Abstract
Objectives:
Secondary prevention trials in subjects with preclinical Alzheimer disease may require documentation of brain amyloidosis. The identification of inexpensive and noninvasive screening variables that can identify individuals who have significant amyloid accumulation would reduce screening costs.
Methods:
A total of 483 cognitively normal (CN) individuals, aged 70–92 years, from the population-based Mayo Clinic Study of Aging, underwent Pittsburgh compound B (PiB)–PET imaging. Logistic regression determined whether age, sex, APOE genotype, family history, or cognitive performance was associated with odds of a PiB retention ratio >1.4 and >1.5. Area under the receiver operating characteristic curve (AUROC) evaluated the discrimination between PiB-positive and -negative subjects. For each characteristic, we determined the number needed to screen in each age group (70–79 and 80–89) to identify 100 participants with PiB >1.4 or >1.5.
Results:
A total of 211 (44%) individuals had PiB >1.4 and 151 (31%) >1.5. In univariate and multivariate models, discrimination was modest (AUROC ∼0.6–0.7). Multivariately, age and APOE best predicted odds of PiB >1.4 and >1.5. Subjective memory complaints were similar to cognitive test performance in predicting PiB >1.5. Indicators of PiB positivity varied with age. Screening APOE ε4 carriers alone reduced the number needed to screen to enroll 100 subjects with PIB >1.5 by 48% in persons aged 70–79 and 33% in those aged 80–89.
Conclusions:
Age and APOE genotype are useful predictors of the likelihood of significant amyloid accumulation, but discrimination is modest. Nonetheless, these results suggest that inexpensive and noninvasive measures could significantly reduce the number of CN individuals needed to screen to enroll a given number of amyloid-positive subjects.
Alzheimer disease (AD) pathophysiology is thought to begin decades before the emergence of overt clinical symptoms.1–3 The many failed treatment trials among patients with AD dementia, despite reducing brain amyloid levels in some studies, highlight the importance of the preclinical phase of AD for the development of disease-modifying therapies.4 As amyloid pathology appears to be the first pathologic entity necessary for the development of AD that is detectable by currently available biomarkers,5–8 the identification of amyloid-positive individuals is critical for drug development. Secondary AD prevention trials in preclinical subjects are now being designed and some of these trials, especially those with an anti-amyloid mechanistic target, require documentation of brain amyloidosis for enrollment. While the exact proportion of amyloid-positive cognitively normal (CN) individuals depends on the age and genetic background of the cohort, it is estimated that approximately 20%–40% of elderly individuals will have brain amyloidosis.9–11 It is not optimally efficient in terms of cost, time, and manpower to screen an entire population with amyloid imaging or a lumbar puncture for CSF amyloid-β to identify eligible individuals with brain amyloidosis. Thus, the aim of the present study was to examine the utility of inexpensive and easily available screening variables including demographic, family history, cognitive performance (domain-specific and self-reported), and APOE genotype to predict amyloid positivity in a population-based cohort of CN individuals. We further sought to estimate and compare the sample size needed to screen for therapeutic trials with and without the addition of these potential predictors.
METHODS
Subjects.
The Mayo Clinic Study of Aging (MCSA) is a population-based study of cognitive aging that was established in Olmsted County, Minnesota, in October 2004. Details of the study design and participant recruitment are provided elsewhere.12,13 All MCSA subjects undergo a clinical and cognitive assessment every 15 months that includes 9 neuropsychological tests. Beginning in 2006, both newly and previously enrolled subjects were offered the opportunity to undergo PET imaging. Severe illness was the only exclusion criterion for participating in imaging. Subjects were not excluded due to neurologic, psychiatric, or systemic illnesses to preserve the representativeness of the study sample. The evaluations of all subjects were reviewed by a consensus panel consisting of behavioral neurologists, geriatricians, neuropsychologists, and study nurses. Subjects were diagnosed by the consensus panel as being CN based on the clinical assessments including mental status examinations, informant interviews, and the neuropsychological testing battery described below. Imaging findings were not used in forming a clinical diagnosis.
Standard protocol approvals, registrations, and patient consents.
The study protocols were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. All subjects provided signed informed consent to participate in the study and in the imaging protocols.
Neuropsychological testing.
The neuropsychological battery was constructed as previously described.12,13 Domain-specific measures were formulated from the Wechsler Adult Intelligence Scale–Revised (WAIS-R), Wechsler Memory Scale–Revised (WMS-R), Auditory Verbal Learning Test (AVLT), Trail Making Test (TMT), category fluency test, and Boston Naming Test (BNT). Four cognitive domains were assessed: executive (TMT: Part B, WAIS-R Digit Symbol); language (BNT, category fluency); memory (WMS-R Logical Memory–II delayed recall, WMS-R Visual Reproduction–II delayed recall, AVLT delayed recall); and visuospatial (WAIS-R Picture Completion, WAIS-R Block Design). Individual test scores were first converted to z scores using the mean and SD from the MCSA 2004 enrollment cohort. The individual z scores were averaged to create 4 domain-specific z scores. A global cognitive summary score was formed from the average of the 4 domain z scores and then converted to a z score by subtracting the mean and dividing by the SD.
