Abstract
Background. How antimalarial antibodies are acquired and maintained during pregnancy and boosted after reinfection with Plasmodium falciparum and Plasmodium vivax is unknown.
Methods. A nested case-control study of 467 pregnant women (136 Plasmodium-infected cases and 331 uninfected control subjects) in northwestern Thailand was conducted. Antibody levels to P. falciparum and P. vivax merozoite antigens and the pregnancy-specific PfVAR2CSA antigen were determined at enrollment (median 10 weeks gestation) and throughout pregnancy until delivery.
Results. Antibodies to P. falciparum and P. vivax were highly variable over time, and maintenance of high levels of antimalarial antibodies involved highly dynamic responses resulting from intermittent exposure to infection. There was evidence of boosting with each successive infection for P. falciparum responses, suggesting the presence of immunological memory. However, the half-lives of Plasmodium antibody responses were relatively short, compared with measles (457 years), and much shorter for merozoite responses (0.8–7.6 years), compared with PfVAR2CSA responses (36–157 years). The longer half-life of antibodies to PfVAR2CSA suggests that antibodies acquired in one pregnancy may be maintained to protect subsequent pregnancies.
Conclusions. These findings may have important practical implications for predicting the duration of vaccine-induced responses by candidate antigens and supports the development of malaria vaccines to protect pregnant women.
How the immune system responds to exposure to infections during pregnancy is poorly understood. Antibody-mediated immunity is essential for protection against many important pathogens, but how antibodies are acquired, maintained, and boosted after re-exposure during pregnancy remains unknown. This knowledge is critical to understanding why pregnant women are more susceptible to and more severely affected by numerous infectious diseases [1] and has translational implications for the development and use of vaccines to protect pregnant women and their babies. In this study, we aimed to address these key questions by studying responses to one of the most important human pathogens during pregnancy: malaria.
It is estimated that every year 125 million women living in areas where malaria is endemic become pregnant [2]. Infection with Plasmodium falciparum and Plasmodium vivax during pregnancy contributes to maternal anemia, can cause severe illness and death, and leads to low infant birth weight, which is a major risk factor for infant mortality and morbidity [3, 4]. At the time of their first pregnancy, women living in areas where malaria is endemic may have developed substantial acquired immunity to malaria, which does not prevent infection per se, but controls high-density parasitemia and associated clinical symptoms [5]. Antibodies against the disease-causing blood stage of malaria have a significant role in protection and target antigens on the surface of merozoites and infected erythrocytes (IE) [6, 7]. Despite pre-existing immunity, pregnant women develop placental and peripheral infections at higher parasite densities, compared with nonpregnant adults [8]. This susceptibility has been attributed to immune modulation resulting in an impaired ability to limit parasite replication during pregnancy and the emergence of specific antigenic variants of P. falciparum that evade existing immunity and accumulate in the placenta [9, 10]. The expression by P. falciparum IEs of the VAR2CSA protein, a specific variant of P. falciparum erythrocyte membrane protein (PfEMP1) that is exposed on the surface of IEs, facilitates the sequestration of IEs in the placenta by mediating adhesion to chondroitin sulfate A and, possibly, other receptors in the intervillous space [9–11]. Levels of antibodies to surface antigens of placental-binding IEs, and VAR2CSA specifically, are generally low before pregnancy and are higher in multigravida women exposed to P. falciparum [9–12].
Little is known about the maintenance and boosting of antimalarial responses over time, particularly during pregnancy, and there is a paucity of studies with repeated sampling over time or studies examining responses to multiple infections. Furthermore, very little is known about antibody responses to non–P. falciparum malaria during pregnancy, particularly P. vivax, which is widespread in Asia and South America. To advance our knowledge on the acquisition and boosting of antibody responses to infections in pregnant women and the maintenance of antibodies in the absence of reinfection, we studied antimalarial responses throughout pregnancy among women exposed to P. falciparum and P. vivax infection in a region of Southeast Asia where malaria is endemic.
MATERIALS AND METHODS
Study Design and Population
This study is a nested case-control study based in the antenatal clinics (ANCs) of the Shoklo Malaria Research Unit (SMRU) in northwestern Thailand [4, 13]. The ANCs were established in the Maela refugee camps to prevent maternal death from malaria, and 90% of pregnant women attend on a weekly basis [13]. Malaria transmission was low, with peak transmission from May through September. The cumulative incidence of malaria during pregnancy in this area is 37%, with the majority of malaria during pregnancy caused by P. falciparum and/or P. vivax [13]. Participants were identified from 1000 Karen women who participated in a placebo randomized controlled trial of chloroquine prophylaxis against P. vivax infection during pregnancy from November 1998 through January 2000 [14]. Women had samples obtained weekly for Plasmodium species infection by microscopic examination of blood smears and fortnightly for serum sample collection. All 136 women with Plasmodium infection detected by light microscopy at any time during pregnancy during the trial were defined as case subjects for the current study; 331 control subjects (3:1 ratio) were then randomly selected from the 864 women with no detectable parasitemia at any time during pregnancy. All detected Plasmodium infections were treated according to the SMRU guidelines [14], and all study women were encouraged to deliver their newborns at the SMRU delivery unit. Estimated gestational age (EGA) at delivery was calculated using the Dubowitz method [15] or, if a woman delivered at home, using a formula developed from a cohort of Karen pregnant women with gestation age from the Dubowitz method [4]. The study was approved by the Ethics Committee of the Faculty of Tropical Medicine of Mahidol University, the London School of Hygiene & Tropical Medicine, and the Walter and Eliza Hall Institute of Medical Research.
