Abstract
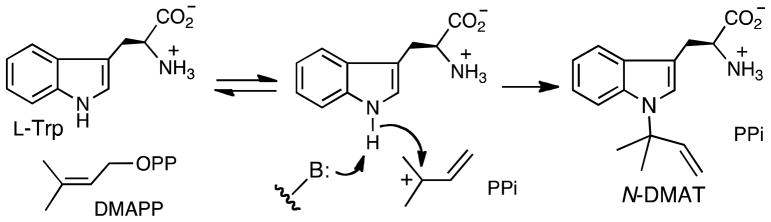
The prenyltransferase CymD catalyzes the reverse N-prenylation of tryptophan using dimethyllallyl diphosphate (DMAPP) in the biosynthesis of the cyclic peptides cyclomarin and cyclomarazine. The mechanism is of interest since a non-nucleophilic indole nitrogen must be alkylated in this process. Three mechanisms were initially considered that included: A) a direct addition of a carbocation to the nitrogen, B) an addition of a carbocation to C-3 followed by an aza-Cope rearrangement, and C) deprotonation of the indole followed by an SN2′ addition to DMAPP. The use of 4-fluorotryptophan and 6-fluorotryptophan revealed that the reaction kinetics are only modestly affected by these substitutions, consistent with the notion that positive charge does not accumulate on the indole ring during catalysis. When E-3-(fluoromethyl)-2-buten-1-yl diphosphate (E-F-DMAPP) was used in place of DMAPP, the maximal rate was reduced by a factor of 100, consistent with the development of positive charge on the dimethylallyl moiety. Positional isotope exchange (PIX) experiments show that the reaction with Trp proceeds without isotopic scrambling of the label in the starting material [1-18O]-DMAPP. However, in the case of 4-fluorotryptophan, significant isotopic scrambling is observed (vPIX/vrxn = 1.1). This is consistent with a mechanism involving a discrete carbocation intermediate. Finally, a significant solvent kinetic isotope effect of 2.3 was observed in D2O, indicating that a proton transfer step is rate limiting. Taken together, these observations support a mechanism that is a hybrid of mechanisms A and C. Ionization of DMAPP generates a dimethylallyl carbocation, and deprotonation of the indole nitrogen accompanies, or precedes, the nucleophilic attack.
Prenylated indole akaloids comprise a large and structurally diverse group of natural products that often possess potent biological properties.1–3 Prenylation is known to occur at all seven of the potential sites around the indole ring system and may occur either in a “normal” (linked via methylene) or “reverse” (linked via tertiary carbon) fashion.4 Within the last ten years, many of the fungal indole prenyltransferases have been identified and characterized.5, 6 They share notable sequence identity (>25%), act in a metal-independent fashion, and bear structural homology to the ABBA family of prenyltransferases.7 Despite these similarities, marked differences in the chemical mechanisms employed by these enzymes are likely to exist given the dramatic differences in reactivity at the various positions of the indole ring.
One recently identified enzyme, CymD, is a reverse N-prenyltransferase involved in the biosynthesis of the cyclic peptides cyclomarin and cyclomarazine (Figure 1).8, 9 This enzyme is found in the marine actinobacterium Salinispora arenicola and has been shown to directly prenylate the indole nitrogen of free tryptophan with dimethylallyl diphosphate (DMAPP) to give N-(1,1-dimethyl-1-allyl)-tryptophan (N-DMAT). The enzyme bears only modest overall sequence homology with the fungal prenyltransferases (< 25%), but appears to share conserved residues and evolutionary origins with this family.
Figure 1.
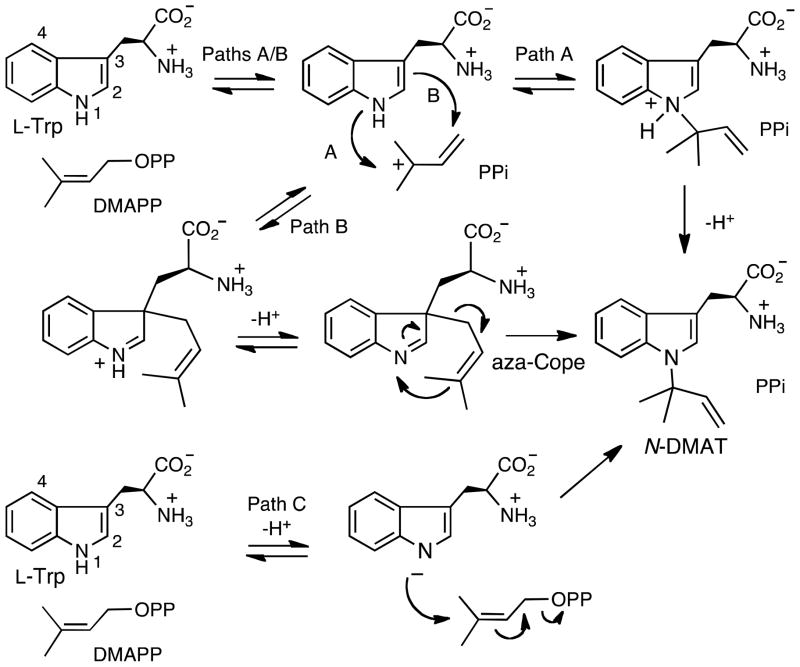
Three initially proposed mechanisms for the reaction catalyzed by CymD.
