Abstract
Objective:
To determine whether the absence of early epileptiform abnormalities predicts absence of later seizures on continuous EEG monitoring of hospitalized patients.
Methods:
We retrospectively reviewed 242 consecutive patients without a prior generalized convulsive seizure or active epilepsy who underwent continuous EEG monitoring lasting at least 18 hours for detection of nonconvulsive seizures or evaluation of unexplained altered mental status. The findings on the initial 30-minute screening EEG, subsequent continuous EEG recordings, and baseline clinical data were analyzed. We identified early EEG findings associated with absence of seizures on subsequent continuous EEG.
Results:
Seizures were detected in 70 (29%) patients. A total of 52 patients had their first seizure in the initial 30 minutes of continuous EEG monitoring. Of the remaining 190 patients, 63 had epileptiform discharges on their initial EEG, 24 had triphasic waves, while 103 had no epileptiform abnormalities. Seizures were later detected in 22% (n = 14) of studies with epileptiform discharges on their initial EEG, vs 3% (n = 3) of the studies without epileptiform abnormalities on initial EEG (p < 0.001). In the 3 patients without epileptiform abnormalities on initial EEG but with subsequent seizures, the first epileptiform discharge or electrographic seizure occurred within the first 4 hours of recording.
Conclusions:
In patients without epileptiform abnormalities during the first 4 hours of recording, no seizures were subsequently detected. Therefore, EEG features early in the recording may indicate a low risk for seizures, and help determine whether extended monitoring is necessary.
Seizures are a common problem in critically ill patients with altered mental status or coma,1 and continuous EEG (cEEG) represents the mainstay of diagnosis. Incidence of seizures in hospitalized patients ranges from 8% in comatose patients without clinical evidence of seizures to 48% in patients with prior convulsive status epilepticus and persistent altered consciousness.1–12 In 1 study of 570 patients, 19% had electrographic seizures.5 The first seizure was detected in <24 hours in 95% of noncomatose patients with seizures, and in <48 hours in 87% of comatose patients. Importantly, greater than 90% of patients with seizures had only nonconvulsive seizures (NCS); while it is uncertain if NCS directly cause damage, their presence is associated with worse outcomes.1,3,4,11–21 Consequently, cEEG is increasingly utilized in the care of critically ill patients.
However, it is unknown if any EEG features can identify patients with a low risk of subsequent seizures. While previous studies have suggested that periodic epileptiform discharges are associated with a high risk of seizures,3–5,7 no studies have explored the question of whether early absence of epileptiform activity signifies a decreased likelihood of seizures. We hypothesized that patients without epileptiform features in the first 30 minutes of EEG monitoring would have a lower incidence of subsequent seizures than those with epileptiform features.
METHODS
Study population.
We retrospectively identified all patients who underwent cEEG monitoring at the Massachusetts General Hospital between August 1, 2010, and September 30, 2011. Patients were drawn from all intensive care and inpatient ward settings, with most from the neurologic intensive care unit. cEEG studies were requested primarily by neurology providers. If not already involved, the neurology service was always consulted at the beginning of cEEG monitoring.
Indications for cEEG monitoring were 1) detection of NCS or evaluation of unexplained depressed level of consciousness in patients without a prior witnessed convulsive seizure or recent history of active epilepsy (seizures within the prior year); 2) detection of subclinical seizures in patients with a witnessed generalized convulsive seizure or recent history of active epilepsy; or 3) characterization of spells in alert patients admitted with new or increased seizure-like spells. As the intent of this study was to evaluate the relationship between early EEG findings and subsequent seizures in subjects with intermediate pretest probability of seizures, patients with witnessed convulsive events or a recent history of active epilepsy were excluded. Patients admitted for characterization of spells (e.g., suspected psychogenic seizures) were also excluded, as they represent a distinct population. Patients reported to have had witnessed unilateral “jerking” or “twitching” movements were included, as the differential diagnosis of such movements is broad, and nonseizure etiologies account for a majority of such events.22 Other exclusion criteria included 1) age <18 years and 2) less than 18 consecutive hours of cEEG recording. If a patient underwent more than 1 cEEG recording, only the first cEEG data were included.
Standard protocol approvals, registrations, and patient consents.
This retrospective review of EEG recordings and associated medical records was carried out with the approval of the local institutional review board. Individual patient consent was not required.
Clinical data.