Antibody Determination
The samples selected were all available samples from 136 case subjects (n = 733) and all control enrollment samples (n = 320). A subset of control subjects was selected for longitudinal antibody determination, based on IgG responses to Schizont extract at enrollment (Supplementary material), to enable the investigation of antibody responses in the absence of infection in those who had relatively high (78 high schizont lysate responder controls, n = 572) and low antibody responses (37 low schizont lysate responder controls, n = 323) at enrollment. Total IgG titer was determined to P. falciparum merozoite antigens (apical membrane antigen, PfAMA1) [16], erythrocyte binding antigen 175 (PfEBA175; region 3–5 [17, 18], merozoite surface protein, PfMSP2 [19], PfMSP3 [20]), schizont extract, PfVAR2CSA (DBL5ε domain) [21], and P. vivax merozoite antigen (PvAMA1) [22] with use of high-throughput enzyme-linked immunosorbent assay (Supplementary material).
Statistical Analysis
Details on statistical approaches are detailed in the Supplementary material. In brief, for enrollment data, the associations between categorical variables were assessed using χ2 tests or Fisher's exact tests and continuous variables by Mann-Whitney U tests, Wilcoxon signed-rank tests, t tests, or Spearman's ρ correlation, where appropriate. In the case-control study, multiple logistic regression determined the association between gravidity, intervention group, and the odds of Plasmodium infection. In case subjects, the association between EGA and odds of each Plasmodium infection outcome was assessed using logistic regression with generalized estimating equations with an exchangeable correlation structure. Linear mixed-effect models were used to investigate the association between antibody levels and gestation time. For the purpose of examining species-specific antibody responses with species-specific infection, a longitudinal exposure group variable was created (4 categories: infected case subjects [species-specific], uninfected case subjects, uninfected control high schizont lysate responders, and uninfected control low schizont lysate responders). The models also included the predefined confounders (gravidity, intervention group, and prior Plasmodium infection [species-specific]) and investigated whether antibody levels over gestation time differed by variables of interest. Antibody response half-life estimates were obtained from the fixed-effects slope component of the mixed-effects model, and the 95% reference range was calculated using the standard deviation of the slope that represents between-woman variability. To assess boosting of antibody responses with each successive species-specific infection, a subgroup linear mixed-effect analysis, as above, was performed in species-specific cases. All statistical analyses were performed using Stata 11.2 (StataCorp) [23].
RESULTS
Antibodies at Enrollment
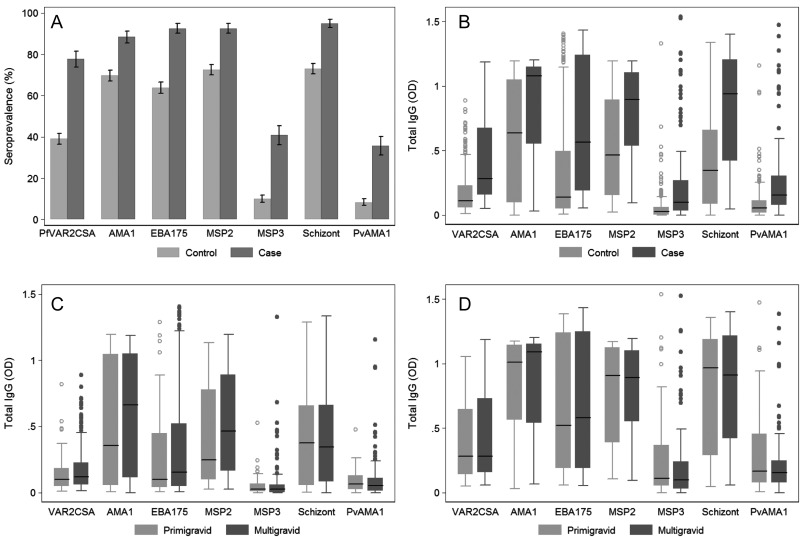
There were 136 case subjects (women infected with P. falciparum and/or P. vivax during pregnancy) and 331 control subjects (women not infected with Plasmodium) included in a nested case-control study evaluating the association between antibody responses and Plasmodium infection during pregnancy (Table 1). A total of 1948 samples were tested for antibodies to 7 different Plasmodium antigens. Antigens were selected on the basis of whether they are targets of protective immunity or biomarkers of immunity [6, 24], include antigens of P. falciparum and P. vivax, and represent antigens of merozoites and IEs. At enrollment (median gestational age, 9.8 weeks [interquartile range (IQR), 7.0–13.9 weeks] for case subjects and 9.56 weeks [IQR, 7.6–11.6 weeks] for control subjects) (Table 1), the prevalence and levels of anti–P. falciparum and P. vivax blood-stage antibodies were higher in case subjects than in control subjects (P < .0001) (Figure 1 A and B), indicative of increased Plasmodium infection before enrollment (Table 1). Although multigravida exhibited some of the highest antibody responses, median levels of merozoite and pregnancy-specific antibodies were comparable to primigravida in case and control subjects (P > .17) (Figure 1C and D). P. falciparum antibody responses were strongly correlated with each other (ρ=0.45–0.87; P < .001) and less strongly with P. vivax responses (ρ=0.31–0.52; P < .001).
Table 1.
