Abstract
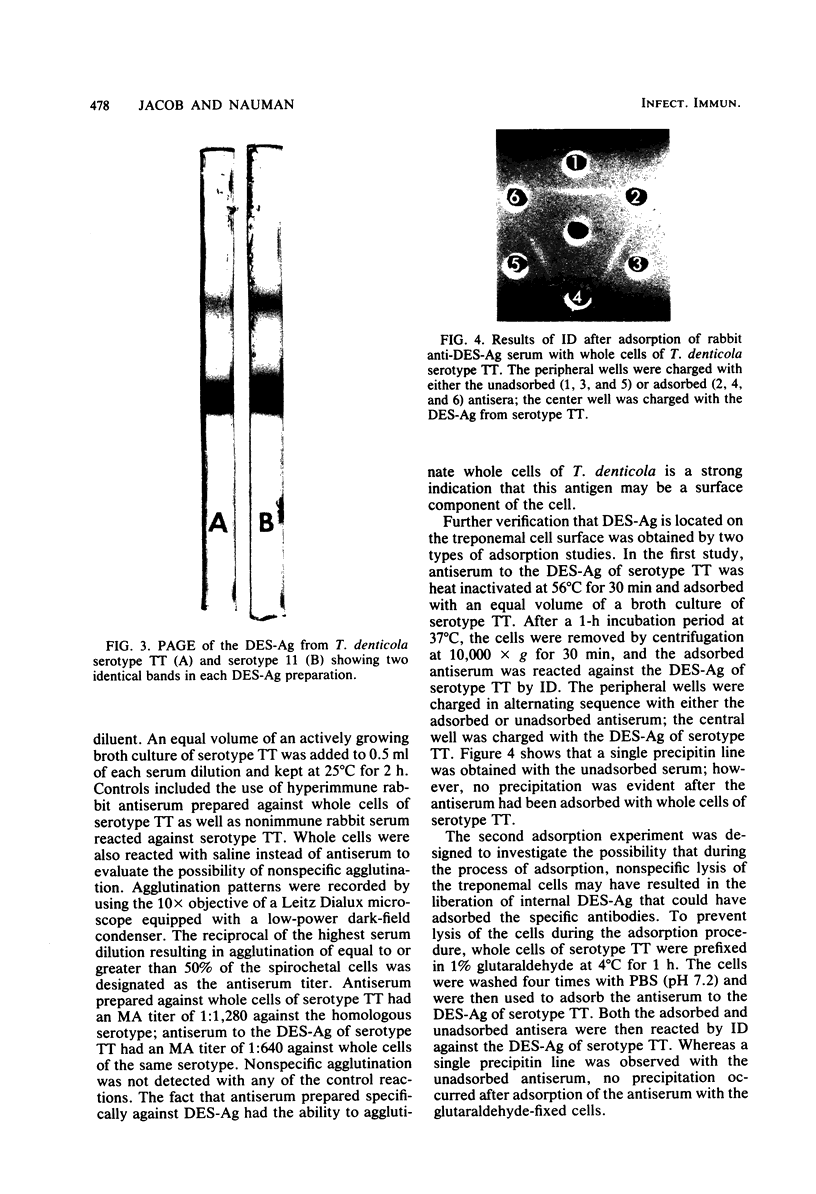
A sodium deoxycholate-ethanol extractable antigen (DES-Ag) was obtained from four serotypes of Treponema denticola and characterized by chemical, physical, and serological procedures. The cross-reactivity of these antigens was demonstrated by indirect microhemagglutination, immunodiffusion, and immunoelectrophoresis with hyperimmune rabbit antiserum against each of the T. denticola serotypes. Antiserum against two nonoral treponemes, T. phagedenis biotype Reieter and T. pallidum Nichols strain, did not cross-react with the DES-Ag of T. denticola. Chemical analysis of the DES-Ag indicated that proteins (84%) and hexoses (12%) accounted for 96% of the total dry weight of the antigen. Trace amounts of N-acetylglucosamine (0.6%) were also detected. Fatty acids, including palmitic, oleic, and stearic, were identified by gas-liquid chromatography. Polyacrylamide gel electrophoresis of the DES-Ag of each serotype revealed the presence of two bands. The molecular weights of the bands were estimated to be 58,000 and 31,000 by comparing the electrophoretic mobility of the DES-Ag to that of five protein standards of known molecular weights. Although only a single precipitin line was observed by immunodiffusion when each antigen was reacted against its homologous antiserum, two precipitin lines were evident by immunoelectrophoresis. Antiserum against the DES-Ag of T. denticola was shown to agglutinate whole cells of the homologous serotype. Adsorption of this anti-DES-Ag serum with whole cells of T. denticola resulted in the elimination of precipitating antibodies to the DES-Ag by immunodiffusion. It is concluded that the DES-Ag is a component of the outer sheath of T. denticola.
