Abstract
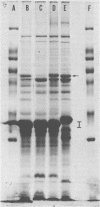
Loci designated penA, penB, and mtr contribute additively to penicillin G resistance in Neisseria gonorrhoeae; the mtr locus also confers resistance to hydrophobic dyes, detergents, and antibiotics, env mutations suppress the phenotypic expression of mtr and penB and are responsible for increased sensitivity to various hydrophobic molecules. We postulated that the host environment is important in the selection of gonococcal strains with these particular outer membrane phenotypes. Thus, mtr strains should predominate in environments that are high in hydrophobic molecules. To test this hypothesis we determined the outer membrane phenotype of 152 strains of N. gonorrhoeae. Rectal and urethral isolates from 58 homosexual men, urethral isolates from 55 heterosexual men, and cervical and rectal isolates from 39 heterosexual women were used in this study. Strains from 43 of the homosexual men were matched with those from heterosexual men with respect to auxotype and year and season of isolation. Cell envelope phenotype was determined for each strain on the basis of its resistance to various hydrophobic compounds. The identity of mtr strains was confirmed by genetic transformation. Among the matched pairs, mtr strains were significantly more prevalent among isolates from homosexual men than among those from heterosexual men (P = 0.03). Serogrouping by coagglutination demonstrated that 17 of 19 mtr strains versus 76 of 131 non-mtr strains belonged to coagglutination group WII (P = 0.01). Coagglutination group WII strains were also associated with homosexuality (P = 0.02). Gonococci were also tested for resistance to fecal lipids, mtr strains were more resistant to growth inhibition by fecal lipids than were non-mtr strains.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Crawford C., Knapp J. S., Hale J., Holmes K. K. Asymptomatic gonorrhea in men: caused by gonococci with unique nutritional requirements. Science. 1977 Jun 17;196(4296):1352–1353. doi: 10.1126/science.405742. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Sparling P. F. Mutations to increased antibiotic sensitivity in naturally-occurring gonococci. Nature. 1978 Jan 19;271(5642):242–244. doi: 10.1038/271242a0. [DOI] [PubMed] [Google Scholar]
- Guymon L. F., Walstad D. L., Sparling P. F. Cell envelope alterations in antibiotic-sensitive and-resistant strains of Neisseria gonorrhoeae. J Bacteriol. 1978 Oct;136(1):391–401. doi: 10.1128/jb.136.1.391-401.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handsfield H. H., Knapp J. S., Diehr P. K., Holmes K. K. Correlation of auxotype and penicillin susceptibility of Neisseria gonorrhoeae with sexual preference and clinical manifestations of gonorrhea. Sex Transm Dis. 1980 Jan-Mar;7(1):1–5. doi: 10.1097/00007435-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Janik A., Juni E., Heym G. A. Genetic Transformation as a tool for detection of Neisseria gonorrhoeae. J Clin Microbiol. 1976 Jul;4(1):71–81. doi: 10.1128/jcm.4.1.71-81.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J., Jefferiss F. J. Treatment of ano-rectal gonorrhoea with ampicillin. Br J Vener Dis. 1973 Aug;49(4):362–363. doi: 10.1136/sti.49.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinghorn G. R., Rashid S. Prevalence of rectal and pharyngeal infection in women with gonorrhoea in Sheffield. Br J Vener Dis. 1979 Dec;55(6):408–410. doi: 10.1136/sti.55.6.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975 Aug;132(2):204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lysko P. G., Morse S. A. Effects of steroid hormones on Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980 Aug;18(2):281–288. doi: 10.1128/aac.18.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysko P. G., Morse S. A. Neisseria gonorrhoeae cell envelope: permeability to hydrophobic molecules. J Bacteriol. 1981 Feb;145(2):946–952. doi: 10.1128/jb.145.2.946-952.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Brown K. E., Morse S. A. Inhibitory action of fatty acids on the growth of Neisseria gonorrhoeae. Infect Immun. 1977 Aug;17(2):303–312. doi: 10.1128/iai.17.2.303-312.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Morse S. A. Binding of progesterone to Neisseria gonorrhoeae and other gram-negative bacteria. Infect Immun. 1977 Apr;16(1):115–123. doi: 10.1128/iai.16.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Hill J. L. Rectal and pharyngeal gonorrhea in homosexual men. JAMA. 1972 Jun 5;220(10):1315–1318. [PubMed] [Google Scholar]
- Sandström E., Danielsson D. Serology of Neisseria gonorrhoeae. Characterization of rabbit hyperimmune antisera by line-rocket immunoelectrophoresis for use in coagglutination. Acta Pathol Microbiol Scand B. 1980 Feb;88(1):17–26. doi: 10.1111/j.1699-0463.1980.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Sandström E., Danielsson D. Serology of Neisseria gonorrhoeae. Classification by co-agglutination. Acta Pathol Microbiol Scand B. 1980 Feb;88(1):27–38. doi: 10.1111/j.1699-0463.1980.tb02600.x. [DOI] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Blackman E., Sparling P. F. Genetic mapping of linked antibiotic resistance loci in Neisseria gonorrhoeae. J Bacteriol. 1974 Dec;120(3):1284–1292. doi: 10.1128/jb.120.3.1284-1292.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Sparling P. F., Blackman E., Lewis E. Loss of low-level antibiotic resistance in Neisseria gonorrhoeae due to env mutations. J Bacteriol. 1975 Nov;124(2):750–756. doi: 10.1128/jb.124.2.750-756.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Sarubbi F. A., Jr, Blackman E. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):740–749. doi: 10.1128/jb.124.2.740-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walstad D. L., Guymon L. F., Sparling P. F. Altered outer membrane protein in different colonial types of Neisseria gonorrhoeae. J Bacteriol. 1977 Mar;129(3):1623–1627. doi: 10.1128/jb.129.3.1623-1627.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington A. E. Experience with various antibiotics for treatment of anorectal gonorrhea. Sex Transm Dis. 1979 Apr-Jun;6(2 Suppl):148–151. doi: 10.1097/00007435-197904000-00020. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Willcox R. R. The rectum as viewed by the venereologist. Br J Vener Dis. 1981 Feb;57(1):1–6. doi: 10.1136/sti.57.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]