Characteristics of Case subjects and Control Subjects at Enrollment
| Characteristic | Case subjects (n = 136) | Control Subjects (n = 331) | P |
|---|---|---|---|
| Age (years) median (IQR) | 24.5 (20–30.5) | 26 (21–31) | .1 |
| Gravidity, median (IQR) | 3 (2–5) | 3 (2–5) | .36 |
| Primigravida, n (%) | 30 (22.1) | 52 (15.7) | .10 |
| Multigravida, n (%) | 106 (77.9) | 279 (84.3) | |
| Parity, median (IQR) | 1 (0–3) | 2 (2–5) | .09 |
| Hematocrit (%), median (IQR) | 32.55 (30–35) | 34.5 (32–36.6) | <.001 |
| Anemiaa, n (%) | 32 (23.5) | 39 (11.8) | .001 |
| Residence in refugee camp | 61 (44.9) | 324 (97.9) | <.001 |
| Receiving chloroquine prophylaxis, n (%) | 56 (41.2) | 168 (50.8) | .06 |
| Estimated Gestational Ageb, median (IQR) | 9.8 (7.0–13.9) | 9.6 (7.6–11.6) | .45 |
| Trimester | |||
| 1 (<14 wk) | 101 (75.0) | 289 (87.3) | .001c |
| 2 (14 to <28 wk) | 32 (23.5) | 42 (12.7) | |
| 3 (28 wk or more) | 2 (1.5) | 0 (0) | |
| Plasmodium species before enrollmentd, n (%) | 76 (55.9) | 117 (35.3) | <.001 |
| P. falciparum | |||
| Proportion women infected, n (%) | 51 (38.3) | 82 (24.8) | .003 |
| Number of episodes, median (IQR) | 1 (1–4) | 1 (1–4) | .89 |
| P. vivax | |||
| Proportion women infected, n (%) | 33 (24.8) | 46 (13.9) | .005 |
| Number of episodes, median [IQR] | 1 (1–3) | 1 (1–3) | .12 |
| Follow up (weeks), median (range) | 28.9 (23.3–32.2) | 30.7 (28.3–32.4) | <.001 |
| Plasmodium screens during pregnancy, median [IQR] | 26 (19–30) | 30 (27–32) | <.001 |
The associations between categorical variables were assessed by χ2 tests or Fisher's exact tests and continuous variables by Mann-Whitney U tests, Wilcoxon signed-rank tests, or t tests.
Abbreviation: IQR, inter-quartile range.
a Haematocrit <30%.
b Determined at enrollment.
c Statistical comparison is first vs second and third trimester (combined).
d Any microscopically confirmed Plasmodium infection documented at SMRU before enrollment into the study.
Figure 1.
Antibodies to Plasmodium species blood-stage antigens at enrollment. Antibody levels were determined in all available case (n = 124) and control (n = 320) samples at enrollment (median 10 weeks gestation). (A) Seroprevalence with standard errors and (B) boxplots of antibody levels in case and control subjects. Horizontal lines represent medians, boxes represent interquartile range, and lines represent ranges with outliers represented as dots. Antibody prevalences and levels were significantly higher in case than in control subjects (P < .001). Antibody levels according to gravidity in control subjects (C) and case subjects (D). In case and control groups, there was no association between gravidity and antibody levels (P > .17).
Plasmodium Infection from Early Pregnancy to Delivery
From enrollment to delivery, the cumulative proportion of case subjects who were infected with P. falciparum and P. vivax was 69.1% and 61.0%, respectively. Multigravida had decreased odds of P. falciparum (0.58; 95% confidence interval [CI], 0.33–1.03; P = .066) and P. vivax (0.53; 95% CI, 0.30–0.96; P = .036) infection, compared with primigravida. A more detailed analysis of infection over gestation in case subjects showed that the occurrence of P. falciparum and P. vivax infection decreased with gestation time (Supplementary material). These associations were not modified by gravidity or chloroquine prophylaxis.
Antibody Dynamics in the Presence or Absence of Infection
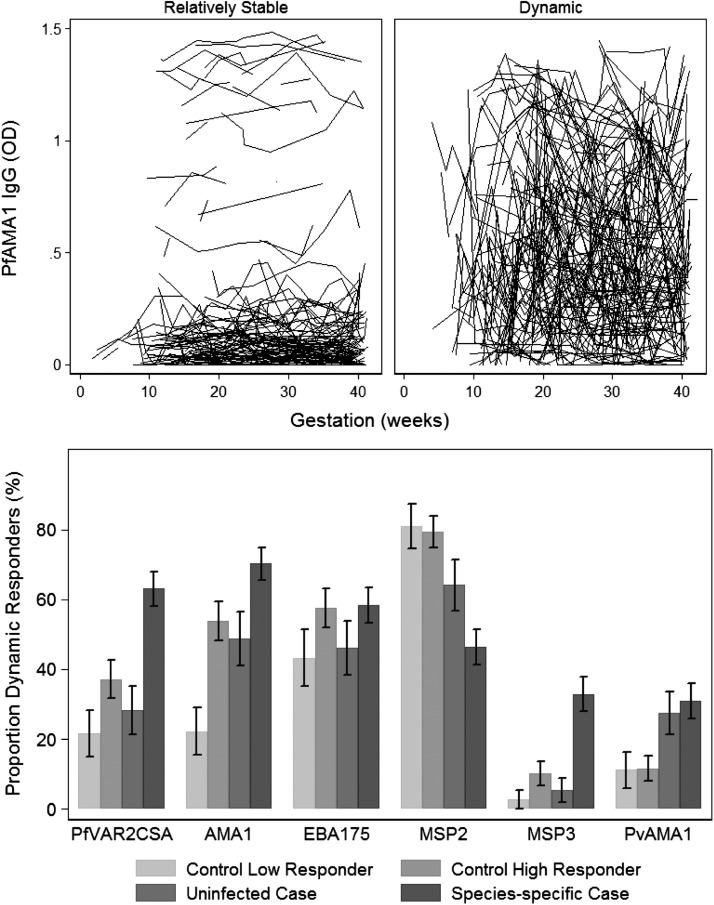
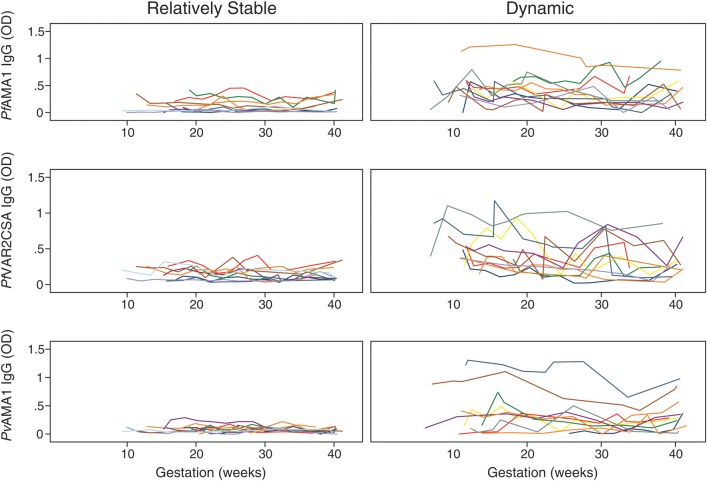
The weekly surveillance for Plasmodium species was coupled with regular antibody determination throughout pregnancy. Antibody responses were assessed from median 10 weeks gestation to delivery in case subjects (in a Plasmodium species–specific manner) and in uninfected control subjects comprising a selection of antibody high schizont lysate responders and low responders. Antibodies to Schizont lysate were used as a marker of exposure to blood-stage malaria. In all longitudinal exposure groups, IgG antibody responses in each woman showed complex dynamics over gestation time. Women could be broadly classified as having dynamic or relatively stable responses (defined as an individual standard deviation ≥0.1 or <0.1, respectively) (Figure 2A). In addition, among individual women, differences in the patterns or dynamics of responses to different