Clinical information was gathered from review of inpatient medical notes, imaging studies and reports, EEG reports, and discharge summaries. Baseline demographic data (age, gender) and any prior medical history of epilepsy were recorded. On the basis of the medical record, the study neurologists determined whether there were witnessed generalized convulsive seizures during or immediately before the acute admission. Seizures were considered convulsive if any of the following terms were in the medical record's description of the reason for monitoring: “generalized tonic-clonic seizures,” “grand mal seizures,” “convulsions,” or other clearcut descriptions of generalized convulsive seizures. Subjects' admission diagnoses were binned into the following categories: ischemic stroke, subarachnoid hemorrhage (SAH), nontraumatic intracerebral hemorrhage, traumatic brain injury (TBI), brain tumor, toxic-metabolic encephalopathy, CNS infection, hypoxic-ischemic encephalopathy (HIE), status-post neurosurgery, unexplained altered mental status, and other miscellaneous causes (such as spontaneous subdural hematomas and posterior reversible encephalopathy syndrome). The data were reanalyzed excluding patients with HIE, as this subpopulation has extremely high early seizure rates and a generally poor prognosis.
EEG recordings.
EEG was recorded using 19 silver/silver chloride electrodes, affixed to the scalp according to the international 10–20 system. A clinical epilepsy fellow and experienced attending neurophysiologist identified the presence and timing of electrographic seizures, periodic epileptiform discharges (PEDs), triphasic waves, and nonperiodic spikes or sharp waves according to previously defined criteria.23 Following routine practice, separate reports were generated for an initial “screening” EEG (range 17–68 minutes, median 22 minutes) and for the subsequent cEEG, ranging from 18 to 70 hours with a median duration of 24 hours for patients without epileptiform discharges on initial EEG. For uniformity, for all patients we analyzed cEEG findings within the initial 30 minutes separately from findings in the later recording. Monitoring was terminated at the discretion of the epilepsy fellow and attending neurophysiologist as part of standard care, with the most common reason being absence of seizures. The primary EEG data were reviewed as necessary to obtain details not included in clinical reports (e.g., timing of the appearance of the first seizure), however, no reclassification of patterns described in the original cEEG clinical reports was made; when the EEG report was ambiguous, the original EEG data were reviewed in order to appropriately classify the findings. For analysis of the relationship between early and later cEEG findings, the findings within the initial 30 minutes of recording were classified into 5 subgroups containing 1) electrographic seizures (regardless of the presence of other abnormalities), 2) PEDs (without organized electrographic seizures, but regardless of isolated spikes and focal or generalized slowing), 3) spikes or sharp waves (without seizures or PEDs), 4) triphasic waves, and 5) EEGs without epileptiform abnormalities.
Statistical analysis.
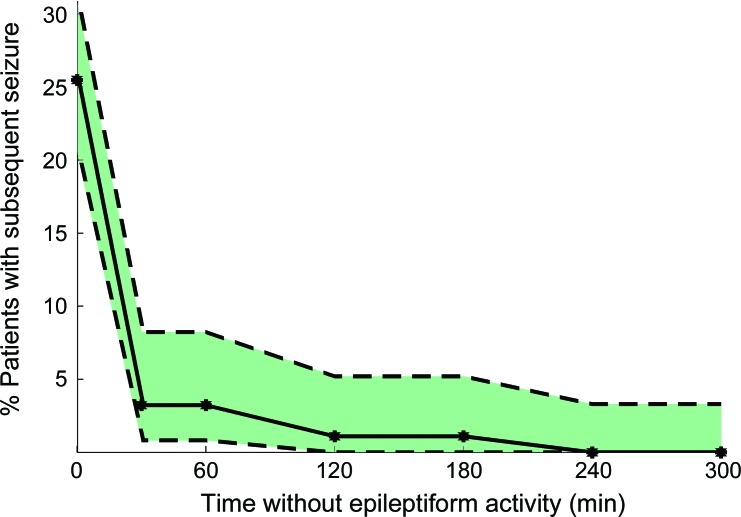
Statistical analysis was performed using Matlab (Natick, MA). In the cohort of patients without seizures during the initial 30 minutes of cEEG monitoring, univariate analysis using χ2 testing or Fisher exact test as appropriate was conducted to identify significant associations with subsequent seizures on cEEG. Estimates for the seizure rate as a function of time (figure 1) were determined from the binomial probability distribution by determining the maximum and minimum rates that could produce the observed seizure frequency within the cohort with at least 5% probability.
Figure 1. Seizure rate as a function of time without epileptiform activity.
The maximum, minimum, and observed seizure rate on subsequent continuous EEG monitoring as a function of time already passed without epileptiform activity (in the cohort of patients without epileptiform activity to that time). The observed seizure rate is indicated with the solid line. The upper dashed line indicates the maximum possible true seizure rate in the underlying population that could have resulted in the observed seizure rate (with a probability p > 0.05); the lower dashed line indicates the minimum possible true seizure rate that could have resulted in the observed seizure rate (with a probability p > 0.05).
