Abstract
Objectives:
We describe temporal trends in stroke incidence stratified by age from our population-based stroke epidemiology study. We hypothesized that stroke incidence in younger adults (age 20–54) increased over time, most notably between 1999 and 2005.
Methods:
The Greater Cincinnati/Northern Kentucky region includes an estimated population of 1.3 million. Strokes were ascertained in the population between July 1, 1993, and June 30, 1994, and in calendar years 1999 and 2005. Age-, race-, and gender-specific incidence rates with 95 confidence intervals were calculated assuming a Poisson distribution. We tested for differences in age trends over time using a mixed-model approach, with appropriate link functions.
Results:
The mean age at stroke significantly decreased from 71.2 years in 1993/1994 to 69.2 years in 2005 (p < 0.0001). The proportion of all strokes under age 55 increased from 12.9% in 1993/1994 to 18.6% in 2005. Regression modeling showed a significant change over time (p = 0.002), characterized as a shift to younger strokes in 2005 compared with earlier study periods. Stroke incidence rates in those 20–54 years of age were significantly increased in both black and white patients in 2005 compared to earlier periods.
Conclusions:
We found trends toward increasing stroke incidence at younger ages. This is of great public health significance because strokes in younger patients carry the potential for greater lifetime burden of disability and because some potential contributors identified for this trend are modifiable.
The Greater Cincinnati/Northern Kentucky Stroke Study (GCNKSS) is a retrospective, population-based epidemiology study designed to study trends in stroke incidence and mortality, with an emphasis on racial disparities.1–4 As in other studies,5,6 we have observed a decrease in overall stroke incidence in white patients over time, although a similar trend was not observed for African Americans.4
Several factors led us to examine stroke incidence trends in younger age groups. We previously reported on diabetes-specific risk for stroke incidence, noting that diabetes is a very powerful risk factor for stroke in those under age 55.7 Given our diabetes stroke incidence results, and given the increasing prevalence of diabetes and obesity among younger people,8–10 more strokes might be expected at younger ages. Furthermore, we made the clinical observation in the early 2000s that a higher proportion of younger stroke patients were being admitted to our main teaching hospital. We thus hypothesized that we would see more strokes in the young over time, specifically hypothesizing that there would be more young strokes in the 2005 study period as compared with earlier study periods with a trend toward younger stroke age over time.
METHODS
We ascertained stroke events that occurred in the population during 3 year-long study periods: between July 1, 1993, and June 30, 1994, and in calendar years 1999 and 2005. Details of our case ascertainment and how methods have been held constant among the 3 periods have been previously published.4 The GCNK region includes 2 Ohio counties and 3 contiguous Northern Kentucky counties that border the Ohio River with 19 hospitals active in 1993/1994, 18 in 1999, and 17 in 2005. Only residents of the 5 study counties are considered for case ascertainment. This study was approved by the Institutional Review Board at all participating hospitals for each study period.
Study nurses screened the medical records of all inpatients and emergency department visits with primary or secondary stroke-related ICD-9 discharge diagnoses (430–436) from all acute care hospitals in the study region. Additional strokes were ascertained by reviewing 1) cases for which stroke was listed as the primary or secondary cause of death by 1 of the 5 county coroners' offices; 2) all stroke-related visits to local public health clinics and hospital-based outpatient clinics; and 3) records of potential stroke cases in a random sample of primary care physicians' offices and nursing homes in the GCNK region. Details of the exact number of sites by study period may be found in table e-1 on the Neurology® Web site at www.neurology.org. Sampling was necessary given the large number of physician offices and nursing homes in the region. Sites were selected randomly, for each study period, by the study statistician from a list generated from a combination of the local yellow pages and the American Medical Association listing of physicians. All events were cross-checked within and between sources to prevent double counting.
To qualify as an incident case, a patient must have met the clinical criteria for one of the stroke categories adapted from the Classification for Cerebrovascular Diseases III11 and used in our previous work1–4: cerebral ischemia, intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), or stroke of uncertain cause. Imaging results were not considered in this clinical definition except for the presence of hemorrhage on CT or MRI for hemorrhagic events. Only first-ever events were included for this analysis. TIA, defined as symptoms lasting <24 hours regardless of imaging results, were not included. The onset of stroke symptoms must have occurred within the study time periods; discharge lists were screened for 60 days beyond the end of each study period to capture patients with a stroke in the period but not discharged until later.