antigens over time also varied (Figure 3), because women could have highly fluctuant responses to ≥1 antigen but relatively stable responses to other antigens. Overall, case subjects were more likely to have dynamic fluctuating antibody responses specific to the infecting Plasmodium species during pregnancy, compared with uninfected women (except for responses to PfMSP2) (Figure 2B). Of interest, a substantial proportion of women demonstrated dynamic responses, even in the absence of detectable Plasmodium infection during pregnancy (Figure 2B), suggesting that exposure to Plasmodium species is not the only factor that determines fluctuations in antibody levels over time.
Figure 2.
Antibody levels over gestation could be classified as relatively stable or dynamic. For each individual woman, the mean antibody response over gestation time and standard deviation (SD; ie, how far the individuals IgG response fluctuated from their mean response) was calculated. Use of a cutoff of a SD of 0.1 broadly classified individual woman as having dynamic (SD ≥ 0.1) or relatively stable responses (SD < 0.1). (A) The categorization of relatively stable and dynamic responses to PfAMA1 is shown as a representative example of antimalarial IgG responses throughout pregnancy (each line represents antibody levels in an individual over time). (B) The proportion (%) of dynamic antibody responses according to longitudinal exposure group. Species-specific cases refers to women who were infected with Plasmodium falciparum or Plasmodium vivax during pregnancy for P. falciparum and P. vivax antigens, respectively. The proportion of women with dynamic responses during pregnancy was associated with species-specific longitudinal exposure groups PfVAR2CSA, P < .001; PfAMA1, P < .001; PfEBA175 P = .24; PfMSP2, P < .001; PfMSP3, P < .001; PvAMA, P = .006.
Figure 3.
Examples of individuals with relatively stable and dynamic antimalarial responses. Each woman was classified as having dynamic (antibody optical density [OD] standard deviation [SD] ≥ 0.1) or relatively stable responses (antibody OD SD < 0.1). Relatively stable responses to PfAMA1, PfVAR2CSA, and PvAMA1 in the same 10 women (one color is the same woman). Dynamic responses to Plasmodium falciparum antigens PfAMA1, PfVAR2CSA in another 10 women, and dynamic responses to Plasmodium vivax antigens in an additional 10 women (with the exception of the yellow individual who had dynamic responses for all 3 antigens).
Maintenance and Boosting of Antibodies in Response to Intermittent Plasmodium Infection
Descriptive univariable analysis suggested that case subjects began pregnancy with higher levels of antibodies, compared with control subjects (Figure 1). Mixed-effect linear models showed that the greater fluctuating antibody responses observed in case subjects was associated with the maintenance of antibody levels at significantly higher mean levels, compared with control subjects or case subjects infected with a Plasmodium species different to the species of the antibody response; mean antibody levels were 0.08 (PvAMA1) to 0.58 (PfAMA1) higher in species-specific case subjects than in uninfected control low schizont lysate responders (P < .0001) (Table 2). Case subjects who were infected with P. vivax only (ie, no P. falciparum infection detected) had mean antibody levels against P. falciparum antigens that were comparable to those of uninfected control low schizont lysate responders (P ≥ .142) (Table 2). Associations with gravidity were seen with the antibodies to the pregnancy-specific PfVAR2CSA antigen (mean levels were 0.09 higher in multigravida than in primigravida; P = .018) but variably with merozoite antigens (Table 2). The maintenance of antibody responses in case subjects infected with P. falciparum and P. vivax at significantly higher levels relative to uninfected individuals suggested that concurrent infection has a role in boosting or maintaining antibodies. To confirm this, further linear mixed-effects analyses were conducted in case subjects infected with P. falciparum and P. vivax. This revealed that antibody levels to the pregnancy-specific PfVAR2CSA and P. falciparum merozoite antigens increased with each successive infection (Figure 4, P ≤ .07), providing evidence of antibody boosting, whereas levels of antibodies to P. vivax AMA1 did not (P = .96).
Table 2.
