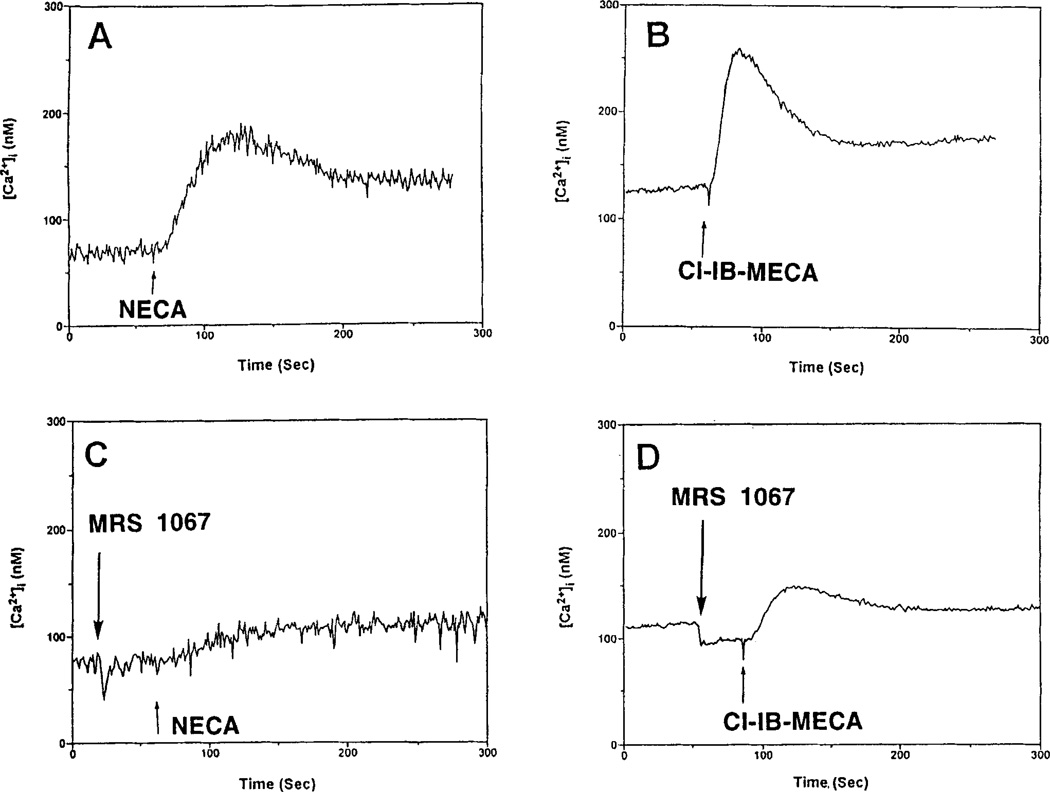
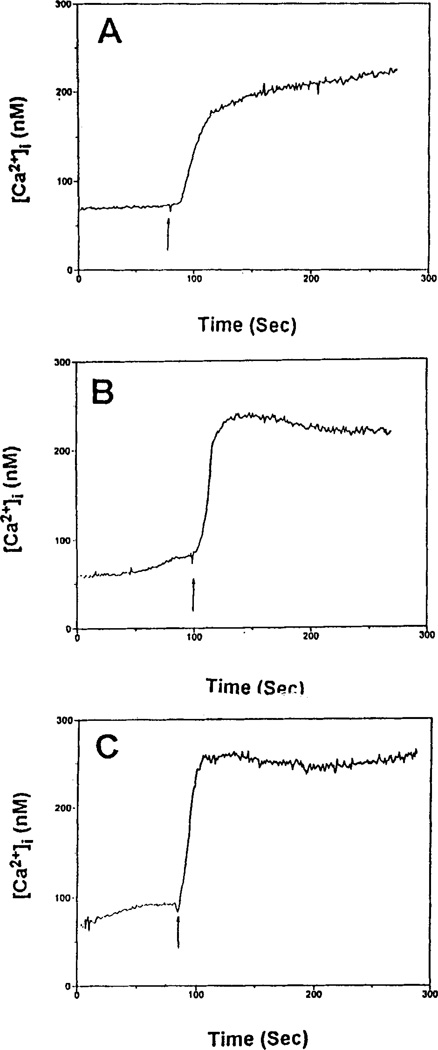
Either 2-CI-IBMECA or NECA caused an increase in [Ca2+]i, in a concentration-dependent manner in RBL-2H3 cells (Fig. 5A,B). 2-CI-IBMECA (EC50 0.07 µM) was much more potent than NECA (EC50 1.37 µM), but was less efficacious. All nucleoside analogs, except 8-bromoadenosine, which was inactive, caused a concentration-dependent increase in [Ca2+]i (data not shown). 2-CI-IBMECA and IBMECA were the most potent. Certain analogs appeared to be significantly less efficacious than either 2-CI-IBMECA or IBMECA. The xanthine DBXRM also caused a concentration-dependent increase in [Ca2+]i but appeared less efficacious than 2-CI-IBMECA (Fig. 5C). The EC50 values are presented in Table 1, as are estimated efficacies relative to NECA. The [Ca2+]i responses to NECA and 2-CI-IBMECA were inhibited by MRS 1067 (Fig. 6). The antigen DNP-HSA alone caused an increase in [Ca2+]i, which was augmented by NECA or 2-CI-IBMECA (Fig. 7).
Figure 5.
Concentration-dependent effects of NECA (A), 2-CI-IBMECA (B), and the xanthine DBXRM (C) on intracellular calcium levels in RBL-2H3 mast cells. Cells were prelabeled with fura-2 as described in Methods. The adenosine analogs or xanthine (0.1, 0.3, 1, 3, 10 µM, from lower to upper trace) were added as indicated (arrows).
Figure 6.
Elevation of intracellular calcium levels by NECA and 2-CI-IBMECA in RBL-2H3 mast cells. Effects of the selective A3- adenosine receptor antagonist MRS 1067. Cells were prelabeled with fura-2 as described in Methods. Additions (arrows) were as follows: A: NECA, 1 µM; B: 2-CI-IBMECA, 1 µM; C: NECA plus MRS 1067, 30 µM. D. 2-CI-IBMECA plus MRS 1067, 30 µM.
Figure 7.
Effect of NECA and 2-CI-IBMECA on the presence of DNP-HSA on intracellular calcium levels in RBL-2H3 mast cells. Cells were prelabeled with fura-2 as described in Methods. Additions (arrows) were as follows: A: DNP-HSA, 20 ng/ml; B: NECA (1 µM) plus DNP-HSA; C: 2-CI-IBMECA (1 µM) plus DNP-HSA.
DISCUSSION
The present study confirms that a xanthine-insensitive adenosine receptor or receptors mediate adenosine analog-stimulated phosphoinositide breakdown in RBL-2H3 mast cells (Figs. 1, 2). A variety of structural modifications of adenosine were examined in an effort to characterize the receptor subtype responsible for the response. Adenosine derivatives (Table 1) selective for A1 (R-PIA, CHA), A2A (CGS 21680), or A3 receptors (2-CI-IBMECA, IBMECA) were utilized, in addition to agents of relatively low selectivity (2-chloroadenosine, N6-benzyladenosine). 