The mechanism employed by CymD is of interest as the nitrogen to be alkylated is extremely non-nucleophilic. One potential mechanism involves an ionization of DMAPP to form the dimethylallyl cation, followed by nucleophilic attack from the indole nitrogen, and a final deprotonation to give the product (Figure 1, Path A). This mechanism has been proposed to be operative with a reverse N-prenyltransferase that accepts cyclic dipeptides.10 This pathway is problematic since the nitrogen lone pair is in a p-orbital and is involved in the aromaticity of the indole ring. The low reactivity of the indole lone pair is reflected in the low basicity of indoles (pKa of protonated indole = −3.5) and the observation that protonation primarily occurs at C-3.11
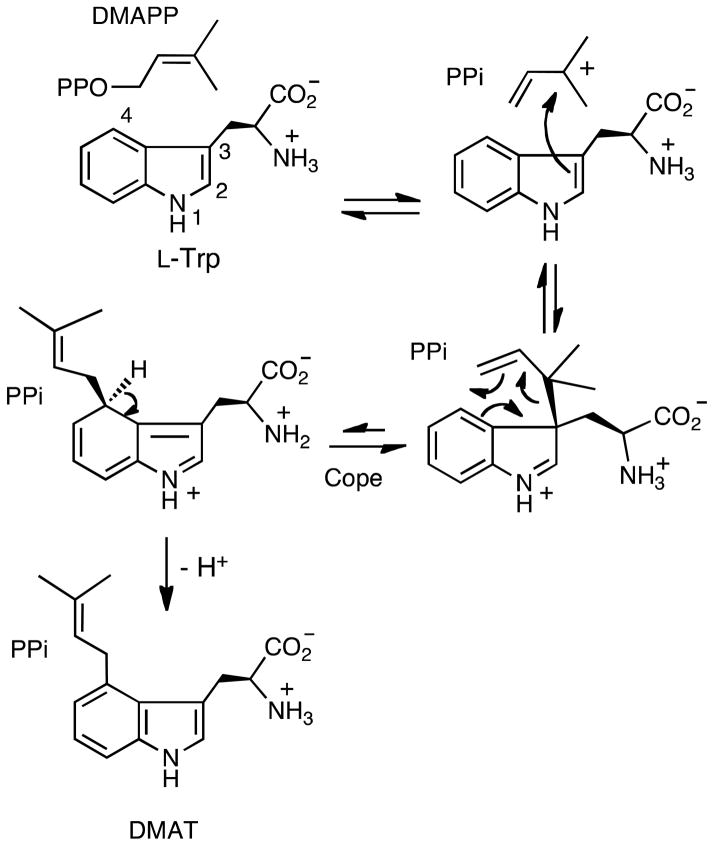
A second mechanism also involves an initial ionization of DMAPP to form the dimethylallyl cation; however, in this case a nucleophilic attack occurs from C-3 of the indole ring to form a “normal” prenylated intermediate (Figure 1, Path B). The latter step is reasonable as indoles are good nucleophiles and C-3 is the preferred site of electrophilic substitution.12–15 Deprotonation of the indole nitrogen then occurs to generate the prenylated intermediate as a neutral imine. A subsequent aza-Cope rearrangement then generates the “reverse” N-prenylated product.16 Precedence for such a Cope rearrangement in non-enzymatic reactions of indoles can be found in the literature, and this rearrangement has been previously postulated to occur during the biosynthesis of indole alkaloids.17–22 Recently, a related Cope rearrangement was proposed to occur in the reaction catalyzed by 4-dimethylallyltyrptophan synthase (DMATS) (Figure 2).23 This mechanism involves the initial formation of a C-3 reverse-prenylated intermediate; however, the Cope rearrangement occurs in the opposite direction to produce the C-4 “normal” prenylated product (DMAT) after deprotonation.
Figure 2.
A Cope rearrangement mechanism proposed for the reaction catalyzed by 4-dimethylallyltyrptophan synthase (DMATS).
A third mechanism involves an initial deprotonation of the indole nitrogen to generate an anionic nucleophile (Figure 1, Path C). This is reasonable as the pKa of the indole nitrogen is approximately 17 in water and 21 in DMSO.24, 25 Once formed, the nucleophilic nitrogen anion could attack DMAPP in an SN2′ fashion to generate the reverse N-prenylated product, N-DMAT. An associative mechanism has been previously been proposed for the mechanism of protein farnesyltransferase in which an excellent nucleophile (a cysteine side chain) is prenylated.26–28
In this manuscript studies that probe the mechanism of CymD are presented. Through the use of fluorinated substrates, positional isotopic scrambling (PIX) experiments, and solvent isotope effect measurements, evidence for a mechanism that is a hybrid of mechanisms A and C is obtained. In this scenario a carbocation intermediate is formed, and nucleophilic attack is accompanied by deprotonation of the indole nitrogen.
MATERIALS AND METHODS
Materials and General Methods
All reagents were purchased from Sigma-Aldrich or Alfa Aesar and used without further purification unless otherwise stated. 6-Fluorotryptophan was purchased from Acros Organics. 1-[18O]-DMAPP was synthesized as described previously.29 E-3-(Fluoromethyl)-2-butenol was synthesized as described previously and converted into E-3-(fluoromethyl)-2-buten-1-yl diphosphate (E-F-DMAPP) using trichloroacetonitrile and bis-triethylammonium phosphate.30, 31 The enzyme kinetic assays were carried out on a Cary 300 UV-Vis spectrophotometer with an attached Cary temperature controller. Proton-decoupled 31P NMR spectra were recorded on a Bruker AV400inv spectrometer at a field strength of 162 MHz. Protein concentrations were determined by the Bradford Assay using a commercial kit (Bio-Rad).
Protein Purification
Recombinant His-tagged CymD was generated using the plasmid pHIS8-cymD and a slightly modified protocol from that described previously.8 After transformation, E. coli. BL21(DE3) pLysS cells (Novagen) containing the pHIS8-cymD plasmid were grown at 37 °C in 500 mL of ZYP-5052 autoinduction medium containing 50 μg/mL kanamycin for 16 h. Cells were harvested and lysed by French Press in buffer A (50 mM Tris-HCl, pH 7.5) containing 20 mM imidazole and 500 mM NaCl). The lysate was cleared by centrifugation at 12 000 rpm for 1 h before the supernatant was loaded onto a column of Chelating Sepharose™ Fast Flow resin (GE Healthcare, 10 mL, loaded with 100 mM NiSO4 and then equilibrated with buffer A containing 500 mM NaCl). The column was washed with wash buffer (first with buffer A containing 500 mM NaCl, then with buffer A containing 125 mM imidazole and 500 mM NaCl) and eluted with elution buffer (buffer A containing 500 mM imidazole and 500 mM NaCl). The resulting eluent was used directly in enzymatic assays as all attempts to remove the imidazole (ultracentrifugation, dialysis, and size exclusion chromatography) resulted in unacceptable loss of enzyme activity. Typically about 10 mg of enzyme was purified from 500 mL of culture.