Full text
PDF





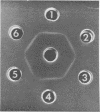
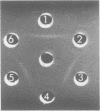
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D., MORGAN W. T. J., WATKINS W. M. Studies in immunochemistry. 11. The action of dilute alkali on the N-acetylhexosamines and the specific blood-group mucoids. Biochem J. 1952 Jun;51(3):379–389. doi: 10.1042/bj0510379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYDEN S. V. The adsorption of proteins on erythrocytes treated with tannic acid and subsequent hemagglutination by antiprotein sera. J Exp Med. 1951 Feb;93(2):107–120. doi: 10.1084/jem.93.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman D. T., Wilson B. L., Moreno E., Angus R. D., Jones L. M. Characterization of Brucella abortus soluble antigen employed in immunoassay. J Clin Microbiol. 1980 Apr;11(4):355–362. doi: 10.1128/jcm.11.4.355-362.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG R. S. M., McCOMB D. E. Erythrocyte sensitizing substance from five strains of Leptospirae. Am J Trop Med Hyg. 1954 May;3(3):481–489. doi: 10.4269/ajtmh.1954.3.481. [DOI] [PubMed] [Google Scholar]
- Jacob E., Allen A. L., Nauman R. K. Detection of oral anaerobic spirochetes in dental plaque by the indirect fluorescent-antibody technique. J Clin Microbiol. 1979 Dec;10(6):934–936. doi: 10.1128/jcm.10.6.934-936.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E., Carter T. B., Nauman R. K. Immunological relationship among oral anaerobic spirochetes as detected by indirect microhemagglutination. J Clin Microbiol. 1980 Oct;12(4):610–613. doi: 10.1128/jcm.12.4.610-613.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob E., Meiller T. F., Nauman R. K. Detection of elevated serum antibodies to Treponema denticola in humans with advanced periodontitis by an enzyme-linked immunosorbent assay. J Periodontal Res. 1982 Mar;17(2):145–153. doi: 10.1111/j.1600-0765.1982.tb01140.x. [DOI] [PubMed] [Google Scholar]
- LOESCHE W. J., SOCRANSKY S. S. Defect in small millipore filters disclosed by new technique for isolating oral treponemes. Science. 1962 Oct 12;138(3537):139–140. doi: 10.1126/science.138.3537.139. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Listgarten M. A., Helldén L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978 May;5(2):115–132. doi: 10.1111/j.1600-051x.1978.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Meyer P. E., Hunter E. F. Antigenic relationships of 14 treponemes demonstrated by immunofluorescence. J Bacteriol. 1967 Mar;93(3):784–789. doi: 10.1128/jb.93.3.784-789.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Lambert M. A., Merwin W. H. Comparison of rapid methods for analysis of bacterial fatty acids. Appl Microbiol. 1974 Jul;28(1):80–85. doi: 10.1128/am.28.1.80-85.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nell E. E., Hardy P. H. Studies on the chemical composition and immunologic properties of a polysaccharide from the Reiter treponeme. Immunochemistry. 1966 May;3(3):233–245. doi: 10.1016/0019-2791(66)90187-x. [DOI] [PubMed] [Google Scholar]
- SCHULTZ-HAUDT S., BRUCE M. A., BIBBY B. G. Bacterial factors in nonspecific gingivitis. J Dent Res. 1954 Aug;33(4):454–458. doi: 10.1177/00220345540330040301. [DOI] [PubMed] [Google Scholar]
- Slots J., Mashimo P., Levine M. J., Genco R. J. Periodontal therapy in humans. I. Microbiological and clinical effects of a single course of periodontal scaling and root planing, and of adjunctive tetracycline therapy. J Periodontol. 1979 Oct;50(10):495–509. doi: 10.1902/jop.1979.50.10.495. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter M. S., Johnson R. C. Treponeme outer envelope: chemical analysis. Proc Soc Exp Biol Med. 1976 Jan;151(1):97–100. doi: 10.3181/00379727-151-39151. [DOI] [PubMed] [Google Scholar]
- Zeigler J. A., VanEseltine W. P. Isolation and chemical characterization of outer envelope of Leptospira pomona. Can J Microbiol. 1975 Jul;21(7):1102–1112. doi: 10.1139/m75-160. [DOI] [PubMed] [Google Scholar]
- Zwaan J. Estimation of molecular weights of proteins by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Nov;21(2):155–168. doi: 10.1016/0003-2697(67)90177-7. [DOI] [PubMed] [Google Scholar]