8-Bromoadenosine is an adenosine derivative that only weakly interacts with adenosine receptors, with Ki values in binding assays within the range of 10–100 µM for A1, A2A and A3-receptors [Van Galen et al., 1994]. DBXRM, a xanthine derivative, has been shown to be a selective and full agonist at a cloned rat brain A3-adenosine receptor [Jacobson et al., 1995], a remarkable activity for a non-adenosine derivative. All these compounds, and adenosine itself, which has been estimated to have a Ki value of roughly 1 µM at the rat A3 receptor [Jacobson et al., 1995], were shown to activate phosphoinositide breakdown, as measured by [3H]IP1 and [3H]IP2 accumulation (Figs. 1, 2) in RBL-2H3 cells with EC50 values within the range of 2–14 µM (Table 1). Thus, analogs much more potent than NECA in binding studies at cloned rat brain A3-adenosine receptors (Table 1) were actually less potent than NECA in stimulating phosphoinositide breakdown in these cells. Clearly, stimulation of phosphoinositide breakdown by adenosine analogs in RBL-2H3 cells, as measured by accumulation of [3H]IP1 or [3H]IP2, shows no correlation with binding data for a rat brain A3-adenosine receptor. In earlier studies on functional responses in RBL-2H3 mast cells, R-PIA and APNEA were twofold more potent than NECA (EC50 = 56 nM) and ninefold more potent than N6-S-phenylisopropyladenosine in enhancing hexosaminidase release [Ramkumar et al., 1993]. However, with respect to enhancing release of serotonin, NECA (EC50 ~1 µM) was more potent and more efficacious than R-PIA [Ali et al., 1990]. Adenosine itself was intermediate in potency and efficacy. In the present study, it was expected that 2-CI-IBMECA would be much more potent than NECA, based on affinities in binding studies at A3-adenosine receptors. Thresholds for both analogs were, however, in the low micromolar range (Figs. l, 2). One possible explanation is that multiple pathways for enhancement of phosphoinositide breakdown by adenosine analogs exist in RBL-2H3 cells and that the dose-response curves, based on accumulations of IP1 and IP2, represent a composite of a requisite high affinity A3-component and another component. Another possibility is that efficacy plays a major role in these functional studies, while, of course, having no role in binding studies. A third possibility is that the apparent A3-adenosine receptor of the RBL-2H3 cells is not identical with the cloned rat brain A3-adenosine receptor.