Enzyme Kinetics
Enzyme kinetics were measured using an EnzChek® Pyrophosphate Assay Kit (Invitrogen) modified from a previously described procedure.29 Solutions (final volume 990 μL in buffer A) containing 20 μM DMAPP, L-tryptophan (variable), 100 μM 2-amino-6-mercapto-7-methylpurine ribonucleoside (MESG), 1 U purine nucleoside phosphorylase (PNPase), 0.1 U inorganic pyrophosphatase (PPase) and 400 μM MgCl2 were prepared in quartz cuvettes and equilibrated for 5 min at 35 °C. The enzymatic reaction was then initiated by addition of 10 μL CymD eluent (4.8 μg) and the rate was calculated from the observed increase of absorption at 360 nm (using ε = 11,000 M−1 cm−1). Kinetic parameters were determined from the fit of the initial velocities to the Michaelis-Menten equation.
Enzyme Kinetics with Fluorinated Substrates
The kinetics with the racemic fluorinated tryptophans were measured as described above with a constant DMAPP concentration of 20 μM. For D,L-4-F-tryptophan and D,L-6-F-tryptophan, 20 μL of CymD eluent containing 6.8 μg and 9.1 μg of enzyme was used per assay, respectively.
The kinetics with E-F-DMAPP were measured with concentrations of DMAPP and E-F-DMAPP at 10 and 20 μM and with L-tryptophan concentration constant at 100 μM. For the analysis it was necessary to concentrate the CymD eluent by ultracentrifugation prior to use and then dilute as required. For DMAPP and E-F-DMAPP, 10 and 20 μL of CymD eluent containing 7.7 μg and 230 μg of enzyme was used per assay, respectively.
PIX Experiments
A solution containing 1-[18O]-DMAPP and unlabeled DMAPP (total concentration of 30 μM in 1.0 mL with 56% 18O incorporation) and L-tryptophan (20 μM) in Tris-HCl buffer (50 mM, pD 7.5, prepared using D2O) was prepared and the 31P NMR spectrum was collected. A solution of CymD (1.5 mg/mL in 0.5 mL of column eluent) was added and the mixture was incubated at 37 °C for 4 h. A 1.0 mL sample of the solution was subjected to ultrafiltration (Amicon Ultra-4, 10000 MWCO, 5000 rpm for 15 min, 4 °C) in order to remove the enzyme. Chelex-100 resin (50 mg of 100–200 mesh, Na+ form, pre-rinsed with D2O) was added to the filtrate and the mixture was vortexed. A second 31P NMR spectrum was then acquired. The proton-decoupled 31P NMR spectra were obtained on a Bruker AV400inv spectrometer operating at a frequency 162 MHz. Acquisition parameters included a 2437 Hz (20 ppm) sweep width centered at −5 ppm with a 27 s acquisition time. Well-resolved spectra were achieved after 200 to 1000 scans. All the spectra were optimized using appodization with exponential and Gaussian functions to achieve higher resolution. Integration of the pyrophosphate and DMAPP signals indicated that 41% of the DMAPP had been consumed during the incubation. PIX experiments with fluorinated substrates were performed under identical conditions with the exception that 3X the amount of CymD was added and the concentration of the racemic substrate was doubled. The value of vPIX/vrxn for 4-fluorotryptophan was calculated as described previously.23
Solvent Kinetic Isotope Effect Determination
A sample of 20X buffer A (8.0 mL) was divided into two equal portions. One portion was lyophilized to dryness and resuspended in an equal volume of D2O to produce the deuterated buffer. Kinetics assays were run using a modification of the coupled enzyme assay. Assay solutions were prepared with either the deuterated or non-deuterated buffer A (final volume 990 μL diluted with either D2O or H2O) containing L-tryptophan (variable), DMAPP (20 μM), 2-amino-6-mercapto-7-methylpurine ribonucleoside (100 μM), purine nucleoside phosphorylase (PNPase) (0.1 units), inorganic pyrophosphatase (PPase) (0.1 units), and MgCl2 (0.4 mM) all of which had been prepared in either the deuterated or non-deuterated buffers, respectively. Note that much lower amounts of the PNPase coupling enzyme was added, yet the enzymatic reactions was still efficiently coupled. These solutions were incubated at 35 °C prior to the addition of CymD (4.8 μg in 10 μL non-deuterated column eluent). The UV absorption change at 360 nm was then recorded on a UV spectrometer to determine the initial velocity.
RESULTS
Enzyme Kinetics
Recombinant His-tagged CymD from S. arenicola was overexpressed in Escherichia coli and purified as described previously.8 In past studies, the product of the CymD reaction was demonstrated to be N-DMAT; however, no kinetic studies were performed. In order to monitor the reaction kinetics, a coupled enzyme assay for phosphate detection was performed in the presence of pyrophosphatase. With DMAPP as the variable substrate it was not possible to measure a value for KM as a maximal rate was observed at 2 μM and the assay was not sufficiently sensitive to measure initial rates at lower concentrations. Kinetic data was therefore obtained with L-Trp as the variable substrate using a fixed 20 μM concentration of DMAPP. Michaelis-Menten kinetics were observed with values of Km,L-Trp = 3.8 μM and kcat = 0.10 s−1 (see Figure S1).
Kinetic Analyses Using Fluorinated Substrates
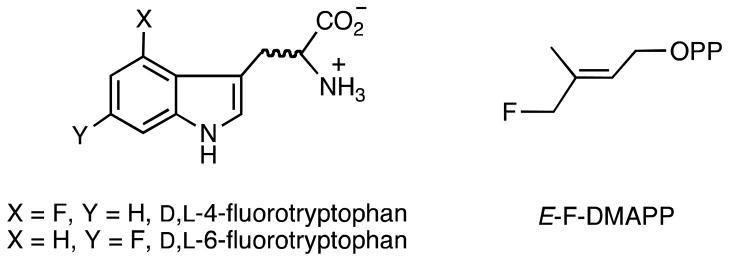
The use of fluorinated substrates has proven to be an effective method of probing the reactions catalyzed by enzymes that generate cationic intermediates and transition states.32 The electron withdrawing properties of fluorine will slow the rate of formation of such electron deficient species. To access the effect of fluorine substitution on the indole ring, racemic 4-fluorotryptophan and 6-fluorotryptophan were kinetically characterized as substrates for CymD (Figure 3). Both compounds served as substrates for the enzyme, and in overnight incubations monitored by 1H NMR spectroscopy a maximal value of 50% reaction was observed, suggesting that only one of the enantiomers (presumably the L-enantiomer) serves as a substrate. Using the coupled enzyme assay, the corresponding kinetic constants were determined to be KM = 7.6 μM, kcat = 0.037 s−1 and KM = 2.6 μM, kcat = 0.011 s−1, respectively (Table 1 and Figure S3). The finding that fluorine substitution has only a modest effect on the rate of the reaction (< 10-fold decrease in the value of kcat) suggests that there is negligible positive charge accumulation on the indole ring during catalysis.