RESULTS
Study cohort.
A total of 394 adult patients underwent at least 18 hours of technically adequate continuous EEG monitoring between August 1, 2010, and September 30, 2011. A total of 39 patients with preserved consciousness admitted for characterization of episodic seizure-like spells were excluded. An additional 113 patients were excluded because of a known history of active epilepsy or a witnessed generalized convulsive seizure prior to monitoring, leaving 242 patients who were included in the study (62%); the mean age was 62 ± 18 years (range 18–98 years), and 108 (45%) were female. The most common admission diagnoses for the included patients were altered mental status and TBI (table e-1 on the Neurology® Web site at www.neurology.org).
Early EEG findings.
During the first 30 minutes of cEEG monitoring, 52 patients (21%) had seizures or status epilepticus; 21 patients (9%) had PEDs; 42 (17%) had spikes or sharp waves; and 24 (10%) had triphasic waves. A total of 103 patients (43% of the cohort) had no epileptiform discharges during the initial 30 minutes of cEEG recording. Seizures within the first 30 minutes of monitoring were most frequently present in patients with HIE, toxic-metabolic encephalopathy, and unexplained altered mental status (table e-1). Absence of epileptiform discharges on initial EEG was common in patients with SAH, brain tumors, and TBI. Excluding patients with HIE, seizures were seen in 18% of the initial EEGs; other categories were changed only minimally (9% PEDs, 18% spikes, 11% triphasic waves, 44% no epileptiform discharges).