The measurement of subjective memory complaints was adapted from the first 5 questions on the Blessed Memory Test. Questions 1–4 were given a score of 2 if subjects reported “definitely worse than when I was younger,” 1 if they were “slightly worse,” and 0 if they were “as good or better.” Item 5 (problems remembering appointments correctly) was scored 1 for yes and 0 for no. Questions 1–5 were summed for a score ranging from 0 (no concern) to 9 (highest concern).
Imaging methods.
PET images14 were acquired using a PET/CT scanner (DRX, GE Healthcare). A CT image was obtained for attenuation correction. The 11C Pittsburgh compound B (PiB)–PET scan, consisting of 4 5-minute dynamic frames, was acquired from 40 to 60 minutes after injection.15,16 Quantitative image analysis for PiB was done using our in-house fully automated image processing pipeline.10 A cortical PiB-PET retention ratio was formed by calculating the median uptake over voxels in the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus regions of interest (ROIs) for each subject and dividing this by the median uptake over voxels in the cerebellar gray matter ROI of the atlas.17 The atlas and image registration steps were based on a 3D T1-weighted volume MRI sequence. We have previously shown in this cohort that 90% diagnostic sensitivity for clinically diagnosed AD dementia corresponds to a PiB-PET cutpoint of 1.5,18 and therefore included this cutpoint as our outcome. However, as a lower amyloid PiB-PET cutpoint may be better suited to answer the question of amyloid positivity in CN subjects who are earlier in the disease pathway, we also examined predictors of a PiB-PET cutpoint of 1.4.
Statistical methods.
Differences in variables between those with and without PiB-PET scans were evaluated using Wilcoxon rank sum test and χ2 tests. Logistic regression models were used to assess predictors of PiB positivity (PiB retention ratio >1.4 or >1.5) in univariate and multivariate models after adjustment for other covariates. Four multivariate models were fit, each building upon the previous model. The models were structured to reflect the ease with which different categories of predictive information can be acquired (i.e., each model is built to reflect the time and effort burden needed to collect data from study participants). As such, the first model included only predictors that could be easily obtained without patient contact (age and sex), the second model would require a phone call (family history of dementia and subjective memory complaint), the third model would require the patient to have a blood draw (APOE genotype), and the final model included predictors that would require the subject to undergo an extensive neuropsychological battery (global z score). Our goal was not to find “the best” multivariable model, but to fit and summarize a prescribed series of models based on increasing patient burden.
We used the area under the receiver operating characteristic curve (AUROC) from the models as a measure of how well the model discriminated between PiB-positive and -negative subjects. The multivariate logistic models allowed us to assign a predicted probability of being PiB-positive to each subject based on values for the variables in the model. Using these estimated probabilities, we defined those with probabilities >0.50 as being more likely to be PiB-positive and those with probabilities ≤0.50 as more likely to be PiB-negative. The positive predictive value (PPV) of the model was then calculated as the percent of true PiB positives, as identified from the PiB scan, out of the total number of subjects who were identified as likely to be PiB-positive from the model. Similarly, the negative predictive value (NPV) was calculated as the percent of true PiB negatives out of the total number of subjects who were identified as likely to be PiB-negative from the model. For each characteristic, we also estimated the number needed to screen to enroll 100 participants with PiB >1.4 or >1.5 into a clinical trial by age group (70–79 and 80–89 years). The number needed to screen was based on the proportions of the characteristics in the MCSA data.
RESULTS
The characteristics of the 483 CN individuals with a PiB-PET scan are shown in table 1. The median age of the sample was 78 years (interquartile range [IQR] 75, 82) and 55% were male. The median Mini-Mental State Examination (MMSE) score was 28 (IQR 27, 29) and global z score was 0.68 (IQR = 0.13, 1.24). There were 124 individuals (26%) with a family history of dementia, 122 (25%) had at least 1 APOE ε4 allele, 211 (44%) had PiB > 1.4, and 151 (31%) had PiB >1.5. Compared to the other CN MCSA participants enrolled in the study but without a PiB-PET scan, those with a scan (included in this analysis) were significantly (p < 0.05) younger (78 vs 80 years), more frequently men (55% vs 49%), and performed slightly better on the MMSE, global cognition, and each of the 4 cognitive domains (table 1).
Table 1.