Figure 3.
Structures of the fluorinated substrates used in this work.
Table 1.
Kinetic constants obtained with fluorinated tryptophans
| Trp Substrate | kcat (s−1) | KM (μM) |
|---|---|---|
| L-Trp | 0.10 ± 0.01 | 3.8 ± 0.7 |
| D,L-4-Fluoro-Trp | 0.037 ± 0.002 | 7.6 ± 1.7 |
| D,L-6-Fluoro-Trp | 0.011 ± 0.001 | 2.6 ± 0.3 |
Fluorinated analogs of prenyl diphosphates have also been used to probe the extent of carbocation formation during catalysis.26, 30, 31, 33–35 In order to apply this test to the CymD reaction, we synthesized E-3-(fluoromethyl)-2-buten-1-yl diphosphate (E-F-DMAPP, Figure 3) using slightly modified literature known procedures.36 Since the value of KM for DMAPP is lower than our assay is able to determine, we were unable to complete a full kinetic characterization, and we simply compared the relative rates at saturating concentrations (10 and 20 μM) of the diphosphates (Table S1). Under otherwise identical conditions, the monofluorinated DMAPP reacted at 0.01 times the rate of DMAPP itself. The fact that fluorine substitution slowed the maximal rate of catalysis indicates that there is a significant degree of carbocationic character to the transition state for C–O bond cleavage.
Positional Isotope Exchange (PIX) Experiments
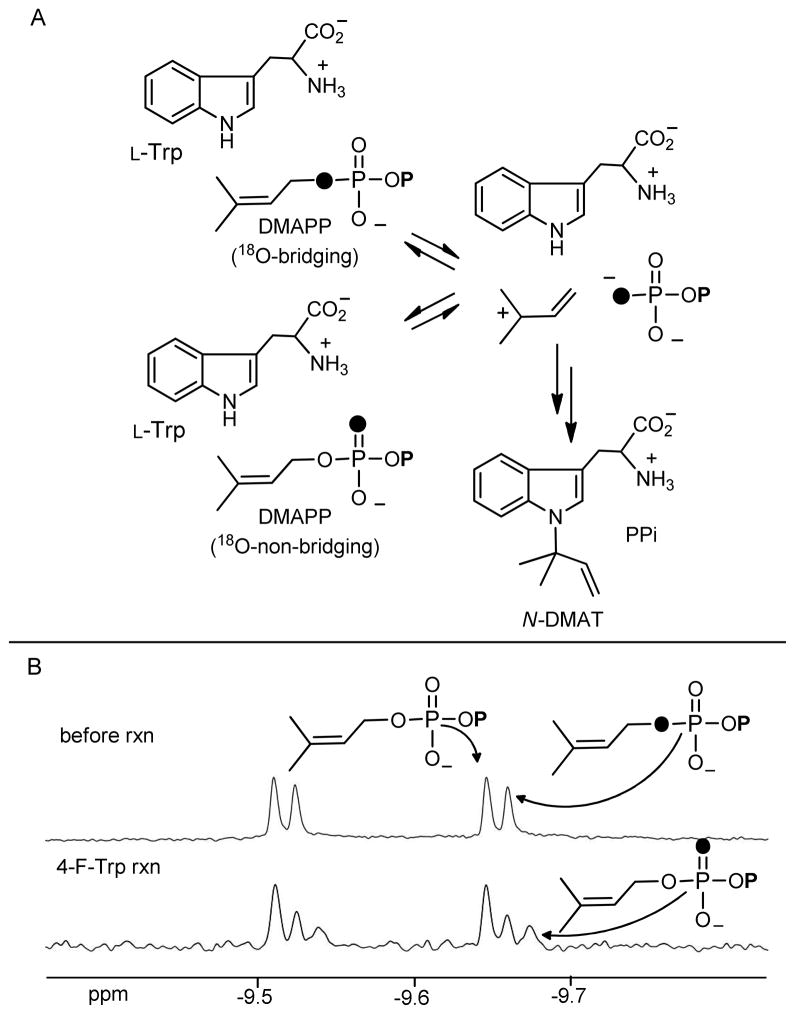
Evidence for the reversible formation of a dimethylallyl cation intermediate can be obtained through the use of positional isotope exchange (PIX) experiments.37 If the 18O-label in 1-[18O]-DMAPP scrambles from a bridging to a non-bridging position during catalysis, one may conclude that a reversible cleavage of the P-O bond has occurred (Figure 4A). The use of PIX experiments has not proven to be informative with metal-dependent prenyltransferases, and it is thought that chelation of the pyrophosphate with the divalent cation prevents any bond rotation from occurring during the lifetime of the carbocation intermediate.38, 39 However, in the case of the metal-independent indole prenyltransferase DMATS, the observed scrambling provided strong support for the existence of the dimethylallylcation intermediate.29 To probe for PIX in the CymD reaction, a sample containing both unlabeled DMAPP and 1-[18O]-DMAPP was incubated with tryptophan and CymD until 41% of the total DMAPP had been consumed. The reaction was then quenched and the remaining DMAPP was analyzed by 31P NMR spectroscopy. Since the magnitude of the isotopic shift on the 31P resonance is dependent on the P-O bond order, any non-bridging label (bond order greater than unity) would result in the appearance of a new upfield doublet.40 The absence of any new upfield signals in the spectrum of this material demonstrated that less than 3% bore isotopic label in a non-bridging position (see Figure S2a), and therefore no PIX was detected. The PIX experiment was also carried out with racemic 4-fluorotryptophan and 6-fluorotryptophan since these indoles should serve as worse nucleophiles and there may be a greater chance for isotopic scrambling to occur. In the case of 4-fluorotryptophan, PIX was clearly observed (Figure 4B). This is evident by the appearance of new upfield 31P NMR signals at −9.61 ppm that are due to 18O-label in a non-bridging position with a P-O bond order of greater than unity.29, 40 After 59% of the DMAPP had been consumed, 44% of the isotopic label had scrambled into a non-bridging position, corresponding to a vPIX/vrxn value of approximately 1.1. In the case of 6-fluorotryptophan, a much smaller extent of PIX was observed and it was not possible to accurately integrate the signals (approximately 10% scrambling after 72% reaction) (Figure S2b). The observation of isotopic scrambling with the fluorinated tryptophans proves that cleavage of the C-O bond in DMAPP is reversible during catalysis and is consistent with the formation of a pyrophosphate-dimethylallyl cation ion pair that can either proceed forward towards product or collapse to reform DMAPP. Given the similarities in rates between the reactions of the fluorinated and unsubstituted tryptophans, it seems unlikely that different mechanisms are operative and we therefore conclude that CymD catalysis proceeds through a carbocation intermediate in all three cases. The scrambling was presumably only observed in the case of the fluorinated tryptophans because of the poorer nucleophilicity of the fluorinated indoles.