Seizures on cEEG and time to epileptiform activity.
In the 190 patients without seizures during the first 30 minutes of cEEG monitoring, 18 (9%) subsequently went on to have seizures during the remainder of cEEG monitoring. Pooling the initial 30-minute and subsequent prolonged cEEG monitoring periods, seizures were detected in 70/242 patients (29%); the rate was lowest in patients with TBI and SAH, and highest in patients with HIE, other miscellaneous neurologic diagnoses, and unexplained altered mental status (table 1). A total of 74% of patients with seizures had their first seizure during the first 30 minutes of cEEG monitoring. Excluding patients with HIE, seizures were detected in 54/212 patients (25%); 37 (69%) had their first seizure within the first 30 minutes. Table 2 shows the number of seizures recorded during cEEG monitoring as a function of initial EEG findings in the 190 patients without seizures in the first 30 minutes of cEEG monitoring. Electrographic seizures were eventually seen in 22% of the patients who had early epileptiform discharges (spikes/sharp waves or PEDs within the first 30 minutes of monitoring), vs only 3% of the patients without early epileptiform discharges (p < 0.001). If epileptiform discharges were more broadly defined to include triphasic waves, the subsequent rate of electrographic seizures was 17%; however, seizure rates in patients without early epileptiform discharges were still significantly lower (p < 0.001). In contrast, no single clinical etiology was significantly associated with later seizures (table 3). Excluding patients with HIE had no impact on these findings.