Descriptive characteristics of Mayo Clinic Study of Aging (MCSA) cognitively normal subjects participating in the current PET study vs the entire MCSA during the period 2009 to 2010
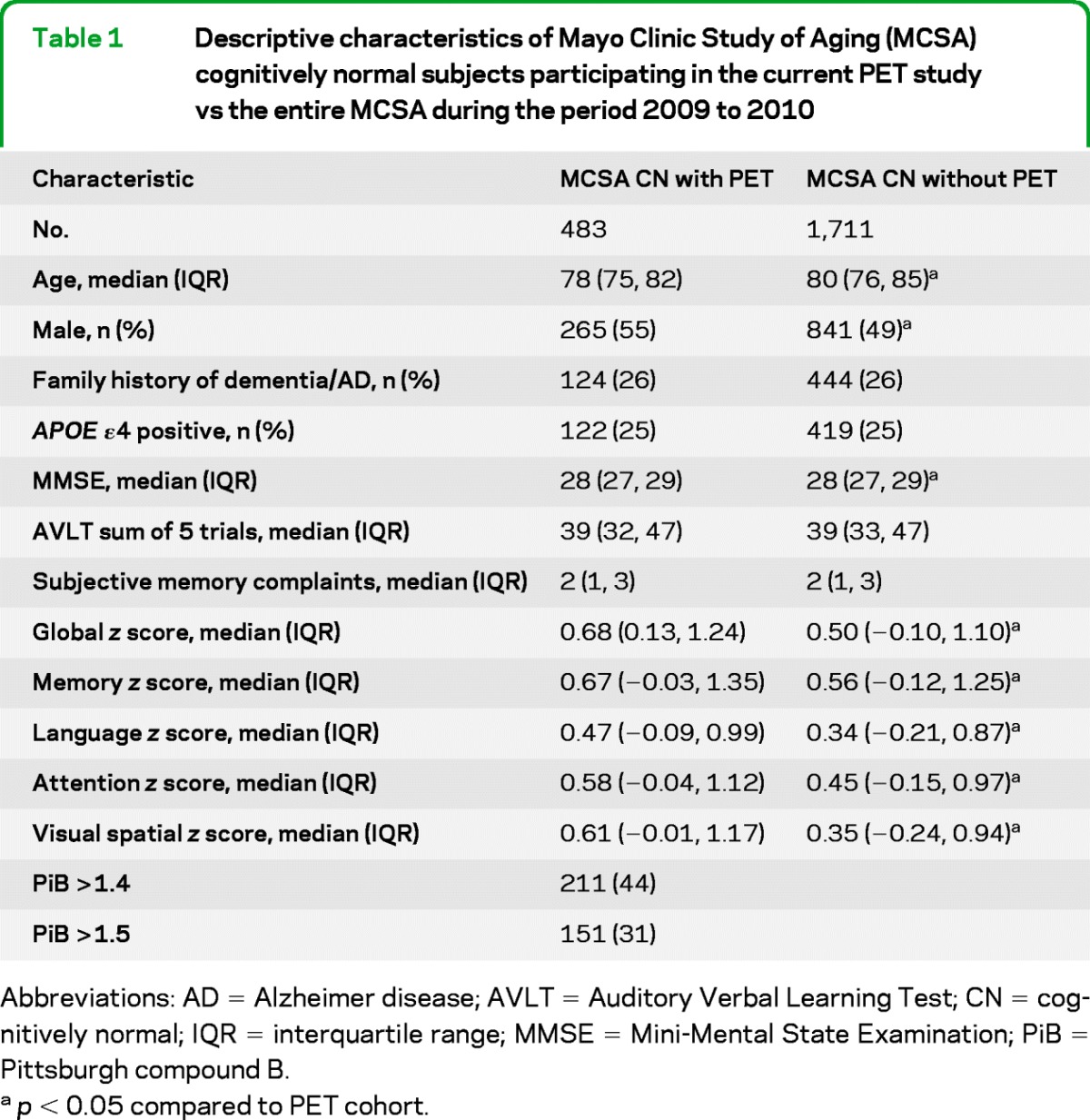
Abbreviations: AD = Alzheimer disease; AVLT = Auditory Verbal Learning Test; CN = cognitively normal; IQR = interquartile range; MMSE = Mini-Mental State Examination; PiB = Pittsburgh compound B.
p < 0.05 compared to PET cohort.
The relative odds of PiB >1.4 and >1.5 are shown in table 2 for the individual effects of age, sex, APOE genotype, family history, cognitive tests, and domain z scores. Participants with an APOE ε4 allele had greater odds of PiB >1.4 (odds ratio [OR] 3.04, 95% confidence interval [CI] 1.98–4.66) and PiB >1.5 (OR 3.16, 95% CI 2.06–4.85). Older age and a family history of dementia/AD were also associated with elevated odds of PiB >1.4 and >1.5, while better cognitive performance across all domains and tests was associated with reduced odds. In univariate models, age had the highest AUROC for discriminating between those with and without PiB >1.4 (AUROC = 0.62), while both age and the presence of an APOE ε4 allele had the highest AUROC for PiB >1.5 (AUROC = 0.61). However, these AUROC values were modest. The AUROCs were similar among the different measures of cognition with the highest AUROC for the global and attention z scores for PiB >1.4 (AUROC = 0.58) and for subjective memory for PiB >1.5 (AUROC = 0.60).