Multivariable Linear Mixed-Effects Modeling of the Outcomes: IgG Responses to Plasmodium falciparum and Plasmodium vivax Recombinant Antigens
| Variable | PfVAR2CSA | PfAMA1 | PfEBA175 | PfMSP2 | PfMSP3 | PvAMA1 |
|---|---|---|---|---|---|---|
| Gestation (weeks)a | −0.0003 (−0.01, 0.01) | −0.01 (−0.02, 0.002) | −0.01** (−0.03, −0.001) | −0.02** (−0.03, −0.01) | −0.01* (−0.02, −0.002) | −0.01# (−0.01, 0.001) |
| Longitudinal exposure groupb | ||||||
| Uninfected control high schizont lysate responder | 0.04 (−0.04, 0.13) | 0.12* (0.003, 0.24) | 0.10 (−0.06, 0.25) | 0.11# (−0.003, 0.22) | 0.02 (−0.05, 0.1) | 0.01 (−0.06, 0.09) |
| Uninfected case | 0.07 (−0.03, 0.17) | 0.05 (−0.08, 0.19) | −0.02 (−0.2, 0.16) | −0.06 (−0.18, 0.07) | 0.00003 (−0.09, 0.09) | 0.19*** (0.11, 0.27) |
| Species−specific infected case | 0.33*** (0.24, 0.42) | 0.58*** (0.47, 0.70) | 0.49*** (0.34, 0.65) | 0.34*** (0.23, 0.45) | 0.18*** (0.1, 0.25) | 0.08* (0.01, 0.16) |
| Gravidityc | ||||||
| Multigravida | 0.09* (0.09, 0.11) | 0.01 (−0.09, 0.11) | 0.14* (0.003, 0.27) | 0.08 (−0.02, 0.17) | 0.03 (−0.03, 0.1) | −0.07* (−0.13, −0.1) |
| Intervention groupd | ||||||
| Chloroquine | 0.02 (−0.04, 0.08) | 0.03 (−0.05, 0.11) | 0.03 (−0.08, 0.13) | −0.005 (−0.08, 0.07) | −0.03 (−0.08, 0.02) | 0.01 (−0.04, 0.06) |
| Prior Plasmodium sppe | ||||||
| Yes | −0.02 (−0.09, 0.04) | −0.01 (−0.09, 0.07) | −0.03 (−0.13, 0.08) | 0.01 (−0.06, 0.09) | −0.01 (−0.06, 0.05) | −0.01 (−0.08, 0.05) |
| Constantf | 0.11* (0.01, 0.21) | 0.18** (0.04, 0.32) | 0.3*** (0.12, 0.48) | 0.57*** (0.44, 0.69) | 0.1* (0.01, 0.2) | 0.16*** (0.08, 0.25) |
Values are coefficients (95% confidence intervals) of optical density values per unit change in covariate.
a Coefficients for gestation weeks is per 10 weeks gestation.
b Vs Uninfected control low schizont lysate responder (controls were selected for longitudinal analysis based on their responses to Schizont extract, Supplementary material).
c Vs Primigravida.
d Vs Placebo.
e Vs no prior Plasmodium spp. (species-specific).
f The mean antibody level for a woman at the start of pregnancy and the reference group of categorical variables = 0.***P < .001, **P ≤ .01, *P ≤ .05, #P < .1. The association between antibody levels with gestation was similar with respect to longitudinal exposure group, gravidity or intervention group (P-values for interaction terms > .1). The greater fluctuating responses in infected species-specific cases were confirmed by applying longitudinal exposure group as a covariate on the residual error of the model (data not shown).
Figure 4.
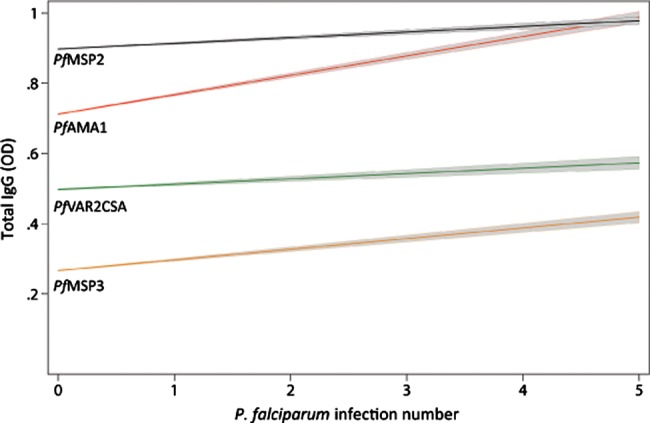
Anti–Plasmodium falciparum levels increase with each successive P. falciparum infection. Multivariable linear mixed-effects modeling of the association between antibody levels and number of episodes in species-specific cases (n = 94 and n = 83 for P. falciparum and P. vivax analysis, respectively). The coefficients (95% confidence interval [CI]) for the estimated mean increase in antibody levels per increase in P. falciparum episode number were as follows: PfVAR2CSA 0.03 (0.01–0.05), P = .011; PfAMA1 0.05 (0.02–0.08), P < .001; PfEBA175 0.05 (0.02–0.07) P < .001; PfMSP2 0.02 (0.003–0.04), P = .025; PfMSP3 0.018 (−0.001 to 0.04), P = .063; PvAMA1 − 0.0004 (−0.01 to 0.01), P = .96; measles −0.003 (−0.015 to 0.01), P = .63.