Study nurses abstracted information on all potential cases regarding stroke symptoms, physical examination findings, past medical/surgical history (including stroke risk factors), prestroke medication use, social history/habits, prehospital evaluation, vital signs and emergency room evaluation, neurologic evaluation, diagnostic test results (testing, EKG/cardiac testing, and all neuroimaging), treatments, outcome, type of insurance, and current address. Classification of race/ethnicity was as self-reported in the medical administrative record; ethnicity was not uniformly collected. The study population of the GCNK region consists of <3% Hispanic and other minorities; all self-identified black or white subjects were included in our analysis. Height and weight information was not collected in 1993/1994, only partially collected in 1999, and collected throughout all of 2005.
Study physicians reviewed every abstract to verify whether a stroke or TIA had occurred, then assigned stroke category and mechanism to each event based on all available information using definitions described previously.
Descriptive statistics included means or medians, as appropriate. In testing for trends over time, we tested for significant changes between periods as well as for overall time trend, with the null hypothesis of no change. We used a mixed-model approach for both categorical and continuous variables to account for the weighting and sampling scheme. We adjusted for multiple comparisons using the Bonferroni correction.
The numerator for incidence rate calculation was the number of first-ever strokes confirmed by physician review, ascertained through inpatient records or emergency departments, plus the number of first-ever strokes ascertained through public health clinics, hospital-based outpatient clinics and family practice centers, and coroners' offices, plus a weighted estimate of the number of strokes ascertained only in the physician's office or nursing home. As an example, events ascertained in physicians' offices and nursing homes for 2005 events were multiplied approximately 16- and 5-fold, respectively, to account for the sampling methodology. Events were considered to be noncases if medical records could not be located to confirm the event.
The denominator for the incidence rate calculation was obtained from the US Census Bureau Web site (www.census.gov). These estimates are based on extrapolation or interpolation of county population between enumerated census years, accounting for births, deaths, and migration. These denominators were not adjusted to exclude those with prior stroke, as we do not have accurate prevalence estimates in our population for each study year, only from the telephone surveys (see next paragraph). The at-risk population included 218,906 black patients and 1,100,950 white patients for 2005.
The 95% confidence intervals for the incidence rates were calculated assuming a Poisson distribution. Age-, race-, and gender-specific rates were also determined. All adjusted rates were standardized to the 2000 US population. Rates were calculated for adults only (age ≥20).
To examine changes in risk factor prevalence, we utilized data from 3 random-digit dialing telephone surveys performed in our study region during 1995,12 2000,13 and 2005,14 as well as risk factor prevalence data from the National Health and Nutrition Examination Surveys (NHANES).15–17 Survey methods have been previously described and our full population survey results have been reported.12–14
RESULTS
The raw number of first-ever strokes ascertained among residents ≥20 years of age was 1,942 in 1993/1994, 2,034 in 1999, and 1,916 in 2005. The numbers of strokes ascertained only via hospital admission were 1,907, 1,995 and 1,883, respectively. The demographics of these defined stroke populations were similar as seen in table 1, although the 2005 first-ever stroke population had a significantly higher proportion of black patients (p = 0.02) and was younger than both the 1993/1994 and 1999 populations (p < 0.0001). Significant increases were seen in the proportion of strokes among those 20–44 and those 20–54 (see table 1), with proportions increasing from 4.1% to 6.4% and from 12.9% to 18.6%, respectively, between 1993/1994 and 2005.
Table 1.
Demographics for first-ever strokes age ≥20 yearsa
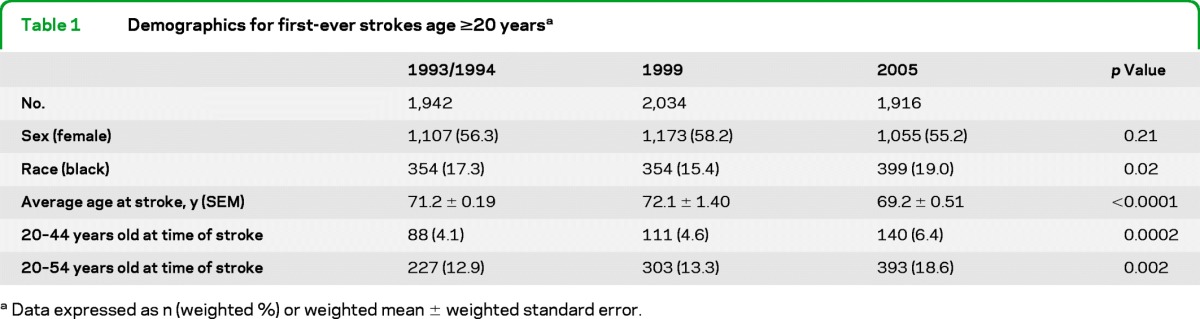
Data expressed as n (weighted) or weighted mean ± weighted standard error.