Table 2.
Summary of univariate logistic regression models
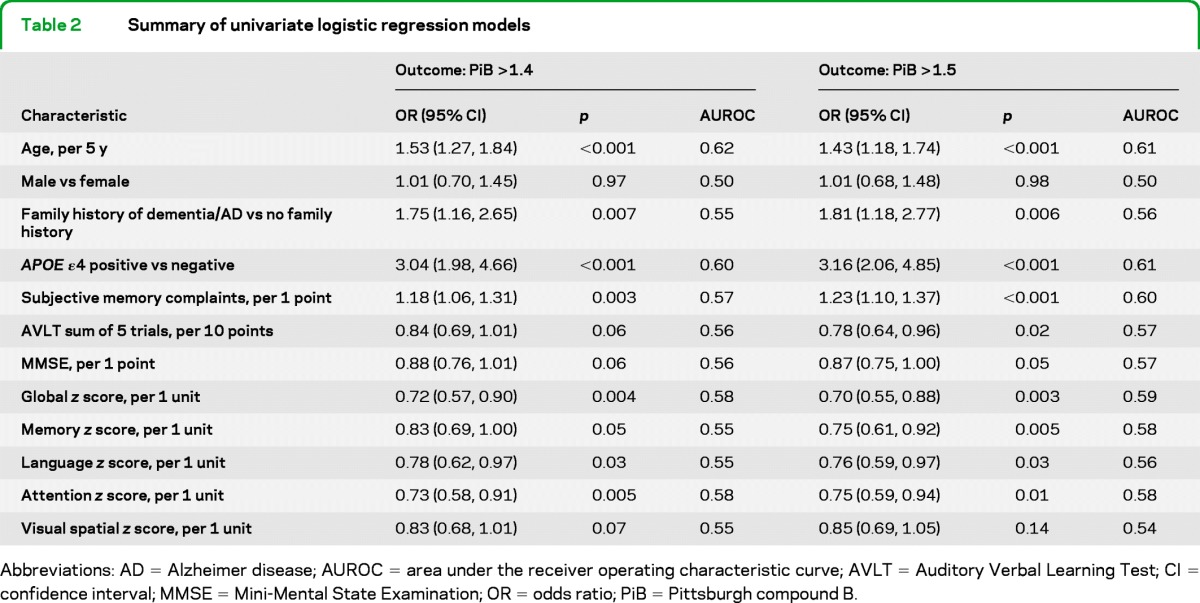
Abbreviations: AD = Alzheimer disease; AUROC = area under the receiver operating characteristic curve; AVLT = Auditory Verbal Learning Test; CI = confidence interval; MMSE = Mini-Mental State Examination; OR = odds ratio; PiB = Pittsburgh compound B.
Tables 3 and 4 provide summaries of multivariate models for discriminating between subjects with and without PiB >1.4 and >1.5. For PiB >1.4, age and sex (model 1) provided only fair discrimination (AUROC = 0.62) for identifying PiB-positive individuals (table 3). However, the PPV of this model was 57%, an increase from the baseline rate of 44% with PiB >1.4. The addition of family history of dementia/AD and subjective memory complaints (model 2) slightly increased the AUROC and reduced the PPV and NPV. Adding information about the presence of an APOE ε4 allele (model 3) appreciably increased the model discrimination (AUROC = 0.70; PPV = 62%; NPV = 66%). The addition of global z score performance (model 4) did not improve the overall model for discrimination, PPV, or NPV.
Table 3.
Summary of multivariate logistic regression models for an outcome of Pittsburgh compound B >1.4
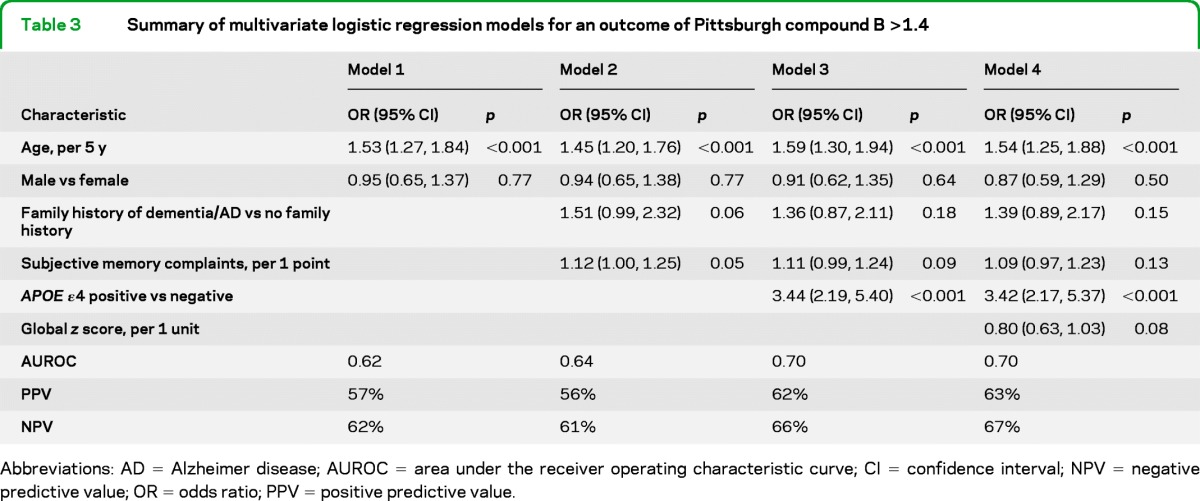
Abbreviations: AD = Alzheimer disease; AUROC = area under the receiver operating characteristic curve; CI = confidence interval; NPV = negative predictive value; OR = odds ratio; PPV = positive predictive value.