Maintenance and Longevity of Responses
Overall, antibody levels to all P. falciparum and P. vivax antigens decreased with increasing gestation time at a similar rate regardless of infection status (Table 2), which paralleled the decreasing point-prevalence of Plasmodium infection with increasing gestation (Supplementary material). To gain insight into the maintenance and durability of malaria immunity, we calculated the duration of Plasmodium antibody responses. In all infection groups, individual women showed antibody responses that persisted for only a few months to individuals with no evidence of decay (95% reference ranges) (Table 3). At the population level, the merozoite antibody response half-life ranged from 0.8 to 7.6 years, varied by antigen, and were generally longer in infected case subjects than in uninfected women. For P. falciparum merozoite antigens, half-lives ranged from 0.8 (PfMSP3) to 3.1 years (PfMSP2) in uninfected control subjects and 2.2 (PfMSP3) to 7.6 years (PfAMA1) in P. falciparum–infected case subjects (Table 3). These responses were relatively short-lived, compared with measles antibodies, which had a half-life of 457 years (95% reference range, 0.4 to ∞), similar to estimates in vaccinated North Americans [25]. The half-life of PfVAR2CSA, expressed on the surface of P. falciparum IEs, was considerably longer than merozoite responses at 142 years in P. falciparum–infected case subjects and 36–57.6 years in uninfected groups (Table 3).
Table 3.
Longevity of Plasmodium species Antibody Responses in Pregnant Women
| Antigen | Uninfected control low schizont lysate responder | Uninfected control high schizont lysate responder | P. falciparum uninfected casea | P. falciparum infected case |
|---|---|---|---|---|
| P. falciparum | ||||
| PfVAR2CSA | 36.0 (0.1–∞) | 50.2 (0.1–∞) | 57.6 (0.2–∞) | 142.0 (0.4–∞) |
| PfAMA1 | 1.8 (0.2–∞) | 3.0 (0.3–∞) | 2.3 (0.2–∞) | 7.6 (0.7–∞) |
| PfEBA175 | 2.0 (0.3–∞) | 2.6 (0.4–∞) | 1.9 (0.3–∞) | 5.3 (0.8–∞) |
| PfMSP2 | 2.6 (0.4–∞) | 3.1 (0.5–∞) | 2.3 (0.4–∞) | 4.1 (0.6–∞) |
| PfMSP3 | 0.8 (0.1–∞) | 1.0 (0.1–∞) | 0.8 (0.1–∞) | 2.2 (0.2–∞) |
| Uninfected control low schizont lysate responder | Uninfected control high schizont lysate responder | P. vivax uninfected casea | P. vivax infected case | |
| P. vivax | ||||
| PvAMA1 | 2.6 (0.4–∞) | 2.8 (0.4–∞) | 5.7 (0.8–∞) | 4.0 (0.5–∞) |
Values represent the estimated mean antibody response half-life in years with the 95% reference range. The predicted mean half-life was determined from the mixed-effects model with covariates set to the mean. The 95% reference ranges were derived from the estimate and between-woman standard deviation of the slope of the linear mixed-effects model. Multigravida had longer antibody half-lives compared to primigravida for VAR2CSA-DBL5 (64.8 [0.2–∞] compared to 36.0 [0.1–∞]).
DISCUSSION
In the most comprehensive study to date of antibody responses to major pathogens during pregnancy, we have advanced several important new concepts regarding the acquisition and maintenance of immunity during pregnancy. We demonstrate that, in most individuals, antibodies against P. falciparum and P. vivax have a highly dynamic profile over time. Maintenance of high levels of antibodies appears to involve highly dynamic fluctuating responses in response to intermittent pathogen exposure, and pregnant women have the ability to boost P. falciparum merozoite and pregnancy-specific responses (anti-PfVAR2CSA), but not P. vivax responses, with each successive infection. Furthermore, we have shown that antibody responses to merozoite antigens have a relatively short half-life in infected and uninfected women, compared with other infectious pathogens, such as measles, or antibodies to the PfEMP1 variant, PfVAR2CSA.
Maintenance of high levels of antimalarial antibody responses during pregnancy was characterized by highly fluctuating responses. Unexpectedly, highly dynamic Plasmodium species–specific responses were observed in some uninfected individuals; it is unlikely that these women were misclassified as uninfected because women were actively monitored weekly for the presence of Plasmodium species during pregnancy (median of 30 times during pregnancy), which would have detected any infections had they been present. These findings are consistent with studies of North American nonpregnant adults, which demonstrated fluctuations in vaccine-induced IgG responses to infectious pathogens in the absence of exposure [25]. These observations, taken together, suggest that antibodies are not maintained at a constant level, but fluctuate around a threshold, even in the absence of pathogen exposure. This has significant implications for our understanding of immune function and how to measure it. The variation in antibody levels over time suggests that measuring antibodies at a single time may not reliably reflect an individual's immune status. This has major translational implications highlighting the need for repeated measures of immune responses over time to accurately assess immune status and exposure in vaccine trials, sero-surveillance in malaria control programs, and studies of human immunity.
Currently, there are very limited data on the nature and existence of antibody maintenance and immunological memory to malaria [26] and boosting of responses after re-exposure. In this study, boosting of previously acquired P. falciparum responses that was observed with the first and successive infections, together with the parity-dependent acquisition of pregnancy-specific antibodies, supports the presence of immunological memory to blood-stage P. falciparum antigens. Previous studies in nonpregnant individuals have shown that P. falciparum–specific memory B cells are acquired after repeated infection [27] and are stably maintained in the absence of reinfection [28]. Of interest, boosting was not observed for P. vivax responses. P. vivax parasite densities are typically lower than P. falciparum, which may account for the differential boosting, assuming boosting is dose-dependent. A further important finding is that antibodies to merozoite antigens were relatively short-lived, compared with antibodies to other pathogens, such as measles, which confers life-long immunity. Hemodilution that occurs in pregnancy cannot account for the antimalarial antibody level decrease observed, because levels of antibodies to other pathogens (eg, measles) did not significantly decrease during pregnancy. The decrease in responses in infected case subjects, despite boosting of antibody responses after re-infection, most likely represents the decrease in Plasmodium species point-prevalence throughout pregnancy in the cohort. This further supports the notion that regular Plasmodium infections are required for antibody maintenance.