Table 1.
Number of patients with seizures on initial or continuous EEG as a function of admission diagnosis
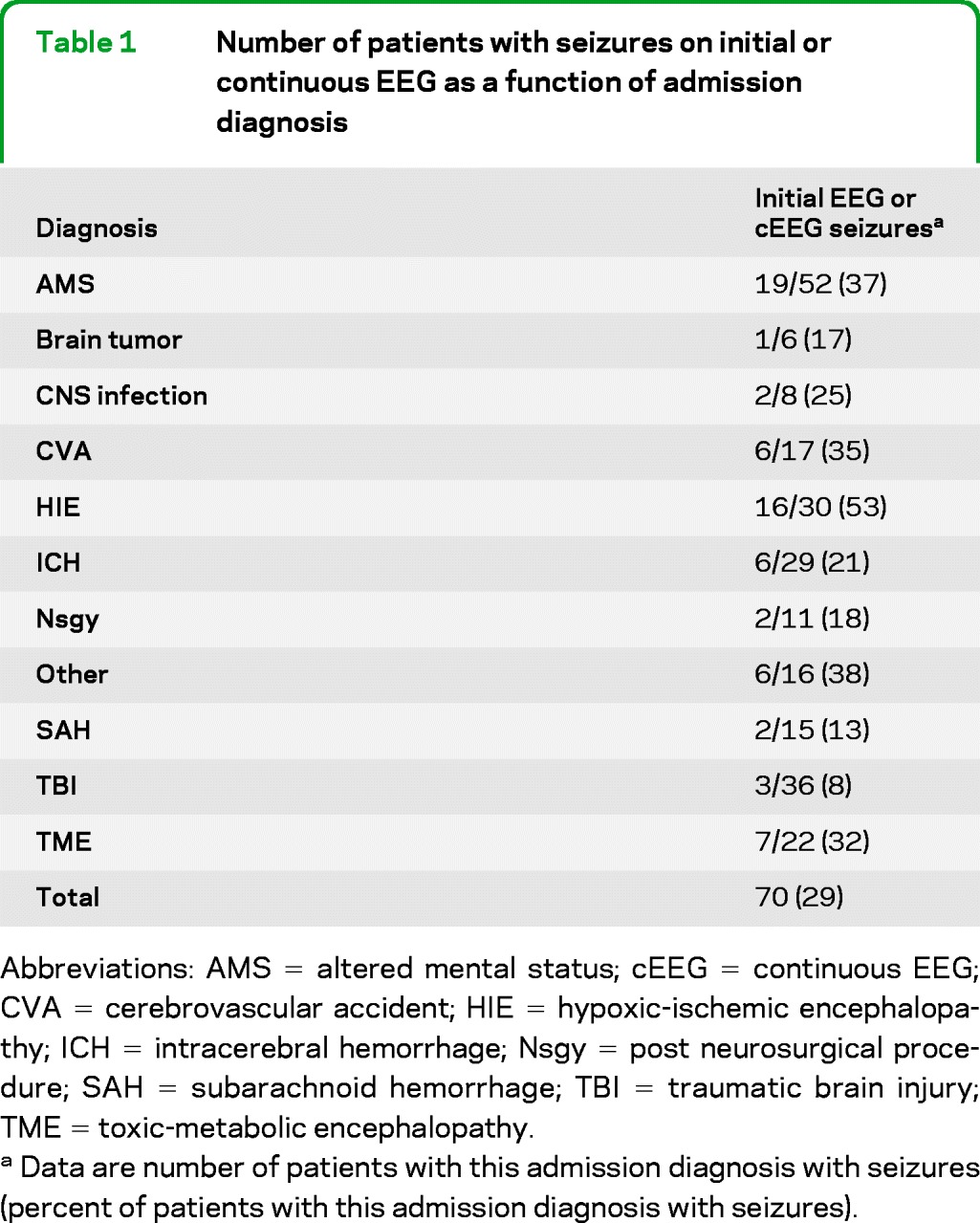
Abbreviations: AMS = altered mental status; cEEG = continuous EEG; CVA = cerebrovascular accident; HIE = hypoxic-ischemic encephalopathy; ICH = intracerebral hemorrhage; Nsgy = post neurosurgical procedure; SAH = subarachnoid hemorrhage; TBI = traumatic brain injury; TME = toxic-metabolic encephalopathy.
Data are number of patients with this admission diagnosis with seizures (percent of patients with this admission diagnosis with seizures).
Table 2.
cEEG seizures as a function of initial EEG findings in the 190 patients without seizures during the first 30 minutes of cEEG monitoring
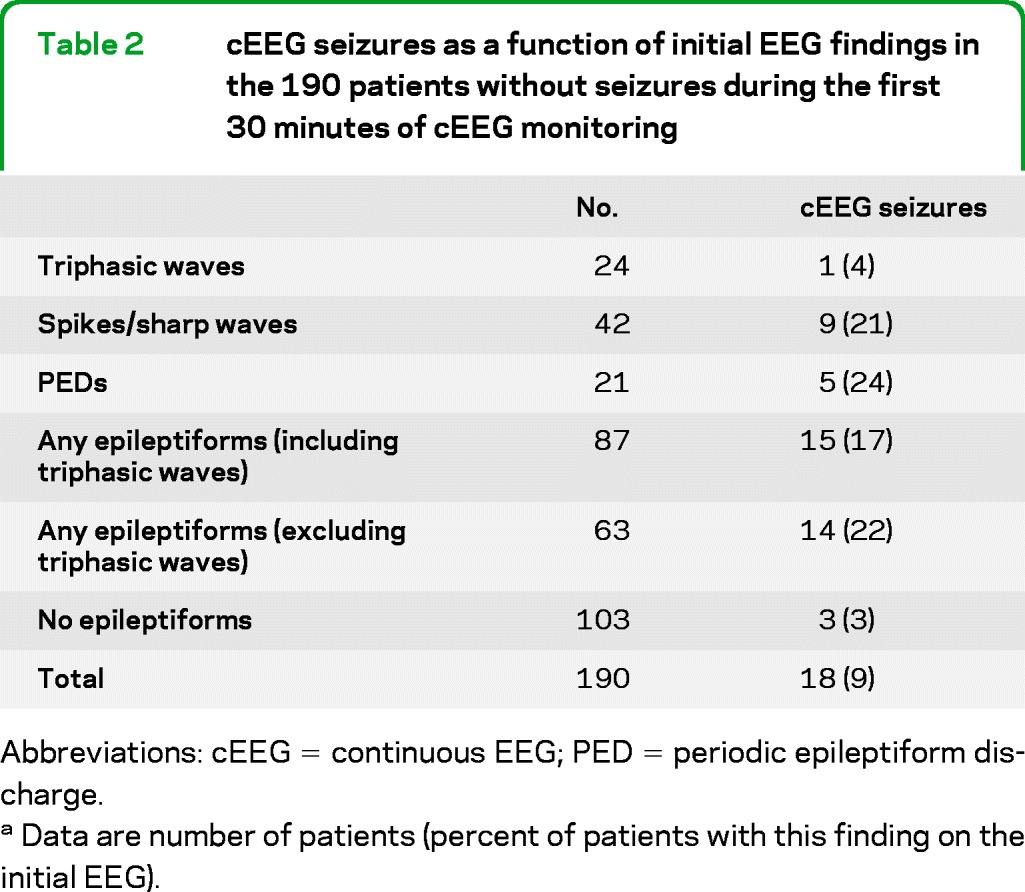
Abbreviations: cEEG = continuous EEG; PED = periodic epileptiform discharge.
Data are number of patients (percent of patients with this finding on the initial EEG).
Table 3.
Delayed seizure rate and associated odds ratios as a function of admission diagnosis and early EEG findings
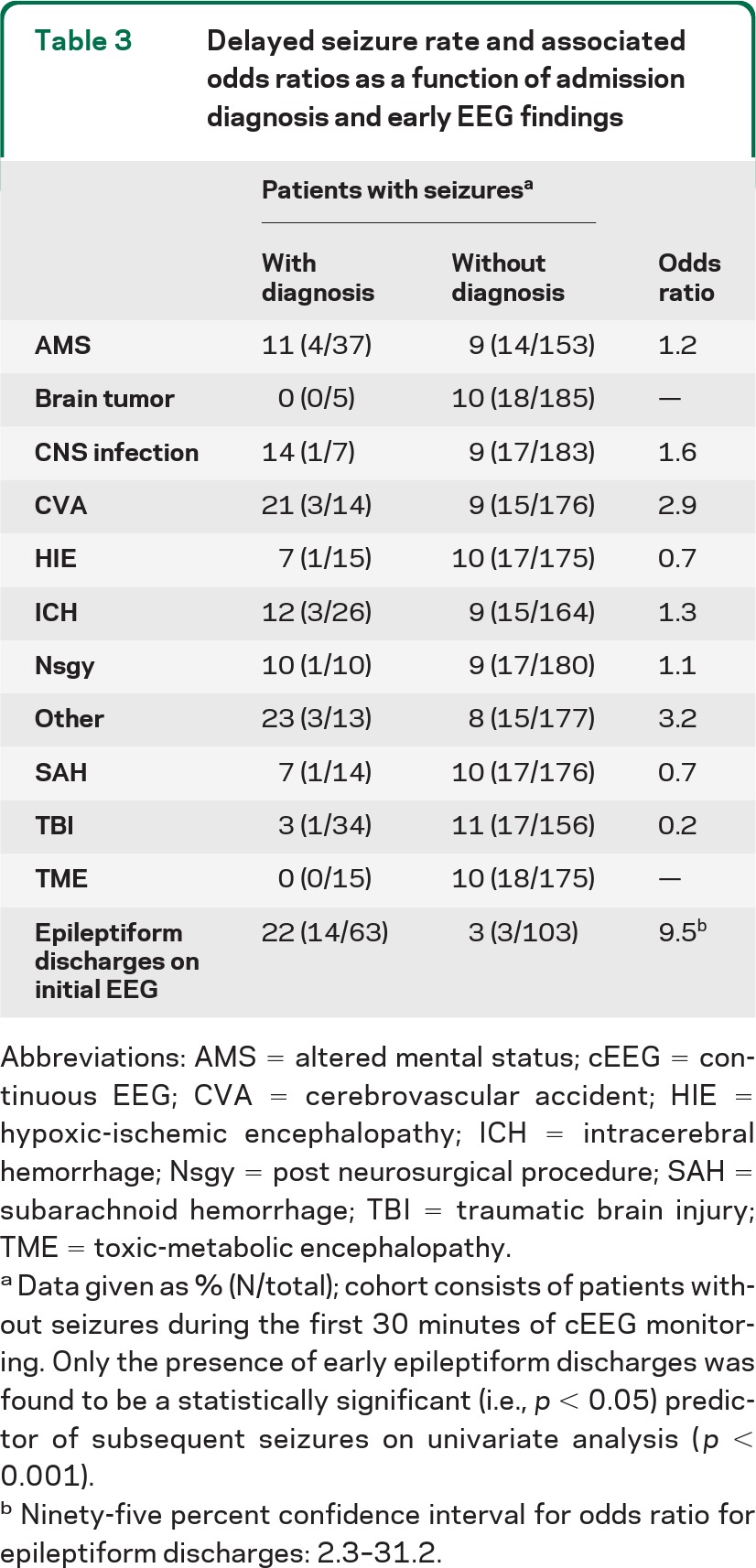
Abbreviations: AMS = altered mental status; cEEG = continuous EEG; CVA = cerebrovascular accident; HIE = hypoxic-ischemic encephalopathy; ICH = intracerebral hemorrhage; Nsgy = post neurosurgical procedure; SAH = subarachnoid hemorrhage; TBI = traumatic brain injury; TME = toxic-metabolic encephalopathy.