Figure 4.
A) A schematic representation of the isotopic scrambling process that would be observed if positional isotope exchange (PIX) occurred in the CymD reaction. B) 31P NMR spectra showing the PIX process during the reaction of 4-fluorotryptophan and [1-18O]-DMAPP with CymD. Only the signals for the α-phosphorus of DMAPP are shown. The upper spectrum shows DMAPP before the addition of enzyme. The lower spectrum shows DMAPP after 59% had been consumed. Darkened atoms represent 18O isotopic labels.
Solvent Isotope Effect Determination
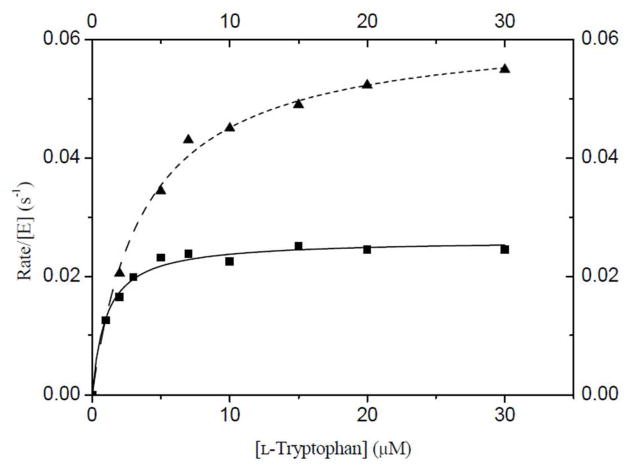
In any proposed mechanism for the CymD reaction, the indole NH bond must be cleaved during catalysis. Since the proton on the indole nitrogen is readily exchangeable with solvent protons, incubation of tryptophan in D2O will rapidly generate the deuterated amine. It is therefore possible to probe whether the proton transfer step is a rate-limiting step of catalysis by measuring a solvent kinetic isotope effect (KIE). To this end, identical samples of the substrates were prepared in both H2O and D2O (> 95%) and assayed in the CymD reaction. While it was not possible to accurately measure the effect of D2O on the value of KM, a clear 2.3-fold decrease in the value of kcat was observed (Figure 5). While it is often difficult to attribute an observed solvent KIE to an individual reaction step in multi-step reactions, it is reasonable to suspect that deprotonation of the indole nitrogen is the isotopically sensitive step in the CymD reaction. It therefore appears that this deprotonation is a rate-limiting step in catalysis.
Figure 5.
Enzyme kinetic plots of initial velocity/[E] vs. substrate concentration demonstrating a solvent kinetic isotope effect. Tryptophan is the variable substrate and saturating DMAPP (20 μM) was employed. Triangles represent data obtained in H2O and squares represent data obtained in > 95% D2O. Data was fit to the Michaelis-Menten equation (dashed line = H2O and solid line = D2O).
DISCUSSION
The recent discovery of a large family of indole prenyltransferases raises interesting questions as to the similarities and differences in the mechanisms employed in installing the prenyl group at the various positions around the indole ring. The reaction catalyzed by CymD is of interest in that a very non-nucleophilic amine must be alkylated during catalysis. In addition, the recent suggestion that DMATS uses a Cope rearrangement during C-4 prenylation (Figure 2), prompted the notion that CymD may use a similar strategy for the prenylation of the indole nitrogen (Figure 1, Path B).23 On the balance of the evidence presented in this work, CymD is proposed to employ a mechanism that involves an initial ionization of DMAPP to form a dimethylallyl carbocation intermediate (Figure 6). A subsequent attack by the indole nitrogen is assisted by deprotonation either prior to, or during, the C-N bond forming process.
Figure 6.
A proposed mechanism for the reaction catalyzed by CymD.
The observation of PIX in the CymD-catalyzed reactions of [1-18O]-DMAPP and the fluorinated tryptophans is consistent with a mechanism that involves the formation of a discrete dimethylallyl cation intermediate. While PIX was not observed with tryptophan itself, it is quite possible that hindered rotation of the pyrophosphate bonds prevents isotopic scrambling during the lifetime of the carbocation intermediate. Since the fluorinated indoles are poorer nucleophiles, the lifetime of the carbocation may be increased sufficiently to allow the scrambling to be detected. A similar observation was made with the enzyme DMATS where partial PIX was observed with tryptophan as the substrate whereas complete PIX was observed with the inactive analog, 6-fluorotryptophan.29 The observation of PIX in the CymD-catalyzed reaction argues against the associative mechanism shown in Path C (Figure 1).