The RBL-2H3 mast cells do contain A1 and A2A receptors, in addition to the A3 receptors [Ji et al., 1994]. However, highly selective A1- (CHA) or A2A- (CGS 21680) agonists had effects on phosphoinositide only at concentrations (Fig. l), far greater than those required for such selective agonists to activate either A1 or A2A receptors. At concentrations of only 1 µM, CHA should selectively and fully activate A1-adenosine receptors and CGS 21680 at 1 µM should selectively and fully activate A2A-adenosine receptors. When the highly selective A3 agonist 2-CI-IBMECA at a subthreshold dose of 1 µM was combined with 1 µM CHA or 1 µM CGS 21680, a marked accumulation of [3H]IPl was observed (Fig. 4). Alone, 2-CI-IBMECA at 1 µM did not cause a significant accumulation of [3H]IP1. The results suggested that activation of either A1- or A2A-receptors could augment the A3- response to 2-CI-IBMECA. However, the same results were obtained when the nonselective A1/A2/A3 agonist NECA was combined with CHA or CGS 21680 (Fig. 4). NECA at 1 µM would, of course, fully activate A1 and A2A-adenosine receptors and would at least partially activate A2B-adenosine receptors. In addition, the synergistic responses to the combinations of agonists were not blocked by XAC at a concentration at which this antagonist should block A1-, A2A- and A2B-adenosine receptor-mediated responses. Thus, it would appear that the apparent potentiation of phosphoinositide breakdown seen with combinations of 2-CI-IBMECA and the selective A1- and A2A adenosine agonists does not involve A1 or A2A-receptors. There remains a possibility that a low-affinity A2B-receptor might be involved, but the lack of blockade of the synergistic response by XAC and the inactivity of CI-IBMECA at A2B receptors (A. IJzerman, unpublished) rule against such an interpretation. It should be stressed that measurement of [3H]IP3 formation might provide a better assessment of receptor-mediated phosphoinositide breakdown than measurement of [3H]IP1 accumulation. But [3H]IP3 formation at both early and late time points was too low for any reliable estimate of threshold or potency of the adenosine analogs. The nature of other pathways or receptors involved in [3H]IP1 accumulation in RBL-2H3 cells is unknown. Adenosine analogs do stimulate formation of [3H]inositol phosphates in rat striatal and hippocampal brain slices with a rank order of potency that correlated to the rank order of affinities in cloned rat A3- receptor binding assays and potency in cloned rat A3-receptor-mediated inhibition of adenylyl cyclase [Abbracchio et al., 1995].