Data given as % (N/total); cohort consists of patients without seizures during the first 30 minutes of cEEG monitoring. Only the presence of early epileptiform discharges was found to be a statistically significant (i.e., p < 0.05) predictor of subsequent seizures on univariate analysis (p < 0.001).
Ninety-five percent confidence interval for odds ratio for epileptiform discharges: 2.3–31.2.
The clinical and EEG features of the 3 patients without early epileptiform discharges who went on to have seizures during cEEG monitoring are presented in table e-2. Of note, the first epileptiform feature occurred relatively early in all recordings. Patient 1 had a right temporal seizure (contralateral to his intracerebral hemorrhage) at 63 minutes of recording (figure e-1a), while patient 2 had a left temporal seizure at 64 minutes (figure e-1b). Patient 3 had a left temporal seizure at 225 minutes, heralded by rhythmic monomorphic left frontotemporal delta activity that was visible approximately 175 minutes into the recording (figure e-1c). None of the remaining 100 patients without epileptiform activity during the first 30 minutes had subsequent seizures during the remainder of cEEG monitoring (median 24 hours, range 18–70 hours).
In figure 1, the percentage of subjects with subsequent seizures is compared to the duration of EEG monitoring without epileptiform abnormalities. At the beginning of the recording (before any cEEG data are available), all monitored patients are included in the dataset; the observed seizure rate in this population is 29% (70/242). After 30 minutes, 103 subjects remained in the cohort without any epileptiform discharges; 3 went on to have a seizure. Only 1 of the 101 subjects without epileptiform abnormalities in the first 2 hours of recording had a subsequent seizure, after 225 minutes of monitoring time. No seizures were observed in the 100 patients without epileptiform abnormalities within the first 4 hours of recording. After 4 hours of cEEG without epileptiform abnormalities, the upper bound of the 95% confidence interval is approximately 3%.
DISCUSSION
The absence of epileptiform activity during the first 30 minutes of cEEG monitoring predicts a low risk of subsequent seizures. Subsequent seizures were seen in only 3% of patients without epileptiform abnormalities within the first 30 minutes of monitoring, compared to 22% in those with epileptiform discharges (but not seizures). Furthermore, none of the 100 patients without epileptiform discharges in the first 4 hours of recording subsequently had seizures. Given a cohort of this size, the maximum plausible true seizure rate consistent with the data is ∼3%. Consequently, our study supports the notion that the absence of epileptiform features early in the record indicates a low risk for subsequent seizures, and suggests that prolonged cEEG may not be necessary when the initial EEG is benign and suspicion for seizures is low.
The overall seizure rate in our population was 29%, within the range reported in prior cEEG studies of critically ill neurologic patients,1–12 although somewhat higher than the 19% recorded in the largest single similar study.5 This higher rate may reflect a greater proportion of patients admitted with HIE; excluding these patients, the rate was 25%. There may also be a higher threshold for requesting cEEG monitoring in our hospital, with the result that only patients with a high likelihood of seizures are monitored. Furthermore, the largest previous study5 appears to have had broader inclusion criteria (no clear minimum duration of cEEG monitoring required, vs a minimum of 18 hours in our study). Patients disconnected before 18 hours tended to be those with low clinical suspicion of seizures, improving clinical status, or bland initial cEEG findings. Including those patients would lower the seizure incidence, and the absence of early epileptiform events would be even more predictive of lowered risk of subsequent seizures. In addition, the rate of 21% for patients with intracerebral hemorrhage is within the range reported in that subpopulation in prior studies,4,12 as is our observed rate of 25% in patients with CNS infections.3 In contrast, the seizure rate in our population of patients admitted with TBI (8%) is lower than previously reported (18% to 28%).5,11 One possible explanation is that the patients with TBI in these prior reports had more severe injuries.