The use of fluorinated tryptophan derivatives in the CymD reaction demonstrated that there is only a negligible effect of this substitution on the rate of catalysis. Our analysis of this observation was largely guided by the computational work of Otero et al. on the nucleophilicity of indole derivatives based on proton affinities and electron densities.12 The proton affinities at each position of the indole ring in substituted indoles were analyzed by DFT using the B3LYP/6–311++G(2d, 3p) level of theory. The proton affinity at the C-3 position of indoles is markedly higher than at any other position and therefore it is not possible to obtain such data through experimental methods. The proton affinity at a given atom should reflect its propensity to add to an electrophilic species such as a carbocation. This is consistent with experimental evidence demonstrating that electrophiles prefer to add to the C-3 position of indoles in solution.12–15 In the active site of an enzyme, however; it is conceivable that positioning of a carbocation could direct the reaction to an alternate site of attack (for an example see the mechanism in Path A). In this scenario, the computationally predicted effects on proton affinities due to substitutions on the indole ring could help predict whether observed trends in reactivity are consistent with a proposed mechanism. A subset of the data presented in the computational study is shown in Table 2. It can be seen that fluorine substitution at either the C-4 or C-6 position causes a greater than 4 kcal/mol decrease in the proton affinity at either the N-1 or C-3 position of the indole ring. The mechanisms shown in Paths A and B, require the addition of a carbocation to the N-1 and C-3 positions of the indole ring, respectively, and if these were operative the rates of reaction should be dramatically lowered by the fluorinations. Such an effect is observed in the case of the DMATS reaction where 6-fluorotryptophan does not serve as a substrate even though 6-methyltryptophan is accepted (even in a direct C-4 prenylation mechanism for the DMATS reaction (not shown in Figure 2), one would expect a dramatic effect since C-6 fluorination is predicted to reduce the proton affinity at C-4 from 211.8 kcal/mol to 205.6 kcal/mol).29, 41 PIX experiments with DMATS, [1-18O]-DMAPP, and 6-fluorotryptophan showed that isotopic scrambling proceeds to completion, indicating that binding and carbocation formation still occurs, but the reduced nucleophilicity of the indole prevents the forward reaction from taking place.29 Overall, the modest effects on the rate of the CymD reaction due to fluorination of the tryptophan ring argue against the electrophilic addition mechanisms shown in Paths A and B where significant positive charge accumulates on the indole ring.
Table 2.
Calculated proton affinities (B3LYP/6–311++G(2d,2p) for substituted indoles (kcal mol−1).a
| compound | N1 | C3 | C4 |
|---|---|---|---|
| indole | 206.8 | 220.1 | 211.8 |
| 4-F-indole | 202.0 | 215.9 | 197.5 |
| 6-F-indole | 202.7 | 216.0 | 205.6 |
Data taken from reference 12.
Fluorinated analogs of DMAPP have previously been used to distinguish between associative (SN2) mechanisms and dissociative 26, 30, 31, 33–35 The (SN1) mechanisms in prenyltransferase reactions. magnitude of the observed rate reductions have been evaluated in terms of a krel value that describes the rate measured with the non-fluorinated compound divided by that measured with the fluorinated compound. In the case of farnesyl diphosphate synthase, a krel of 3.7 × 10−4 was determined (for the rate constant of the ionization step) with the mono-fluoro derivative, 3′-fluorogeranyl diphosphate.34 This value agrees well with that obtained for the solvolysis of the corresponding mesylates (krel = 7.7 × 10−4); reactions that proceed via an SN1 mechanism. This was forwarded as evidence for a carbocationic intermediate in the synthase reaction. Alternatively, in the case of protein farnesytransferase, a krel of 1.8 × 10−2 was determined for the mono-fluoro derivative, 3′-fluorofarnesyl diphosphate.26 This value agrees well with that obtained for the SN2 reaction between dimethylallyl p-methoxybenzene sulfonates (with and without a monofluoro substituent at C-3) and azide (krel = 6.1 × 10−2).33 This was forwarded as evidence for an associative mechanism with this enzyme. Fluorinated DMAPP analogs have also been tested with the indole prenyltransferase DMATS, although the mechanistic implications were less clear-cut.30 A krel value of 1.1 × 10−2 was measured when comparing DMAPP to E-F-DMAPP and this was compared to a krel value of 1.5 × 10−3 for the unimolecular solvolysis rates of the corresponding mesylates. The data was interpreted as supporting an electrophilic mechanism for catalysis, and in subsequent studies strong evidence has been presented for the existence of a dimethylallyl carbocation intermediate in the DMATS reaction.29 In this work, a krel value of 1.0 × 10−2 was determined when comparing DMAPP to E-F-DMAPP in the CymD reaction. While this is consistent with an associative mechanism involving an “exploded” transition state bearing considerable carbocation character,42 it is also compatible with a dissociative mechanism involving a discrete carbocation intermediate. Given the observation of PIX in the CymD reaction, it seems likely that the later scenario is at play.
The observation of a primary solvent KIE indicates that a proton transfer is a rate-determining step of catalysis. This very likely corresponds to the removal of the indole NH proton as this is the only proton transfer that must occur during catalysis. Deprotonation would be expected to greatly increase the nucleophilicity of the indole nitrogen, and would directly generate the product N-DMAT without producing a high-energy indolinium species. Deprotonation of the indole NH (pKa 17) generates a reasonably strong base and might be expected to constitute a rate-determining step of catalysis. Such a step is not an unreasonable proposition as there is ample precedence for enzymatic deprotonations of carbon acids with similar pKa’s.43. The deprotonation events depicted in Paths A and C would likely not be rate-determining steps as the NH functionality is much more acidic (particularly in the case of Path A).
Taken together, we suggest that these results best support a mechanism that is a hybrid of Paths A and C (Figure 6). An initial ionization of DMAPP forms a carbocation intermediate. The indole nitrogen then attacks the carbocation with the assistance of a deprotonation by a general base. This deprotonation could occur prior to the attack to form an indole anion, or in concert with the attack. The formation of a dimethylallyl carbocation intermediate is strongly supported by the observation of PIX. The proposed timing of the deprotonation step is supported by the insensitivity of the reaction towards fluorination of the indole ring and by the observation of a solvent kinetic isotope effect. Neither of these latter two observations would be expected if a positively charged indolinium intermediate were formed during catalysis. The deprotonation step also helps to explain how the poorly nucleophilic nitrogen participates in an alkylation reaction. While it is not possible to rule out a similar mechanism involving a deprotonation-assisted reverse prenylation at C-3, followed by a Cope rearrangement, we feel that the barrier to the Cope rearrangement would likely be high enough to be cleanly rate-limiting and mask the solvent isotope effect.