Table 4.
Summary of multivariate logistic regression models for an outcome of Pittsburgh compound B >1.5
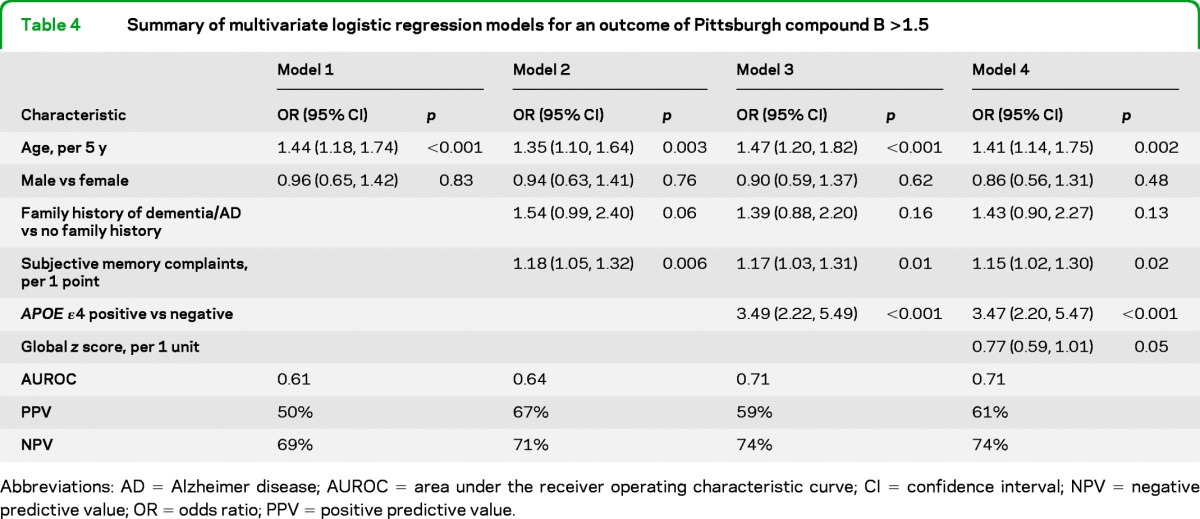
Abbreviations: AD = Alzheimer disease; AUROC = area under the receiver operating characteristic curve; CI = confidence interval; NPV = negative predictive value; OR = odds ratio; PPV = positive predictive value.
For the prediction of PiB >1.5, age and sex (model 1, table 4) again provided only fair discrimination (AUROC = 0.61) for identifying PiB-positive individuals. However, the PPV of this model was 50%, an increase from the baseline rate of 31% in this population. Information concerning family history of dementia/AD and subjective memory complaints (model 2) slightly increased the discrimination of PiB >1.5 (AUROC = 0.64) and greatly increased the PPV (67%). The addition of information on APOE genotype (model 3) and global z score performance (model 4) further increased the discrimination, but the AUROC value (0.71) for model 4 remained modest. In additional analyses, we substituted domain-specific z scores, the AVLT, and MMSE for the global z score, but they did not improve model prediction or discrimination (data not shown).
We also estimated the number needed to screen to enroll 100 individuals with PiB >1.4 or >1.5, based on the prevalence of each characteristic in the MCSA and stratified by age group (70–79 vs 80–89). The predictability of multiple factors varied with age and PiB cutoff (table 5). For example, screening on APOE genotype alone reduced the number needed to screen to enroll 100 participants with PiB >1.5 by 48% in persons aged 70–79 and 33% in those aged 80–89. To enroll 100 participants with PiB >1.4, screening on APOE genotype reduced the number needed to screen by 37% in persons aged 70–79 and 32% in persons aged 80–89. There was some difference by age for subjective memory complaints with PiB >1.4. However, for PiB >1.5, screening individuals with memory complaints reduced the number needed to screen to enroll 100 participants by 37% for persons aged 70–79, and by only 10% for persons aged 80–89. Finally, the effect of sex varied by age for both cutpoints such that men aged 70–79, and women aged 80–89, were more likely to be PiB positive.