Of the 70 patients with seizures during EEG monitoring, 52 (74%) had a seizure within the first 30 minutes, an early seizure rate higher than in similar prior retrospective and prospective studies, in which the rate within the first hour is typically 50%–60%.2,5,17 One possible explanation for this discrepancy is the higher incidence of patients with HIE (whose seizures were often present on initiation of EEG) in our initial dataset; excluding these patients, the early seizure rate was 69%. Only 55% of the patients with acute focal brain lesions and seizures had their first seizure recorded during the initial EEG, similar to prior studies.2,5,17 In contrast, the first seizure was recorded early (within 30 minutes) in patients admitted with altered mental status (15/19 patients, 79%) and toxic-metabolic encephalopathy (7/7 patients, 100%). Thus, another reason for the elevated early seizure rate in our study may be that there is a higher threshold for ordering cEEG monitoring in patients without acute focal brain lesions in our institution, such that these patients only undergo monitoring when suspicion for ongoing seizure activity is high and alternative explanations for altered mental status have been generally ruled out; consequently, in these patients, seizures are present early in the recording. Other possible explanations include differences between study populations, sample size, and interinstitutional variability in defining periodic patterns, particularly periodic epileptiform discharges, as ictal or not. Finally, another important possibility is that since the standard duration of cEEG monitoring in patients without epileptiform discharges was 24 hours in this study, patients with a very delayed onset of epileptiform activity and seizures would not have been identified (see below).
Key limitations of our study include the retrospective design, lack of a strict and uniform protocol for ordering cEEG studies, and variability in treatment adopted for similar cEEG findings, all of which may potentially influence the final statistics. For example, the high early seizure rate noted in patients without acute brain injuries in this study suggests that many such patients not undergoing cEEG monitoring may be having undetected seizures. As in other retrospective studies of seizures in critically ill patients, selection bias probably results in the referral of patients with at least moderate a priori risk of seizures. If all patients were to be included, regardless of suspicion for seizures, the number without epileptiform discharges and without subsequent seizures would presumably be higher, strengthening our results. Between-subject variability in the duration of cEEG monitoring represents another likely confounding factor. Subjects without epileptiform discharges were monitored for a median duration of 24 hours; while previous studies5 suggest that this is sufficient to capture a substantial majority of seizures, there could still be late (undetected) seizures in this population. Therefore, one important hypothesis that cannot be ruled out is that a lack of epileptiform discharges early in the recording signifies a low seizure risk within the next 24 hours, but the subsequent delayed seizure rate is higher (perhaps due to the time course of evolution of the underlying cerebral injury).
As the purpose of this study was to evaluate the relationship between early EEG findings and subsequent seizures in subjects with intermediate pretest probability of seizures, patients with witnessed convulsive events or a recent history of active epilepsy were excluded. We suspect that these patients would have a higher rate of seizures, both early and delayed, regardless of initial EEG findings, and therefore the early EEG would be less informative.
Finally, our data do not permit firm recommendations regarding how the required duration of cEEG monitoring should vary as a function of pretest probability (as informed, e.g., by etiology or clinical data). Indeed, one might expect that longer recording times would be required to detect seizures in patients with a high probability of infrequent events. The data obtained in this and other studies could be used to generate predictions regarding the transition between seizure risk categories. Survival curve analysis could be used to generate detailed statistical models of expected seizure rates as a function of EEG features observed during monitoring. Nevertheless, while our results suggest that early EEG findings predict whether seizures will not be seen during subsequent cEEG monitoring, prospective disease-specific studies with clearly defined indications for starting cEEG and predefined times for assessment of relevant findings are needed to confirm and extend these findings, to clearly define the relationship between cEEG features and the temporal evolution of seizure risk, and for application to patient care. Ultimately, the optimal duration of monitoring will depend on available resources and the maximum tolerable rate of undetected seizures. If resources are constrained and the findings described herein are replicated in prospective studies, then a relatively short duration of monitoring (30 minutes to 4 hours, depending on tolerance for undetected seizures) may be adequate in patients without epileptiform discharges.
Supplementary Material
GLOSSARY
- cEEG
continuous EEG
- HIE
hypoxic-ischemic encephalopathy
- NCS
nonconvulsive seizure
- PED
periodic epileptiform discharge
- SAH
subarachnoid hemorrhage
- TBI
traumatic brain injury
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
M.M.S., M.B.W., and S.S.C. conceptualized and designed the study. M.M.S. and M.B.W. analyzed the data, conducted the statistical analysis, and drafted the original manuscript. A.J.C., R.D.K., D.B.H., and S.S.C. reviewed and revised the manuscript. Principal author: Sydney S. Cash.