Table 2 shows age- and race-specific incidence trends. In both black and white patients, there were trends toward stroke incidence rates increasing in the younger ages and decreasing among the oldest subjects. Statistically significant increases in the rates of first-ever stroke were seen for white patients aged 20–44, and for both black and white patients when combining our 2 lowest age groups (for those 20–54 years old). Statistically significant decreases were seen only for white patients aged 75–84 and ≥85. Similar directional trends were seen in black patients but the smaller number of events limits power.
Table 2.
Age-specific incidence rates for first-ever stroke, excluding TIAs, per 100,000 (for 1993/1994, 1999, 2005; sex-adjusted to 2000 US population)
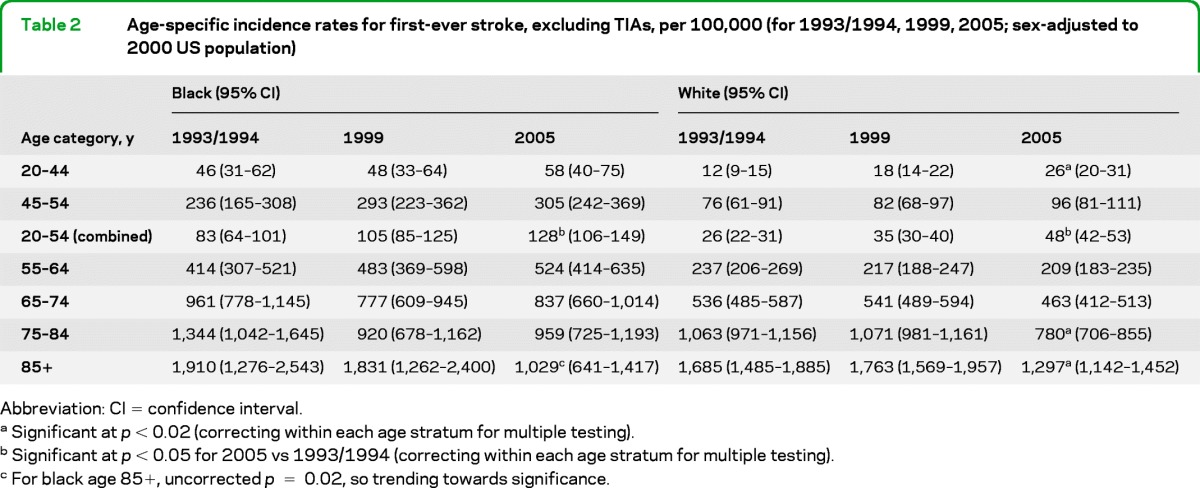
Abbreviation: CI = confidence interval.
Significant at p < 0.02 (correcting within each age stratum for multiple testing).
Significant at p < 0.05 for 2005 vs 1993/1994 (correcting within each age stratum for multiple testing).
For black age 85+, uncorrected p = 0.02, so trending towards significance.
Increases were seen primarily in ischemic stroke. Among our first-ever strokes we found that there is a trend toward a higher proportion of ischemic stroke and lower hemorrhagic stroke in ages 20–44, especially in 2005 compared to earlier periods (table 3). Among those 45–54 years old, the proportion of hemorrhagic stroke increased slightly from 1993/1994 to later periods. The only statistically significant change was a decrease in ischemic stroke for the 45- to 54-year-olds. Incidence rates for ICH and SAH did not change significantly over time (data not shown).
Table 3.