Table 5.
Proportion of Pittsburgh compound B–positive cognitively normal participants by individual characteristics and stratified by age group (70–79 vs 80–89)
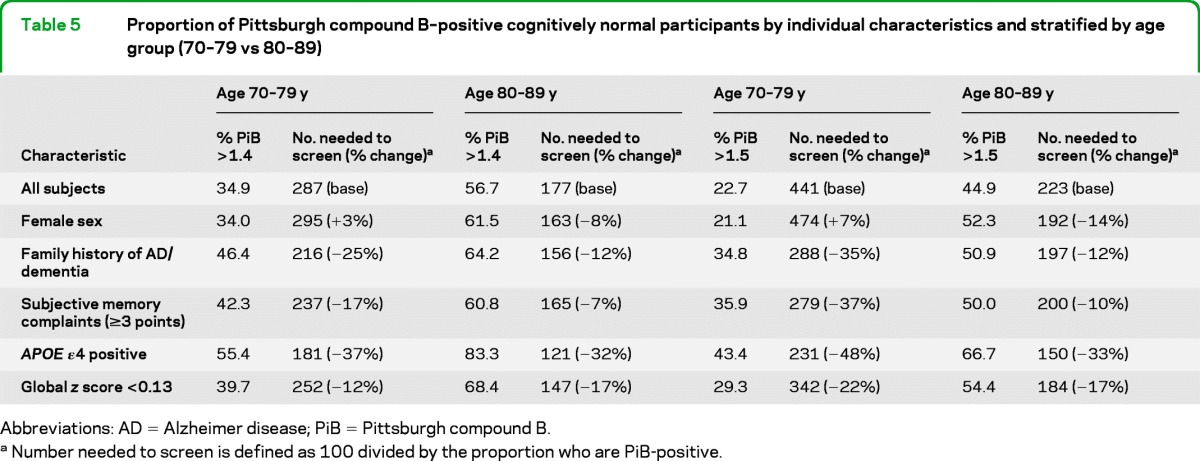
Abbreviations: AD = Alzheimer disease; PiB = Pittsburgh compound B.
Number needed to screen is defined as 100 divided by the proportion who are PiB-positive.
DISCUSSION
In this study, we examined indicators of PiB positivity (defined as either PiB retention ratio >1.4 or >1.5) among 483 individuals enrolled in the population-based MCSA. Information on age, sex, APOE genotype, family history of dementia, and subjective memory complaints, all easily obtainable information with little participant burden, would greatly reduce costs of screening the population for individuals with brain amyloidosis at both PiB cutoffs. Age and APOE genotype were the most useful and efficient indicators of PiB positivity (amyloid accumulation). However, the discrimination was modest, and the efficiency might come at the expense of narrowing the population of the labeling granted by a regulatory agency. Screening on subjective memory (in particular) and a positive family history would still improve efficiency, albeit more modestly than APOE and old age, with a minimal effect on regulatory labeling restrictions. Notably, many of these characteristics vary by age so it will be important to consider the age range of the population to optimally reduce screening costs when planning for a clinical trial.
There are currently no disease-modifying treatments for AD dementia. Although it could be that the right molecular target has yet to be identified, another ominous possibility is that the downstream pathophysiologic events dominate in the fully symptomatic stage and cannot be halted by anti-amyloid intervention alone.19 AD pathophysiology begins to appear in the brain years before the clinical symptoms become apparent. This long preclinical phase provides a critical opportunity for the prevention of future symptoms with disease-modifying therapies.20 Brain amyloid accumulation is considered the earliest pathologic feature of AD that is detectable by currently available biomarkers3,5,8,21 and is described as stage 1 by the new National Institute on Aging-Alzheimer's Association research criteria for preclinical AD.8 Indeed, both postmortem and epidemiologic studies report that 20%–40% of CN elderly individuals have significant amyloid accumulation.9–11,18 Since cerebral amyloidosis is necessary, albeit not sufficient, for an AD diagnosis, and increases the risk of conversion to mild cognitive impairment,22–27 secondary prevention trials are now being designed that require documentation of brain amyloidosis for enrollment.4 This requires an expensive amyloid PET scan or an invasive lumbar puncture for CSF amyloid levels. The identification of noninvasive, low-cost measures that would reduce the number of subjects needed to be screened with brain scans or lumbar punctures would greatly improve the efficiency of clinical trials targeting the preclinical disease phase.
The literature examining the relationship between cognitive performance and brain amyloidosis has been inconsistent. Some studies show a cross-sectional correlation between cognitive performance, particularly memory,28–30 while others do not find an association.31–33 The logical conclusion is that the association exists, but is fairly weak. In the present study, better performance across all tests and domains was associated with reduced odds of PiB >1.4 and >1.5. However, the discrimination of each test was modest (AUROC ∼0.6) and the global z score was similar to tests of delayed memory recall in indicating PiB positivity. Moreover, subjective memory complaints were as good of a predictor of PiB positivity as cognitive test performance, especially for PiB >1.5. Thus, extensive cognitive testing of CN elderly individuals with a standardized test battery may not be a cost-effective screening tool for PiB positivity. While it is possible that other cognitive tests could be more sensitive, our full battery included assessments of all domains with an emphasis on delayed memory recall.