DISCLOSURE
M. Shafi receives research support from the National Center for Research Resources: Harvard Clinical and Translational Science Center and the Center for Integration of Medicine and Innovative Technology (CIMIT). M.B. Westover receives research support from the NIH/NINDS (NS062092). A.J. Cole receives research support from the NIH/NINDS (NS062092). R. Kilbride reports no disclosures. D. Hoch receives research support from the NIH/NINDS (NS062092). S. Cash receives research support from the NIH/NINDS (NS062092). Go to Neurology.org for full disclosures.
REFERENCES
- 1. Friedman D, Claassen J, Hirsch LJ. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg 2009; 109: 506– 523 . [DOI] [PubMed] [Google Scholar]
- 2. Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology 2011; 76: 1071– 1077 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carrera E, Claassen J, Oddo M, Emerson RG, Mayer SA, Hirsch LJ. Continuous electroencephalographic monitoring in critically ill patients with central nervous system infections. Arch Neurol 2008; 65: 1612– 1618 . [DOI] [PubMed] [Google Scholar]
- 4. Claassen J, Jetté N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology 2007; 69: 1356– 1365 . [DOI] [PubMed] [Google Scholar]
- 5. Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004; 62: 1743– 1748 . [DOI] [PubMed] [Google Scholar]
- 6. DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia 1998; 39: 833– 840 . [DOI] [PubMed] [Google Scholar]
- 7. Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol 2006; 63: 1750– 1755 . [DOI] [PubMed] [Google Scholar]
- 8. Jordan KG. Neurophysiologic monitoring in the neuroscience intensive care unit. Neurol Clin 1995; 13: 579– 626 . [PubMed] [Google Scholar]
- 9. Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res 1994; 18: 155– 166 . [DOI] [PubMed] [Google Scholar]
- 10. Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology 2000; 54: 340– 345 . [DOI] [PubMed] [Google Scholar]
- 11. Vespa PM, Nuwer MR, Nenov V, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg 1999; 91: 750– 760 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vespa PM, O'Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology 2003; 60: 1441– 1446 . [DOI] [PubMed] [Google Scholar]
- 13. Claassen J, Hirsch LJ, Frontera JA, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care 2006; 4: 103– 112 . [DOI] [PubMed] [Google Scholar]
- 14. Drislane FW. Evidence against permanent neurologic damage from nonconvulsive status epilepticus. J Clin Neurophysiol 1999; 16: 323– 331 . [DOI] [PubMed] [Google Scholar]
- 15. Jordan KG, Hirsch LJ. In nonconvulsive status epilepticus (NCSE), treat to burst-suppression: pro and con. Epilepsia 2006; 47 (suppl 1): 41– 45 . [DOI] [PubMed] [Google Scholar]
- 16. Knake S, Rochon J, Fleischer S, et al. Status epilepticus after stroke is associated with increased long-term case fatality. Epilepsia 2006; 47: 2020– 2026 . [DOI] [PubMed] [Google Scholar]
- 17. Pandian JD, Cascino GD, So EL, Manno E, Fulgham JR. Digital video-electroencephalographic monitoring in the neurological-neurosurgical intensive care unit: clinical features and outcome. Arch Neurol 2004; 61: 1090– 1094 . [DOI] [PubMed] [Google Scholar]
- 18. Rossetti AO, Logroscino G, Liaudet L, et al. Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology 2007; 69: 255– 260 . [DOI] [PubMed] [Google Scholar]
- 19. Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology 2003; 61: 1066– 1073 . [DOI] [PubMed] [Google Scholar]
- 20. Waterhouse EJ, Vaughan JK, Barnes TY, et al. Synergistic effect of status epilepticus and ischemic brain injury on mortality. Epilepsy Res 1998; 29: 175– 183 . [DOI] [PubMed] [Google Scholar]
- 21. Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology 1996; 47: 83– 89 . [DOI] [PubMed] [Google Scholar]
- 22. Benbadis SR, Chen S, Melo M. What's shaking in the ICU? The differential diagnosis of seizures in the intensive care setting Epilepsia 2010; 51: 2338– 2340 . [DOI] [PubMed] [Google Scholar]
- 23. Kilbride RD, Costello DJ, Chiappa KH. How seizure detection by continuous electroencephalographic monitoring affects the prescribing of antiepileptic medications. Arch Neurol 2009; 66: 723– 728 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.