Stroke etiologya
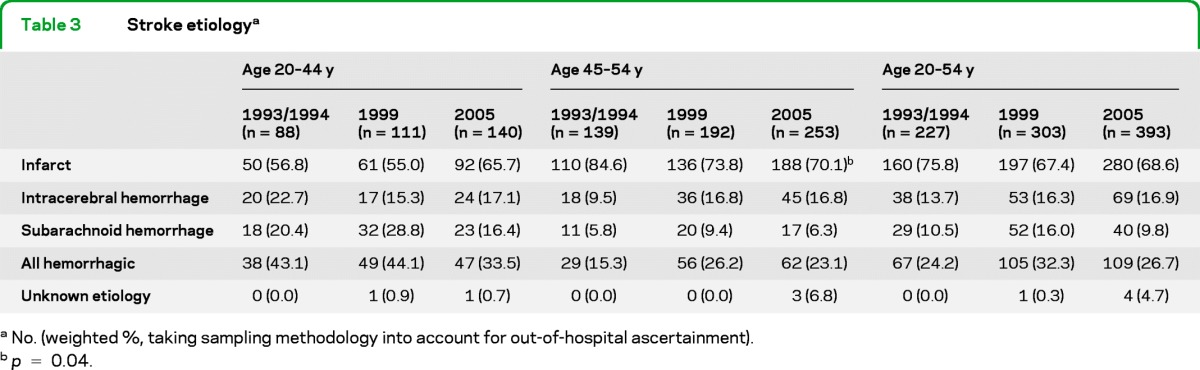
No. (weighted, taking sampling methodology into account for out-of-hospital ascertainment).
p = 0.04.
National data show that stroke risk factors (especially diabetes and obesity) are increasing at younger ages,18–20 and thus likely increasing stroke risk in the young. Table 4 shows risk factor prevalence results of a telephone-based random-digit dialing survey in our region for subjects 20–54 years old (middle panel) in comparison to risk factor prevalence from stroke subjects in these same periods (left panel). These data show that among the survey respondents in our population, there was a significant increase over time in the prevalence of high cholesterol (p = 0.04 for trend). Surprisingly, the prevalence of diabetes was statistically unchanged over time among survey participants, although the number surveyed in the younger age group is small. Among our stroke patients (left panel) the only significant trend was more coronary heart disease (CHD) reported in 1999 and 2005 as compared to 1993/1994; this is in contrast to our population survey results (middle panel) where the prevalence of CHD did not change over time. As would be expected, the prevalence of stroke risk factors including hypertension, diabetes, CHD, and current smoking are all elevated in the younger stroke population compared to the population survey, whereas the prevalence of high cholesterol surprisingly is lower. Table 4 shows NHANES national prevalence data in the right-hand panel for comparison.
Table 4.
Risk factor prevalence in Cincinnati population-based surveys, comparable Cincinnati stroke populations, and NHANES for age 20 to 54 y
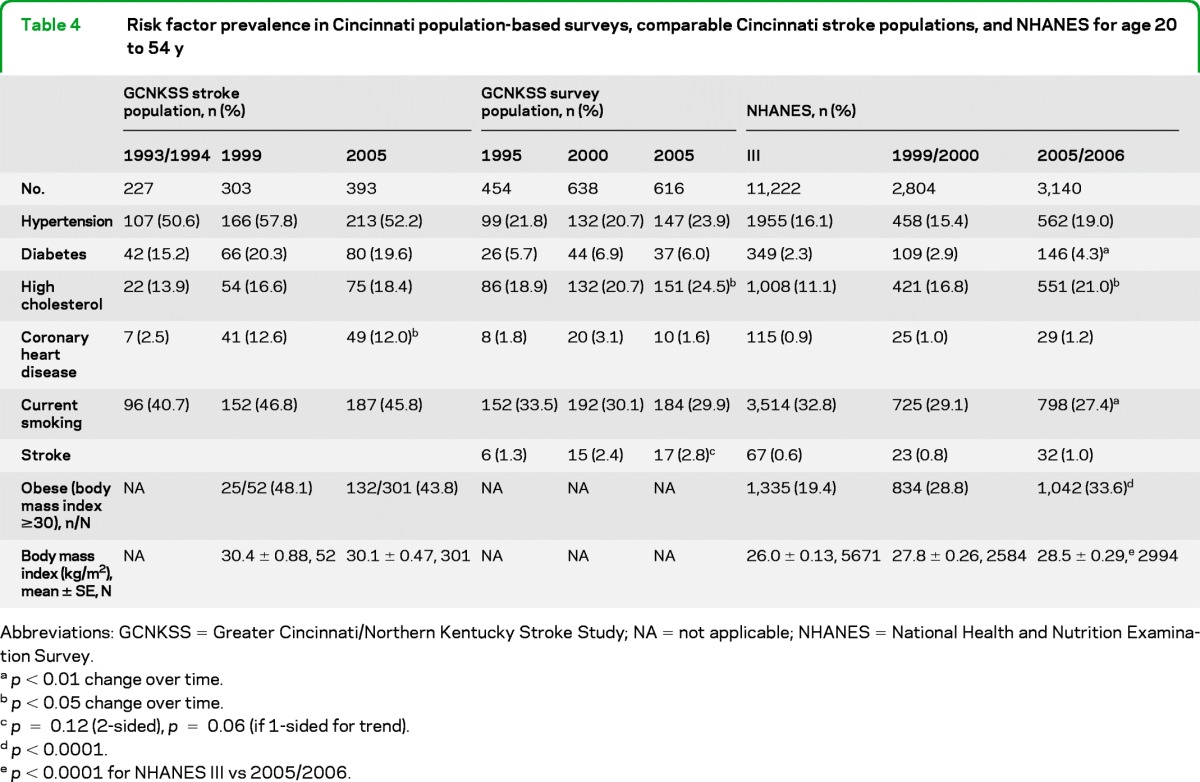
Abbreviations: GCNKSS = Greater Cincinnati/Northern Kentucky Stroke Study; NA = not applicable; NHANES = National Health and Nutrition Examination Survey.