As expected, the presence of an APOE ε4 allele was associated with 3-fold increased odds of PiB >1.4 and >1.5. Requiring the presence of an ε4 allele for enrollment into a trial of preclinical AD could be helpful as a screening measure, and in restricting enrollees to those most likely to progress to clinical symptoms. A blood draw for APOE genotype is noninvasive and low-cost. However, selecting subjects based on APOE genotype would exclude a large portion of individuals at risk for AD who do not have an ε4 allele.34 From a regulatory perspective, additional studies in non-ε4 carriers would also be necessary. Thus, the findings that prescreening on the easily obtainable and cost-effective information of age, sex, family history of dementia/AD, and self-reported memory (all of which can be assessed over the phone) will still reduce the number of individuals needed to screen for brain amyloidosis is significant.
Notably, while screening on these factors will help reduce the costs of identifying individuals with amyloidosis, the NPVs (60%–70%) are modest. Thus, a number of individuals with amyloidosis, defined by either cutpoint, will screen negative and thus be excluded from the trial. While this may be concerning, the costs of screening and excluding the false negatives are minimal compared to screening all potential individuals with amyloid imaging or a lumbar puncture for CSF to identify eligible individuals. Clearly, screening with these factors would not be an acceptable option for determining AD pathology in the clinical practice setting. However, from a clinical trial standpoint, it would greatly reduce the costs of identifying individuals with amyloidosis for AD prevention trials. Notably, the results for both cutoffs of PiB >1.4 and >1.5 were similar. This suggests that a cutoff of PiB >1.4 is biologically relevant and could be incorporated into preclinical AD trials.
Strengths of this study include a large sample size of individuals with PiB-PET recruited from the population-based MCSA. While participants with a PiB-PET scan were younger and performed slightly better on cognitive tests, the percentage of individuals in the MCSA with an APOE ε4 allele or a family history of dementia/AD did not differ in those who participated in PET scanning compared to the whole MCSA population (table 1). This is an advantage over most biomarker studies, which are not representative of the general older population. That is, volunteer cohorts typically have higher rates of family history and APOE ε4 genotypes and tend to come from highly educated and higher socioeconomic status backgrounds. A potential limitation of the study is that findings from the residents of Olmsted County may not precisely generalize to other populations. In particular, Olmsted County residents are primarily of European ancestry, thus the reported estimates may differ in other ethnic groups.
Supplementary Material
GLOSSARY
- AD
Alzheimer disease
- AUROC
area under the receiver operating characteristic curve
- AVLT
Auditory Verbal Learning Test
- BNT
Boston Naming Test
- CI
confidence interval
- CN
cognitively normal
- IQR
interquartile range
- MCSA
Mayo Clinic Study of Aging
- MMSE
Mini-Mental State Examination
- NPV
negative predictive value
- OR
odds ratio
- PiB
Pittsburgh compound B
- PPV
positive predictive value
- ROI
region of interest
- TMT
Trail Making Test
- WAIS-R
Wechsler Adult Intelligence Scale–Revised
- WMS-R
Wechsler Memory Scale–Revised.
Footnotes
Editorial, page 1530
AUTHOR CONTRIBUTIONS
Dr. Mielke: drafting/revising the manuscript, including medical writing for content, and generated the first and final drafts; study concept or design, analysis and interpretation of data. Ms. Wiste: drafting/revising the manuscript, statistical analysis, interpretation of the data. Mr. Weigand: drafting/revising the manuscript, statistical analysis, interpretation of the data. Dr. Knopman: drafting/revising the manuscript, study concept or design, analysis and interpretation of data. Dr. Lowe: drafting/revising the manuscript, study concept or design, study supervision. Dr. Roberts: drafting/revising the manuscript, acquisition of data. Dr. Geda: drafting/revising the manuscript, acquisition of data. Ms. Swenson-Dravis: drafting/revising the manuscript, study supervision. Dr. Boeve: analysis and interpretation of data, acquisition of data. Mr. Senjem: drafting/revising the manuscript, performed analyses of imaging data. Dr. Vemuri: drafting/revising the manuscript, analysis and interpretation of data. Dr. Petersen: drafting/revising the manuscript, obtaining funding. Dr. Jack: drafting/revising the manuscript, obtaining funding, acquisition of data, study supervision, study concept and design, analysis and interpretation of data.