There are few published cohort data that address the question of antibody longevity, particularly during pregnancy. It is widely said that antimalarial antibodies are very short-lived (eg, weeks or months) [29, 30]. Methodological differences most likely underpin the differences in these estimates (Supplementary material); however, our findings suggest that they persist much longer, at least in pregnant women. Of interest, the response half-life of anti-merozoite antibodies to P. falciparum conserved (PfEBA175, PfMSP3) and variant (PfAMA1, PfMSP2) antigens and responses to P. vivax AMA1 were similar regardless of antigenic or species diversity. Conversely, antibody response half-life to the pregnancy-specific antigen PfVAR2CSA was considerably longer and was maintained throughout pregnancy. This is an important finding and suggests that antibodies acquired during one pregnancy would be maintained over multiple pregnancies in most women; this is supported by data in this study that describe that PfVAR2CSA prevalence was 79% in multigravida case subjects at enrollment (median 10 weeks gestation). Maintenance of PfVAR2CSA antibodies across pregnancy advocates the case for PfVAR2CSA vaccines to protect pregnant women against malaria [31]. Furthermore, data on antibody longevity may be valuable for predicting the duration of responses induced by candidate malaria vaccines. The shorter duration of antimerozoite may be an important issue to address in vaccine development if vaccines are to provide extended protection over many years. The different longevity of antibody responses to PfVAR2CSA relative to merozoite antigens could be attributable to differences in immunogenic properties or exposure to the immune system. Previous studies in this population have shown that, during weekly active surveillance, peripheral P. falciparum infection is typically associated with placental infection [32]. Placental parasites may maintain an antigenic reservoir and influence PfVAR2CSA antibody persistence. This may cause an overestimation of antibody longevity in P. falciparum–infected case subjects but indicate longer-lived antibodies in the presence of parasitemia. Furthermore, estimates of PfVAR2CSA lasting decades in uninfected women (negative peripheral smear results are always accompanied with placental negative smear results [32]) in this study supports the long-lived nature of these pregnancy-specific responses relative to merozoite antigens. Additional studies of post-partum women are required to better understand antibody duration.
In the present study, we selected samples from women participating in a chloroquine prophylaxis trial. The active monitoring and subsequent treatment of any Plasmodium infection for medical and ethical reasons introduced a limitation into our study, because we were unable to examine how antibodies fluctuate during the course of a natural infection relative to changes in parasite density and the length of infection. Additional studies examining the relationship between the peaks and troughs of responses (and the mechanism that produces them) and functional antibody studies will help dissect the mechanisms by which these antibodies constitute protective immunity to malaria. Better boosting and maintenance may be required to overcome the dramatic fluctuations observed. Greater exposure in high-transmission settings because of more frequent or prolonged infections might lead to greater boosting and maintenance of responses. However, the burden of malaria has recently decreased in many populations [33], and the use of intermittent preventive treatment during pregnancy is widespread in many populations, reducing the exposure of pregnant women to malaria in medium- and high-transmission areas. It is therefore important to understand immunity to malaria in low-exposure settings, particularly because many areas where malaria is endemic are transitioning from high to low malaria transmission intensity with recent strengthening of malaria control efforts.
Our findings also have important practical implications for malaria control and elimination campaigns. First, chloroquine prophylaxis was not immunosuppressive [34], with maintenance and boosting of anti–P. falciparum and P. vivax immunity similar in those receiving and not receiving prophylaxis. Second, higher antibody levels were found in women who had been exposed to Plasmodium infection during pregnancy; therefore, antibodies to P. falciparum and P. vivax in pregnant women may serve as useful tools for population sentinel surveillance in this high-risk group. Furthermore, the decrease in antibody levels over time, particularly for merozoite antigens, would suggest that these immune biomarkers could be used for assessing the impact of malaria control interventions, because decreasing rates of malaria during pregnancy would be reflected in decreasing levels and prevalence of antibodies to merozoite antigens in the medium and long term.
New insights from this study have major implications for our fundamental understanding of the function of the immune system during pregnancy and are particularly relevant to understanding the susceptibility and immunity of pregnant women to infectious pathogens. Additional studies of post-partum women and nonpregnant individuals are required to determine the longevity of antibody responses, in addition to studies aimed at understanding the reasons for differences in antibody maintenance of different malaria antigens. This is crucial for the development of new interventions, particularly vaccines, and tools for malaria surveillance in the global challenge of malaria control and elimination.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the participants and the Karen staff of the SMRU; Maelenn Gouillou, Ulrich Terheggen, and Margaret Tiong, for technical assistance; and Joseph Smith, for providing the recombinant PfVAR2CSA-DBL5 antigen.