p < 0.01 change over time.
p < 0.05 change over time.
p = 0.12 (2-sided), p = 0.06 (if 1-sided for trend).
p < 0.0001.
p < 0.0001 for NHANES III vs 2005/2006.
Table 5 shows imaging rates across the 3 study periods, and demonstrates a significantly higher rate of MRI obtained over time. Only 18% of all first-ever stroke patients received an MRI in 1993/1994 compared to 27% in 1999 and 58% in 2005. Importantly, the proportion receiving MRI varied significantly by age, with younger patients more likely to receive MRI in 2005 vs prior periods. There were significant differences in obtaining MRI by age (p < 0.0001) and study period (p < 0.0001), but the age × period interaction was not statistically significant. Thus while younger patients had higher rates of receiving MRI, the relative increase in imaging over time was similar across all ages. It is important to note that the clinical stroke definition used for calculating incidence rates did not include imaging findings except for assessing hemorrhage.
Table 5.
Rates of CT and MRI obtained in first-ever stroke patientsa
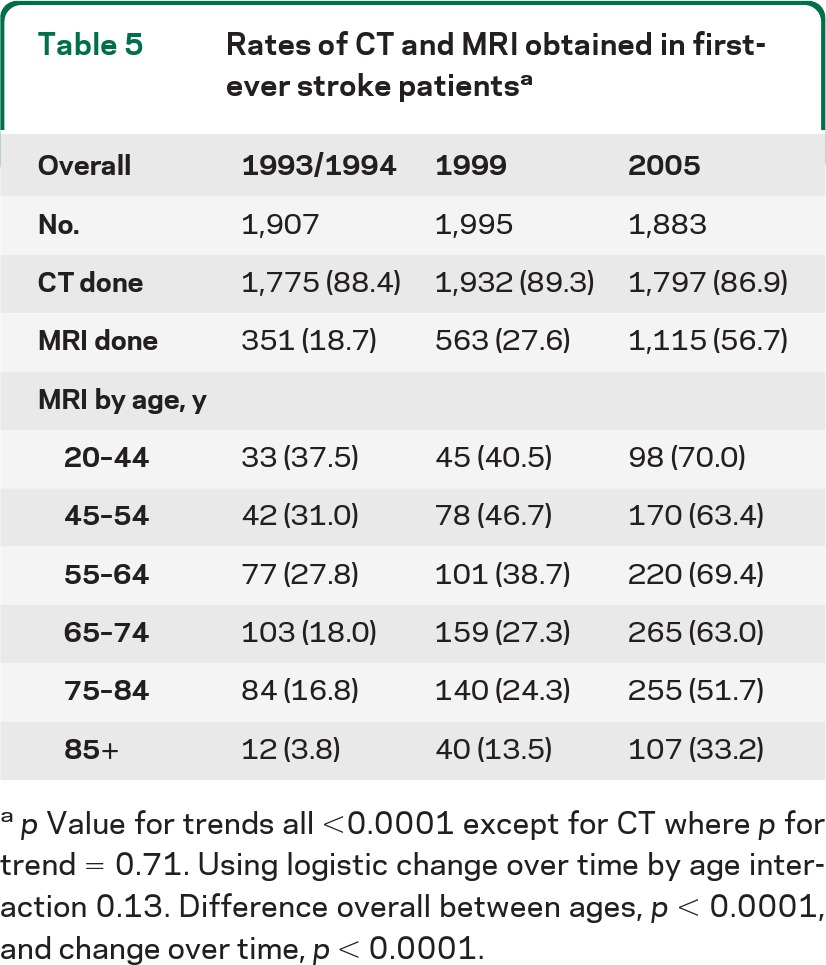
p Value for trends all <0.0001 except for CT where p for trend = 0.71. Using logistic change over time by age interaction 0.13. Difference overall between ages, p < 0.0001, and change over time, p < 0.0001.