DISCLOSURE
M. Mielke receives research support from the NIH/NIA. H. Wiste and S. Weigand report no disclosures. D. Knopman serves as Deputy Editor for Neurology®; serves on a Data Safety Monitoring Board for Lilly Pharmaceuticals; is an investigator in clinical trials sponsored by Baxter, Elan Pharmaceuticals, and Forest Pharmaceuticals; and receives research support from the NIH. V. Lowe serves on scientific advisory boards for Bayer Schering Pharma and GE Healthcare and receives research support from GE Healthcare, Siemens Molecular Imaging, the NIH (NIA, NCI), the MN Partnership for Biotechnology and Medical Genomics, and the Leukemia & Lymphoma Society. R. Roberts receives research support from the NIH/NIA and from Abbott Research Labs. Y. Geda receives research support from NIH, the Robert Wood Johnson Foundation, and the European Union Regional Development Fund. D. Swenson-Dravis reports no disclosures. B. Boeve receives research support from Cephalon, Inc., the NIH/NIA, the Alzheimer's Association, and the Center for Inherited Disease Research. M. Senjem and P. Vemuri report no disclosures. R. Petersen serves on scientific advisory boards for Pfizer, Inc., Janssen Alzheimer Immunotherapy, Elan Pharmaceuticals, and GE Healthcare; and receives research support from the NIH /NIA. C. Jack serves on scientific advisory boards for Elan/Janssen AI, Johnson & Johnson, Eli Lilly & Company, GE Healthcare, Bristol Meyer Squib, and Eisai Inc.; and receives research support from Baxter International Inc., Allon Therapeutics, Inc., the NIH/NIA, and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239– 259 . [DOI] [PubMed] [Google Scholar]
- 2. Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov 2007; 6: 295– 303 . [DOI] [PubMed] [Google Scholar]
- 3. Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010; 9: 119– 128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med 2011; 3: 111– 133 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature 2009; 461: 916– 922 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain 2009; 132: 1355– 1365 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002; 297: 353– 356 . [DOI] [PubMed] [Google Scholar]
- 8. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 280– 292 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 2010; 31: 1275– 1283 . [DOI] [PubMed] [Google Scholar]
- 10. Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain 2008; 131: 665– 680 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mintun MA, Larossa GN, Sheline YI, et al. [11C]PiB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006; 67: 446– 452 . [DOI] [PubMed] [Google Scholar]
- 12. Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008; 30: 58– 69 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men: The Mayo Clinic Study of Aging. Neurology 2010; 75: 889– 897 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol 2004; 55: 306– 319 . [DOI] [PubMed] [Google Scholar]
- 15. Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab 2005; 25: 1528– 1547 . [DOI] [PubMed] [Google Scholar]
- 16. McNamee RL, Yee SH, Price JC, et al. Consideration of optimal time window for Pittsburgh compound B PET summed uptake measurements. J Nucl Med 2009; 50: 348– 355 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lopresti BJ, Klunk WE, Mathis CA, et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med 2005; 46: 1959– 1972 . [PubMed] [Google Scholar]
- 18. Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to NIA-AA criteria for preclinical Alzheimer's disease. Ann Neurol 2012; 71: 765– 775 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hyman BT. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch Neurol 2011; 68: 1062– 1064 . [DOI] [PubMed] [Google Scholar]
- 20. Selkoe DJ. Resolving controversies on the path to Alzheimer's therapeutics. Nat Med 2011; 17: 1060– 1065 . [DOI] [PubMed] [Google Scholar]
- 21. Mormino EC, Kluth JT, Madison CM, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain 2009; 132: 1310– 1323 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 2007; 64: 343– 349 . [DOI] [PubMed] [Google Scholar]
- 23. Fagan AM, Head D, Shah AR, et al. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol 2009; 65: 176– 183 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol 2009; 66: 1469– 1475 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li G, Sokal I, Quinn JF, et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology 2007; 69: 631– 639 . [DOI] [PubMed] [Google Scholar]
- 26. Jack CR, Jr, Wiste HJ, Vemuri P, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain 2010; 133: 3336– 3348 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knopman DS, Jack CR, Jr, Wiste HJ, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology 2012; 78: 1576– 1582 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chetelat G, Villemagne VL, Pike KE, et al. Independent contribution of temporal beta-amyloid deposition to memory decline in the pre-dementia phase of Alzheimer's disease. Brain 2011; 134: 798– 807 . [DOI] [PubMed] [Google Scholar]
- 29. Kantarci K, Lowe V, Przybelski SA, et al. APOE modifies the association between Aβ and cognition in cognitively normal older adults. Neurology 2012; 78: 232– 240 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain 2007; 130: 2837– 2844 . [DOI] [PubMed] [Google Scholar]
- 31. Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 2008; 65: 1509– 1517 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Resnick SM, Sojkova J, Zhou Y, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology 2010; 74: 807– 815 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sperling RA, Laviolette PS, O'Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 2009; 63: 178– 188 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aisen PS. Clinical trial methodologies for disease-modifying therapeutic approaches. Neurobiol Aging 2011; 32 (suppl 1): S64– S66 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