An alternate indirect assay of IP3 formation would be to assess the elevation of intracellular calcium elicited by adenosine analogs, since such elevations should be the result of IP3-elicited release of calcium from an intracellular pool. Adenosine analogs did increase [Ca2+]i in a concentration-dependent manner (Fig. 5). Because of variability in different batches of fura-2-labeled RBL-2H3 cells, precise EC50 values were difficult to determine. The EC50 values and efficacy for the adenosine analogs and DBXRM are presented in Table 1, along with the EC50 values for phosphoinositide breakdown and previously reported binding affinities (Ki) at cloned rat brain A3 receptors. Unlike the case of phosphoinositide breakdown, there is a relatively good correlation between the relative potencies in elevating [Ca2+]i and relative affinities in binding at A3-adenosine receptors (Table 1). Clearly, the 2-CI-IBMECA and IBMECA are most potent in both assays. Thus, it appears likely that increases in [Ca2+]i by adenosine analogs in the rat RBL-2H3 mast cell line are mediated by an A3-adenosine receptor similar in structure–activity relationships to a cloned rat brain A3-adenosine receptor. In contrast, the stimulation of phosphoinositide breakdown in RBL-2H3 cells, as assessed by accumulation of [3H]IP1 shows no such correlation, suggesting that multiple pathways and/or receptors may be involved. It should be noted that the thresholds and EC50 values for stimulation of phosphoinositide breakdown as assessed by accumulation of [3H]IP3 or [3H]IP3 (Figs. 1, 2; Table 1) are much higher than the thresholds and EC50 values for elevation of [Ca2+]i (Table l), the latter probably a better measure of extent of breakdown of polyphosphoinositides and IP3 formation. An alternate pathway for calcium mobilization in RBL-2H3 cells, involving sphingosine kinase has recently been reported [Choi et al., 1996]. However, it appears not to be involved in the NECA-response, since dihydrosphingosine, an inhibitor of the kinase, had no effect at 25 µM on the NECA-response (data not shown). The apparent low efficacy of many adenosine analogs and the xanthine DBXRM in elevating calcium is in contrast to full agonist activity of such analogs and of DBXRM with a cloned rat brain A3-adenosine receptor coupled to inhibition of adenylate cyclase [Jacobson et al., 1995]. Consonant with involvement of an A3- receptor in the calcium-response in RBL-2H3 cells is the inhibition of the response by a A3-selective adenosine receptor antagonist, MRS 1067 [Karton et al., 1996], as demonstrated versus both 2-CI-IBMECA and NECA (Fig. 6). MRS 1067 also inhibited the accumulation of [3H]IP3 elicited by either NECA or 2-CI-IBMECA (Fig. 3). In toto, the present results suggest that adenosine analogs and the xanthine DBXRM interact with an A3-adenosine receptor in an rat RBL-2H3 mast cell line to cause elevation of intracellular calcium via generation of IP3. The levels of IP3, however, are too low to measure reliably either as [3H]IP3 or using a binding assay with [3H]IP3 (data not shown). The adenosine analogs and DBXRM do significantly stimulate phosphoinositide breakdown as measured by [3H]IP1 and [3H]IP2 accumulation, but structure–activity relationships are inconsistent with those expected of a rat A3-adenosine receptor.
ACKNOWLEDGMENTS
The authors thank Dr. Hea Ok Kim for synthesis of two agents used in this study, and Dr. Michael A. Beaven for comments and Dr. Oksoon Choi for suggestions and for advice on binding assays with [3H]IP3.
Abbreviations
- APNEA
N6-p-aminophenylethyladenosine
- CHA
N6-cyclohexyladenosine
- CGS 21680
2-(p-carhoxethylphenylethylamino
- DBXRM
1,3-dibutylxanthine 7-rihoside-5’-N-methylcarboxamide
- DNP
3,5-dinitrophenol
- DNP-HSA
antigen consisting of DNP conjugated with human serum albumin
- IP1, IP2, IP3
inositol monobis- and trisphophate
- IgE
immunoglobulin E
- NECA
N-ethylcarhoxamidoadenosine
- R-PIA
N6-R-phenylisopropyladensine
- XAC
8-(p-arninoethylaminocarhoxymethyloxyphenyl)-1,3-dipropylxanthine.