Documentation of street drug abuse in the medical record of stroke patients increased from 0.6% to 1.9% to 5.9% in 1993/1994, 1999, and 2005, respectively. Street drug abuse documentation was more prevalent among stroke patients in the youngest ages. In 2005, 23% of first-ever stroke patients age 20–44 used street drugs, compared to 21% of the 45- to 54-year-olds and 2.2% for those 55 and older. Street drug abuse was reported in 11% of ICH and 15% of SAH, but only 4% of ischemic stroke. Given the lesser frequency of hemorrhagic stroke, substance abuse contributes to the total burden of young stroke in only a minor way. However, the relatively high proportion of street drug use by hemorrhage patients might explain the increase in ICH seen in later periods (table 3).
DISCUSSION
In our population-based study, we found increased incidence of ischemic stroke in the young (age 20–54) for both black and white patients over time, especially in 2005 compared to earlier study periods. We also saw declining stroke incidence rates in the oldest age groups for black and white patients. Our incidence rate changes are supported by a trend toward higher rates of prevalent stroke among 20- to 54-year-olds in our population survey.
Our results must be taken within the context of changing trends in stroke incidence. Stroke incidence rates are declining over time in white patients within our population and others,4–6 although a similar trend was not seen in black patients within the Greater Cincinnati region.4 Any decline in stroke incidence is positive from a public health prospective, but reduced incidence in older ages is counterbalanced by the worrisome trend of younger strokes with substantial productive life years lost and immense health care expenses over time.
Other studies regarding stroke in younger age groups have reported incidence rates ranging from 3–23 per 100,000 over the last 3 decades.21–24 None of these studies presented incidence trends over time.
George et al.25 reported trends in younger stroke. They utilized the Nationwide Inpatient Sample of the Healthcare Cost and Utilization Project and identified hospitalized strokes between 1995 and 2008 using ICD-9 codes. They reported an increased prevalence of hospitalizations for ischemic stroke in all age groups <45 years over this time period except for females age 5–14. Prevalence of stroke risk factors including hypertension, diabetes, obesity, hyperlipidemia, and smoking increased during their study period among hospitalized patients aged 15–44.
The reasons for our incidence trends are not clear. SAH is more common in young adults, and hemorrhages account for a higher proportion of childhood and young adult strokes,26–29 but the younger age of strokes in our population is not attributable to an increase in hemorrhagic stroke. Young persons with stroke have significantly different etiologic subtype patterns as compared to older patients.21,23–24 There was a notable increase in CHD prevalence in our young stroke subjects. A detailed analysis of ischemic stroke etiologic subtype will be forthcoming; whether more cardioembolic strokes can explain some of the incidence trends is beyond the scope of this article. Increasing rates of drug abuse were seen in our young stroke patients over time. In another study, recent illicit drug use occurred in 12.1% of young stroke patients30 whereas another report documented cocaine use in 27%–38% of young stroke patients.27 Reported rates of illicit drug use in our work, and others, must be viewed with caution as documentation is not uniform in the medical record.
It is possible that the trend toward younger stroke is related to changing risk factor prevalence. We compared our local prevalence data to the national NHANES survey periods in 1988–1994, 1999–2000, and 2005–2006.15–17 These NHANES periods, comparable to our study periods, show a significant trend toward more diabetes, high cholesterol, and obesity over time among survey respondents age 20–54 (table 4, right panel), consistent with known national trends from other studies.23,31,32 The prevalence changes seen in our population did not exactly match those in the nationally representative NHANES study; this may be due to differences in our community or by chance given the small numbers of young subjects sampled in our survey. Many young adults do not see a doctor regularly and prevalence estimates may thus be underestimates. Among our stroke subjects, we saw small increases in stroke risk factors and a significant increase in CHD in the last 2 study periods, consistent with George et al.25
Increasing stroke risk factors in the young should lead to earlier strokes, assuming that stroke is often the end result of sustained risk factors. Stroke prevention treatments may have been applied preferentially to the elderly where physicians expect stroke to occur, and less so in young adults where stroke is considered unlikely. This may partially explain trends in incidence seen over time, but it is not possible to make causal inferences from our population-level data. Given the increase in stroke among those <55 years old, an important public health message is that younger adults should see a physician regularly to monitor their health and risk for stroke and heart disease. Furthermore, physicians seeing young adults must identify and treat risk factors for macrovascular events such as stroke.