Supplementary Material
Acknowledgments
We thank the Natural Sciences and Engineering Research Council of Canada (to M.E.T.) and the NIH (Grant GM085770 to B.S.M.) for operating funds. We thank Louis Y. P. Luk for synthesizing E-F-DMAPP. We are grateful to both reviewers for very helpful suggestions concerning additional experiments and interpretations that greatly improved the quality of this work.
Abbreviations used
- DFT
density functional theory
- DMAPP
dimethylallyl diphosphate
- DMAT
dimethylallyltryptophan
- DMATS
dimethylallyltryptophan synthase
- E-F-DMAPP
E-3-(fluoromethyl)-2-buten-1-yl diphosphate
- N-DMAT
N-(1,1-dimethyl-1-allyl)-tryptophan
- KIE
kinetic isotope effect
- MESG
2-amino-6-mercapto-7-methylpurine ribonucleoside
- PIX
positional isotope exchange
- PNPase
purine nucleoside phosphorylase
- PPase
pyrophosphatase
Footnotes
This research was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC to M.E.T) and the NIH (Grant GM085770 to B.S.M).
SUPPORTING INFORMATION AVAILABLE
Includes graphical depictions of kinetic data and spectra for selected PIX experiments. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Lindel T, Marsch N, Adla SK. Indole prenylation in alkaloid synthesis. Top Curr Chem. 2011;309:67–130. doi: 10.1007/128_2011_204. [DOI] [PubMed] [Google Scholar]
- 2.Li SM. Prenylated indole derivatives from fungi: structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat Prod Rep. 2010;27:57–78. doi: 10.1039/b909987p. [DOI] [PubMed] [Google Scholar]
- 3.Williams RM, Stocking EM, Sanz-Cervera JF. Biosynthesis of prenylated alkaloids derived from tryptophan. Topics Curr Chem. 2000;209:97–173. [Google Scholar]
- 4.Yu X, Liu Y, Xie X, Zheng XD, Li SM. Biochemical characterization of indole prenyltransferases: Filling the last gap of prenylation positions by a 5-dimethylallyltryptophan synthase from Aspergillus clavatus. J Biol Chem. 2012;287:1371–1380. doi: 10.1074/jbc.M111.317982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonitz T, Alva V, Saleh O, Lupas AN, Heide L. Evolutionary relationships of microbial aromatic prenyltransferases. PLoS ONE. 2011;6:e27336. doi: 10.1371/journal.pone.0027336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li SM. Evolution of aromatic prenyltransferases in the biosynthesis of indole derivatives. Phytochemistry. 2009;70:1746–1757. doi: 10.1016/j.phytochem.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Tello M, Kuzuyama T, Heide L, Noel JP, Richard SB. The ABBA family of aromatic prenyltransferases: broadening natural product diversity. Cell Mol Life Sci. 2008;65:1459–1463. doi: 10.1007/s00018-008-7579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz AW, Lewis CA, Luzung MR, Baran PS, Moore BS. Functional characterization of the cyclomarin/cyclomarazine prenyltransferase CymD directs the biosynthesis of unnatural cyclic peptides. J Nat Prod. 2010;73:373–377. doi: 10.1021/np9006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz AW, Oh DC, Carney JR, Williamson T, Udwary DW, Jensen PR, Gould SJ, Fenical W, Moore BS. Biosynthesis and structures of cyclomarins and cyclomarazines, prenylated cyclic peptides or marine actinobacterial origin. J Am Chem Soc. 2008;130:4507–4516. doi: 10.1021/ja711188x. [DOI] [PubMed] [Google Scholar]
- 10.Zou HX, Xie XL, Linne U, Zheng XD, Li SM. Simultaneous C7- and N1-prenylation of cyclo-L-Trp-L-Trp catalyzed by a prenyltransferase from Aspergillus oryzae. Org Biomol Chem. 2010;8:3037–3044. doi: 10.1039/c002850a. [DOI] [PubMed] [Google Scholar]
- 11.Hinman RL, Lang J. The protonation of indoles. Basicity studies The dependence of acidity functions on indicator strength. J Am Chem Soc. 1964;86:3790–3806. [Google Scholar]
- 12.Otero N, Mandado M, Mosquera RA. Nucleophilicity of indole derivatives: Activating and deactivating effects based on proton affinities and electron density properties. J Phys Chem A. 2007;111:5557–5562. doi: 10.1021/jp0708953. [DOI] [PubMed] [Google Scholar]
- 13.Westermaier M, Mayr H. Electrophilic allylations and benzylations of indoles in neutral aqueous or alcoholic solutions. Org Lett. 2006;8:4791–4794. doi: 10.1021/ol0618555. [DOI] [PubMed] [Google Scholar]
- 14.Lakhdar S, Westermaier M, Terrier F, Goumont R, Boubaker T, Ofial AR, Mayr H. Nucleophilic reactivies of indoles. J Org Chem. 2006;71:9088–9095. doi: 10.1021/jo0614339. [DOI] [PubMed] [Google Scholar]
- 15.Jackson AH, Lynch PP. Electrophilic aromatic substitution in indoles. Part 12. J Chem Soc Perkin Trans II. 1987:1215–1219. [Google Scholar]
- 16.Wu PL, Chu M, Fowler FW. The 1-aza-Cope rearrangement. J Org Chem. 1988;53:963–972. [Google Scholar]
- 17.Voute N, Philip D, Slawin AMZ, Westwood NJ. Studies on the Claisen rearrangements in the indolo[2,3-b]quinoline system. Org Biomol Chem. 2010;8:442–450. doi: 10.1039/b915677a. [DOI] [PubMed] [Google Scholar]
- 18.Gorst-Allman CP, Steyn PS, Vleggaar R. The biosynthesis of roquefortine. Chem Commun. 1982:652–653. [Google Scholar]