Financial support. This work was supported by the National Health and Medical Research Council of Australia (to J.B., F. F., and J. R.), Infrastructure for Research Institutes Support Scheme Grant, Australian Research Council (to J. B.), and Victorian State Government Operational Infrastructure Support. SMRU is part of the Mahidol Oxford University Tropical Medicine Research Unit supported by the Wellcome Trust of Great Britain. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis. 2006;12:1638–43. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS medicine. 2010;7:e1000221. doi: 10.1371/journal.pmed.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 4.Nosten F, McGready R, Simpson JA, et al. Effects of Plasmodium vivax malaria in pregnancy. Lancet. 1999;354:546–9. doi: 10.1016/s0140-6736(98)09247-2. [DOI] [PubMed] [Google Scholar]
- 5.Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunology. 2006;28:51–60. doi: 10.1111/j.1365-3024.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 6.Fowkes FJ, Richards JS, Simpson JA, Beeson JG. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS medicine. 2010;7:e1000218. doi: 10.1371/journal.pmed.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hviid L. The role of Plasmodium falciparum variant surface antigens in protective immunity and vaccine development. Hum Vaccin. 2010;6:84–9. doi: 10.4161/hv.6.1.9602. [DOI] [PubMed] [Google Scholar]
- 8.Brabin BJ. Geneva: WHO; 1991. The risks and severity of malaria in pregnant women; pp. 1–34. Applied field research in malaria. [Google Scholar]
- 9.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science (New York, NY. 1996;272:1502–4. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 10.Beeson JG, Brown GV, Molyneux ME, Mhango C, Dzinjalamala F, Rogerson SJ. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J Infect Dis. 1999;180:464–72. doi: 10.1086/314899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salanti A, Dahlback M, Turner L, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med. 2004;200:1197–203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beeson JG, Ndungu F, Persson KE, et al. Antibodies among men and children to placental-binding Plasmodium falciparum-infected erythrocytes that express var2csa. The American Journal of Tropical Medicine and Hygiene. 2007;77:22–8. [PubMed] [Google Scholar]
- 13.Nosten F, ter Kuile F, Maelankirri L, Decludt B, White NJ. Malaria during pregnancy in an area of unstable endemicity. Trans R Soc Trop Med Hyg. 1991;85:424–9. doi: 10.1016/0035-9203(91)90205-d. [DOI] [PubMed] [Google Scholar]
- 14.Villegas L, McGready R, Htway M, et al. Chloroquine prophylaxis against vivax malaria in pregnancy: a randomized, double-blind, placebo-controlled trial. Trop Med Int Health. 2007;12:209–18. doi: 10.1111/j.1365-3156.2006.01778.x. [DOI] [PubMed] [Google Scholar]
- 15.Dubowitz L. A clinical manual: gestational age of the newborn. USA: Addison Wesley; 1977. [Google Scholar]
- 16.Hodder AN, Crewther PE, Anders RF. Specificity of the protective antibody response to apical membrane antigen 1. Infect Immun. 2001;69:3286–94. doi: 10.1128/IAI.69.5.3286-3294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards JS, Stanisic DI, Fowkes FJ, et al. Association between Naturally Acquired Antibodies to Erythrocyte-Binding Antigens of Plasmodium falciparum and Protection from Malaria and High-Density Parasitemia. Clin Infect Dis. 2010;51:e50–60. doi: 10.1086/656413. [DOI] [PubMed] [Google Scholar]
- 18.Reed MB, Caruana SR, Batchelor AH, Thompson JK, Crabb BS, Cowman AF. Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid-independent pathway of invasion. Proc Natl Acad Sci USA. 2000;97:7509–14. doi: 10.1073/pnas.97.13.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanisic DI, Richards JS, McCallum FJ, et al. IgG subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infection and Immunity. 2009;77:1165–74. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roussilhon C, Oeuvray C, Muller-Graf C, et al. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS medicine. 2007;4:e320. doi: 10.1371/journal.pmed.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avril M, Cartwright MM, Hathaway MJ, et al. Immunization with VAR2CSA-DBL5 recombinant protein elicits broadly cross-reactive antibodies to placental Plasmodium falciparum-infected erythrocytes. Infect Immun. 2010;78:2248–56. doi: 10.1128/IAI.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yildiz Zeyrek F, Palacpac N, Yuksel F, et al. Serologic markers in relation to parasite exposure history help to estimate transmission dynamics of Plasmodium vivax. PLoS One. 2011;6:e28126. doi: 10.1371/journal.pone.0028126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.StataCorp. Vol. 87. College Station, Texas: StataPress; 2009. Stata statistical software: release 11; pp. 377–90. [Google Scholar]
- 24.Richards JS, Beeson JG. The future for blood-stage vaccines against malaria. Immunology and Cell Biology. 2009 doi: 10.1038/icb.2009.27. [DOI] [PubMed] [Google Scholar]
- 25.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. The New England Journal of Medicine. 2007;357:1903–15. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 26.Wykes M, Good MF. Memory B cell responses and malaria. Parasite immunology. 2006;28:31–4. doi: 10.1111/j.1365-3024.2006.00768.x. [DOI] [PubMed] [Google Scholar]
- 27.Weiss GE, Traore B, Kayentao K, et al. The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog. 2010;6:e1000912. doi: 10.1371/journal.ppat.1000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wipasa J, Suphavilai C, Okell LC, et al. Long-lived antibody and B Cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog. 2010;6:e1000770. doi: 10.1371/journal.ppat.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malaria Journal. 2007;6:82. doi: 10.1186/1475-2875-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akpogheneta OJ, Duah NO, Tetteh KK, et al. Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infection and Immunity. 2008;76:1748–55. doi: 10.1128/IAI.01333-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hviid L. The case for PfEMP1-based vaccines to protect pregnant women against Plasmodium falciparum malaria. Expert Rev Vaccines. 2011;10:1405–14. doi: 10.1586/erv.11.113. [DOI] [PubMed] [Google Scholar]
- 32.McGready R, Davison BB, Stepniewska K, et al. The effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmission. The American Journal of Tropical Medicine and Hygiene. 2004;70:398–407. [PubMed] [Google Scholar]
- 33.WHO. Geneva: WHO Press, World Health Organization; 2010. World malaria report 2010; pp. 1–63. [Google Scholar]
- 34.Rogerson SJ, Wijesinghe RS, Meshnick SR. Host immunity as a determinant of treatment outcome in Plasmodium falciparum malaria. Lancet Infect Dis. 2010;10:51–9. doi: 10.1016/S1473-3099(09)70322-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.