A final possibility is that more strokes in the young are being detected due to greater use of MRI. Our data show substantially increased MRI use over time, and younger patients were more likely to receive MRI than older patients. We did not use imaging results in physician adjudications for this incidence analysis, although imaging results could have biased ascertainment via changes in ICD-9 discharge coding, which we used to identify potential cases.
Limitations include the possibility of methodologic drift, especially related to ascertainment (as above) or case determination. Our methodology captures the effect of imaging on incidence, maintaining our case definition while creating a second variable to capture imaging-proven cases that do not meet the clinical case definition. While we remained steadfast in our clinical case definition, adjudicators were not blinded to imaging results and we cannot completely exclude drift in physician adjudication. Further potential limitations include incomplete case ascertainment or differential bias in ascertainment by age independent of imaging. Due to the large size of our metropolitan population, we only study incidence once every 5 years, and cannot look at continuous trends. We cannot therefore rule out that our 2005 incidence rates are aberrant, although our findings match the population survey trend regarding prevalent stroke and the work of George et al.25 Both our risk factor prevalence data and those of NHANES are limited to self-report, and are subject to limitations of telephone surveys and healthy participant bias. We did not capture body mass index in our population survey, and cannot comment on the effect of obesity in our population over time. Each survey period included approximately 600 respondents under age 55, and our ability to measure changes in risk factor prevalence in these age groups is limited.
The strengths of our article include a population-based epidemiologic study that has been carried out periodically over 15 years. Our study includes physician confirmation of suspected cases and we have made a conscientious effort to prevent methodology changes over time. The core group of nurse abstractors and physician adjudicators has remained relatively constant.
Supplementary Material
GLOSSARY
- CHD
coronary heart disease
- GCNKSS
Greater Cincinnati/Northern Kentucky Stroke Study
- ICD
International Classification of Diseases
- ICH
intracerebral hemorrhage
- NHANES
National Health and Nutrition Examination Survey
- SAH
subarachnoid hemorrhage
Footnotes
Editorial, page 1752
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
B. Kissela obtained funding for this study. For this manuscript, he provided conception and design, data collection, data analysis, data interpretation, drafting, and critical review. J. Khoury provided data analysis, conception and design, data interpretation, manuscript drafting, and critical review for this manuscript. K. Alwell provided data collection, conception and design, data interpretation, and critical review for this manuscript. C. Moomaw provided data collection, data analysis, data interpretation, and critical review for this manuscript. D. Woo provided data collection and critical review for this manuscript. O. Adeoye provided data collection and critical review for this manuscript. M. Flaherty provided data collection and critical review for this manuscript. P. Khatri provided data collection and critical review for this manuscript. S. Ferioli provided data collection and critical review for this manuscript. F. De Los Rios La Rosa provided data collection and critical review for this manuscript. J. Broderick provided conception and design and critical review for this manuscript. D. Kleindorfer obtained funding for this study. For this manuscript, she provided conception and design, data collection, data interpretation, drafting, and critical review.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Broderick J, Brott T, Kothari R, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever total incidence rates of stroke among blacks. Stroke 1998;29:415–421 [DOI] [PubMed] [Google Scholar]
- 2. Kissela B, Schneider A, Kleindorfer D, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke 2004;35:426–431 [DOI] [PubMed] [Google Scholar]
- 3. Kleindorfer D, Broderick J, Khoury J, et al. The unchanging incidence and case-fatality of stroke in the 1990s: a population-based study. Stroke 2006;37:2473–2478 [DOI] [PubMed] [Google Scholar]
- 4. Kleindorfer DO, Khoury J, Moomaw CJ, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke 2010;41:1326–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carandang R, Seshadri S, Beiser A, et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA 2006;296:2939–2946 [DOI] [PubMed] [Google Scholar]