- 19.Grundon MF, Hamblin MR, Harrison DM, Logue JND, Maguire M, McGrath JA. Biosynthesis of aromatic isoprenoids. Part 5. J Chem Soc Perkin Trans I. 1980:1294–1298. [Google Scholar]
- 20.Inada S, Nagai K, Takayanagi Y, Okazaki M. The acid-catalyzed rearrangement of 1-allylindoles. A hypothesis for the biogenesis of echinulin-type compounds. Bull Chem Soc Japan. 1976;49:833–834. [Google Scholar]
- 21.Floss HG. Biosynthesis of ergot alkaloids and related compounds. Tetrahedron. 1976;32:873–912. [Google Scholar]
- 22.Seiler M-P. PhD Dissertation No 4574. ETH Zürich; 1970. [Google Scholar]
- 23.Luk LYP, Qian Q, Tanner ME. A Cope rearrangement in the reaction catalyzed by dimethylallyltryptophan synthase? J Am Chem Soc. 2011;133:12342–12345. doi: 10.1021/ja2034969. [DOI] [PubMed] [Google Scholar]
- 24.Balon M, Carmona MC, Munoz MA, Hidalgo J. The acid-base properties of pyrrole and its benzologs indole and carbazole. A reexamination of the excess acidity method. Tetrahedron. 1989;45:7501–7504. [Google Scholar]
- 25.Bordwell FG, Drucker GE, Fried HE. Acidities of carbon and nitrogen acids: The aromaticity of the cyclopentadienyl anion. J Org Chem. 1981;46:632–635. [Google Scholar]
- 26.Huang CC, Hightower KE, Fierke CA. Mechanistic studies of rat protein farnesyltransferase indicate an associative transition state. Biochemistry. 2000;39:2593–2602. doi: 10.1021/bi992356x. [DOI] [PubMed] [Google Scholar]
- 27.Harris CM, Poulter CD. Recent studies of the mechanism of protein prenylation. Nat Prod Rep. 2000;17:137–144. doi: 10.1039/a904110i. [DOI] [PubMed] [Google Scholar]
- 28.Weller VA, Distefano MD. Measurement of the α-secondary kinetic isotope effect for a prenyltransferase by MALDI mass spectrometry. J Am Chem Soc. 1998;120:7975–7976. [Google Scholar]
- 29.Luk LYP, Tanner ME. Mechanism of dimethylallyltryptophan synthase: Evidence for a dimethylallyl cation intermediate in an aromatic prenyltransferase. J Am Chem Soc. 2009;131:13932–13933. doi: 10.1021/ja906485u. [DOI] [PubMed] [Google Scholar]
- 30.Gebler JC, Woodside AB, Poulter CD. Dimethylallyltryptophan synthase. An enzyme-catalyzed electrophilic aromatic substitution. J Am Chem Soc. 1992;114:7354–7360. [Google Scholar]
- 31.Poulter CD, Argyle JC, Mash EA. Prenyltransferase. New evidence for an ionization-condensation-elimination mechanism with 2-fluorogeranyl pyrophosphate. J Am Chem Soc. 1977;99:957–959. doi: 10.1021/ja00445a056. [DOI] [PubMed] [Google Scholar]
- 32.Pongdee R, Liu H-w. Elucidation of enzyme mechanisms using fluorinated substrate analogs. Bioorg Chem. 2004;32:393–437. doi: 10.1016/j.bioorg.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Dolence JM, Poulter CD. A mechanism for posttranslational modifications of proteins by yeast protein farnesyltransferase. Proc Natl Acad Sci USA. 1995;92:5008–5011. doi: 10.1073/pnas.92.11.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poulter CD, Wiggins PL, Le AT. Farnesylpyrophosphate synthetase. A stepwise mechanism for the 1′-4 condensation reaction. J Am Chem Soc. 1981;103:3296–3927. [Google Scholar]
- 35.Poulter CD, Argyle JC, Mash EA. Farnesyl pyrophosphate synthetase. Mechanistic studies of the 1′-4 coupling reaction with 2-fuorogeranyl pyrophosphate. J Biol Chem. 1978;253:7227–7233. [PubMed] [Google Scholar]
- 36.Davisson VJ, Woodside AB, Neal TR, Stremler KE, Meuehlbacher M, Poulter CD. Phosphorylation of isoprenoid alcohols. J Org Chem. 1986;51:4768–4779. [Google Scholar]
- 37.Raushel FM, Villafranca JJ. Positional isotope exchange. CRC Crit Rev Biochem. 1988;23:1–26. doi: 10.3109/10409238809103118. [DOI] [PubMed] [Google Scholar]
- 38.Croteau RB, Shaskus JJ, Renstrom B, Felton NM, Cane DE, Saito A, Chang C. Mechanism of the pyrophosphate migration in the enzymatic cyclization of geranyl and linalyl pyrophosphates to (+) and (−)-bornyl pyrophosphates. Biochemistry. 1985;24:7077–7085. doi: 10.1021/bi00346a009. [DOI] [PubMed] [Google Scholar]
- 39.Mash EA, Gurria GM, Poulter CD. Farnesylpyrophosphate synthetase. Evidence for a rigid geranyl cation-pyrophosphate anion pair. J Am Chem Soc. 1981;103:3927–3929. [Google Scholar]
- 40.Cohn M, Hu A. Isotopic (18O) shift in 31P nuclear magnetic resonance applied to a study of enzyme-catalyzed phosphate-phosphate exchange and phosphate (oxygen)-water exchange reactions. Proc Natl Acad Sci USA. 1978;75:200–203. doi: 10.1073/pnas.75.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steffan N, Unsold IA, Li SM. Chemoenzymatic synthesis of prenylated indole derivatives by using a 4-dimethylallyltryptophan synthase from Aspergillus fumigatus. ChemBioChem. 2007;8:1298–1307. doi: 10.1002/cbic.200700107. [DOI] [PubMed] [Google Scholar]
- 42.Richard JP, Jencks WP. Concerted bimolecular substitution reactions of 1-phenylethyl derivatives. J Am Chem Soc. 1984;106:1383–1396. [Google Scholar]
- 43.Richard JP, Aymes TL. Proton transfer at carbon. Curr Opin Chem Biol. 2001;5:626–633. doi: 10.1016/s1367-5931(01)00258-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.