REFERENCES
- Abbracchio MP, Brambilla R, Ceruti S, Kim HO, von Lubitz DKJE, Jacobson KA, Cattabeni F. G-protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol Pharmacol. 1995;48:1038–1045. [PubMed] [Google Scholar]
- Ali H, Cunha-Melo JR, Saul WF, Beaven MA. Activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen-stimulated RBL-2H3 cells. Evidence for a novel adenosine receptor. J Biol Chem. 1990;265:745–753. [PubMed] [Google Scholar]
- Beaven MA, Ramkumar V, Ali H. Adenosine A3 receptors in mast cells. Trends Pharmacol Sci. 1994:1513–1514. doi: 10.1016/0165-6147(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Dawson RM, Downes CP, Heslop JP, Irvine RF. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983;212:473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi OH, Kim J-H, Kinet J-P. Calcium mobilization via sphingosine kinase in signaling by the FcRI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- Gallo-Rodriguez C, Ji X-D, Melman N, Siegman BD, Sanders LH, Orlina J, Pu Q, Olah ME, van Galen PJM, Stiles GL, Jacobson KA. Structure–activity relationships of N6-benzyladenosine-5′-uronamides as A3-selective adenosine agonists. J Med Chem. 1994;37:636–646. doi: 10.1021/jm00031a014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Jacobson KA, Kim HO, Siddiqi SM, Olah ME, Stiles G, von Lubitz DKJE. A3 adenosine receptors: Design of selective ligands and therapeutic prospects. Drugs Future. 1995;20:689–699. doi: 10.1358/dof.1995.020.07.531583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X-D, Gallo-Rodriguez C, Jacobson KA. A selective agonist affinity label for A3 adenosine receptors. Biochem Biophys Res Commun. 1994;203:570–576. doi: 10.1006/bbrc.1994.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karton Y, Jiang J-I, Ji X-D, Melman N, Olah ME, Stiles GL, Jacobson KA. Synthesis and biological activities of flavonoid derivatives as A3 adenosine receptor antagonists. J Med Chem. 1996;39:2293–2301. doi: 10.1021/jm950923i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HO, Ji X-D, Siddiqi SM, Olah ME, Stiles GL, Jacobson KA. 2-Substitution of N6-benzyladenosine-5′-uronamides enhances selectivity for A3-adenosine receptors. J Med Chem. 1994a;37:3614–3621. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HO, Ji X-D, Melman N, Olah ME, Stiles GL, Jacobson KA. Selective ligands for rat A3-adenosine receptors: Structure–activity relationships of 1,3-dialkylxanthine-7-riboside derivatives. J Med Chem. 1994b;37:4020–4030. doi: 10.1021/jm00049a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt DL. Adenosine and mast cell secretion. In: Cooper DMF, Londos C, editors. Adenosine Receptors. New York: Alan R. Liss; 1988. pp. 87–95. [Google Scholar]
- Marquardt DL, Walker LL. Pretreatment with phorbol esters abrogates mast cell adenosine responsiveness. J Immunol. 1989;142:1268–1273. [PubMed] [Google Scholar]
- Marquardt DL, Walker LL. Inhibition of protein kinase A fails to alter mast cell adenosine responsiveness. Agents Actions. 1994;43:7–12. doi: 10.1007/BF02005755. [DOI] [PubMed] [Google Scholar]
- Marquardt DL, Walker LC, Heinemann S. Cloning of two adenosine receptor subtypes from mouse bone marrow-derived mast cells. J Immunol. 1994;152:4508–4515. [PubMed] [Google Scholar]
- Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993;268:16887–16890. [PubMed] [Google Scholar]
- Van Galen PJM, van Bergen AH, Gallo-Rodriguez C, Melman N, Olah ME, IJzerman AP, Stiles GL, Jacobson KA. A binding site model and structure–activity relationships for the rat A3 adenosine receptor. Mol Pharmacol. 1994;45:1101–1111. [PMC free article] [PubMed] [Google Scholar]