- 6. Rothwell PM, Coull AJ, Giles MF, et al. , Oxford Vascular Study. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 2004;363:1925–1933 [DOI] [PubMed] [Google Scholar]
- 7. Kissela BM, Khoury J, Kleindorfer D, et al. Epidemiology of ischemic stroke in patients with diabetes: the Greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care 2005;28:355–359 [DOI] [PubMed] [Google Scholar]
- 8. Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D'Agostino RBS. Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: the Framingham Heart Study. Circulation 2006;113:2914–2918 [DOI] [PubMed] [Google Scholar]
- 9. Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care 2006;29:2114–2116 [DOI] [PubMed] [Google Scholar]
- 10. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 11. National Institute of Neurological Disorders and Stroke Classification of Cerebrovascular Diseases III. Stroke 1990;21:653 [DOI] [PubMed] [Google Scholar]
- 12. Pancioli AM, Broderick J, Kothari R, et al. Public perception of stroke warning signs and knowledge of potential risk factors. JAMA 1998;279:1288–1292 [DOI] [PubMed] [Google Scholar]
- 13. Schneider A, Pancioli A, Khoury J, et al. Stroke warning signs and risk factors: a comparative survey of community knowledge. Stroke 2002;33:391–392 [Google Scholar]
- 14. Kleindorfer D, Khoury J, Broderick JP, et al. Temporal trends in public awareness of stroke: warning signs, risk factors, and treatment. Stroke 2009;40:2502–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey Data. NHANES III. Available at: http://www.cdc.gov/nchs/nhanes/nh3data.htm Accessed December 14, 2004
- 16. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey Data. NHANES 1999–2000. Available at: http://www.cdc.gov/nchs/nhanes/nhanes1999–2000/nhanes99_00.htm Accessed June 2, 2005
- 17. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey Data. NHANES 2005–06. Available at: http://www.cdc.gov/nchs/nhanes/nhanes2005–2006/nhanes05_06.htm; Accessed August 7, 2009
- 18. Lipscombe LL, Hux JE. Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995–2005: a population-based study. Lancet 2007;369:750–756 [DOI] [PubMed] [Google Scholar]
- 19. Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA 2008;299:2401–2405 [DOI] [PubMed] [Google Scholar]
- 20. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010;303:235–241 [DOI] [PubMed] [Google Scholar]
- 21. Kristensen B, Malm J, Carlberg B, et al. Epidemiology and etiology of ischemic stroke in young adults aged 18 to 44 years in northern Sweden. Stroke 1997;28:1702–1709 [DOI] [PubMed] [Google Scholar]
- 22. Jacobs BS, Boden-Albala B, Lin IF, Sacco RL. Stroke in the young in the Northern Manhattan Stroke Study. Stroke 2002;33:2789–2793 [DOI] [PubMed] [Google Scholar]
- 23. Putaala J, Metso AJ, Metso TM, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke 2009;40:1195–1203 [DOI] [PubMed] [Google Scholar]
- 24. Kittner SJ, McCarter RJ, Sherwin RW, et al. Black-white differences in stroke risk among young adults. Stroke 1993;24:I13–I15; discussion I20–I21 [PubMed] [Google Scholar]
- 25. George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995–2008. Ann Neurol Epub 2011 [DOI] [PubMed] [Google Scholar]
- 26. Kleindorfer D, Khoury J, Kissela B, et al. Temporal trends in the incidence and case fatality of stroke in children and adolescents. J Child Neurol 2006;21:415–418 [DOI] [PubMed] [Google Scholar]
- 27. Qureshi AI, Safdar K, Patel M, Janssen RS, Frankel MR. Stroke in young black patients: risk factors, subtypes, and prognosis. Stroke 1995;26:1995–1998 [DOI] [PubMed] [Google Scholar]
- 28. Broderick J, Talbot G, Prenger E, Leach A, Brott T. Stroke in children within a major metropolitan area: the surprising importance of intracerebral hemorrhage. J Child Neurol 1993;8:250–255 [DOI] [PubMed] [Google Scholar]
- 29. Bevan H, Sharma K, Bradley W. Stroke in young adults. Stroke 1990;21:382–386 [DOI] [PubMed] [Google Scholar]
- 30. Sloan MA, Kittner SJ, Feeser BR, et al. Illicit drug-associated ischemic stroke in the Baltimore-Washington Young Stroke Study. Neurology 1998;50:1688–1693 [DOI] [PubMed] [Google Scholar]
- 31. Centers for Disease Control and Prevention (CDC) Office of Surveillance, Epidemiology, and Laboratory Services. Behavioral Risk Factor Surveillance System. Available at: http://apps.nccd.cdc.gov/brfss/index.asp Accessed June 26, 2011
- 32. Rohr J, Kittner S, Feeser B, et al. Traditional risk factors and ischemic stroke in young adults: the Baltimore-Washington Cooperative Young Stroke Study. Arch Neurol 1996;53:603–607 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


