Abstract
Small shifts in circadian timing occur frequently as a result of daylight saving time or later weekend sleep. These subtle shifts in circadian phase have been shown to influence subjective sleepiness, but it remains unclear if they can significantly affect performance. In a retrospective analysis we examined performance on the Psychomotor Vigilance Test before bedtime and after wake time in 11 healthy adults on fixed sleep schedules based on their habitual sleep times. The dim light melatonin onset, a marker of circadian timing, was measured on two occasions. An average 1.1 hour shift away from a proposed optimal circadian phase angle (6 hours between melatonin onset and midpoint of sleep) significantly slowed mean, median and fastest 10% reaction times before bedtime and after wake time (p<0.05). These results add to previous reports that suggest that humans may be sensitive to commonly occurring small shifts in circadian timing.
Keywords: circadian, melatonin, performance
1.1 Introduction
The deleterious effect of major circadian misalignment on cognitive performance, such as that which occurs during night shift work, is well recognized (e.g. Ferguson et al., 2012; Wright et al., 2006). However the effect of more subtle changes in circadian phase, such as those that commonly occur in the general population after daylight saving time or returning to work after later weekend sleep, have not been well studied. Daylight saving time affects a quarter of the world’s population twice a year and humans do not always adjust to the 1 hour time shift (Kantermann et al., 2007). Other reports suggest the transition to daylight saving time can have significant health consequences, such as a 6–10% increase in myocardial infarction (Janszky and Ljung, 2008). Later sleep times on the weekend are very common, with a recent population survey suggesting on average adults delay bedtime by up to 1 hour and delay wake time by up to 2 hours on the weekend or on non-work days (National Sleep Foundation, 2011). Later sleep on the weekend leads to circadian phase delays of up to 1 hour (Taylor et al., 2008; Yang et al., 2001), and worsens subjective sleepiness and well-being on Monday morning (Yang et al., 2001), possibly until Wednesday afternoon (Taylor et al., 2008). One research group reported reduced performance on memory and verbal fluency tasks on Monday morning after delayed weekend sleep (Yang and Spielman, 2001), but later failed to replicate this finding (Yang et al., 2001). Otherwise, the effect of such common but small shifts in circadian phase on objective performance measures has received little attention.
To assess the effects of small circadian phase shifts on performance, we conducted a posthoc analysis of circadian phase and performance data we collected from the same individuals on two separate occasions. We used what others have proposed to be an optimal phase angle of 6 hours between the melatonin onset and midpoint of sleep (Lewy et al., 2006), which is equivalent to a 2 hour interval between melatonin onset and bedtime in 8 hour sleepers. This phase angle is commonly observed in healthy subjects (Burgess and Eastman, 2005) and is also associated with better mood in healthy subjects (Emens et al., 2009b). Therefore, we examined the change in performance when people were closer to versus further from this optimal circadian phase angle.
1.2 Material and Methods
Baseline data was selected from two previous studies (Burgess, 2010; Smith and Eastman, 2009) and an ongoing unpublished study in our laboratory. In all studies subjects had two baseline periods in which their dim light melatonin onset, the most reliable circadian phase marker (Klerman et al., 2002), was assessed. For this analysis all subjects who had at least a 30 minute difference in the timing of their two melatonin onsets were selected. The sample included eleven healthy subjects (8 men, 3 women, mean age ± S.D. 31.0 ± 5.6 years, body mass index 26.6 ± 2.9 kg/m2), free of medication (including hormonal contraception), non-smokers and not extreme chronotypes (Horne and Ostberg, 1976). All subjects passed a urine drug screen and reported low habitual caffeine (≤ 300 mg) and alcohol (≤ 2 drinks) daily use. All subjects gave written informed consent prior to participation and the studies were approved by the Rush University Medical Center Institutional Review Board.
In each baseline period, subjects followed an 8 hour sleep schedule at home for 6 to 9 days, matched to their habitual sleep times recorded in the week before the study start. Subjects completed sleep logs and their activity was sampled every 30 seconds by a wrist actigraph (Actiwatch-L, Respironics, USA). Compliance to the sleep schedule was confirmed with actigraphy every 1–3 days. The average sleep schedule was 00:22 to 8:22 hours. Subjects completed a 5 minute Psychomotor Vigilance Test (PVT) 15 minutes before and after assigned bed and wake time (Burgess, 2010) or 1 hour before assigned bed time and 2 hours after assigned wake time (Smith and Eastman, 2009), on a portable handheld device. The PVT is one of the most widely used and sensitive measures of sustained performance.
After the baseline sleep at home, subjects participated in a circadian phase assessment in the laboratory. Saliva samples were collected in dim light (< 5 lux at angle of gaze) every 30 minutes, beginning 7–8 hours before bedtime. Non-steriodal anti-inflammatory drugs (NSAIDs), alcohol and caffeine were prohibited for at least 72, 40 and 29 hours before saliva collection, respectively to avoid contamination of the data. Pharmasan Labs (Osceola, WI) radioimmunoassayed the samples for melatonin, with assay sensitivity of 0.7 pg/ml and intra- and inter-assay variability 12.1% and 13.2%, respectively. The circadian phase angle was calculated as the interval between the melatonin onset (3 pg/ml threshold with linear interpolation) and midpoint of sleep.
The PVT tests completed before and after the last night of baseline sleep, just prior to each circadian phase assessment, were analyzed. Mean and median reaction time (RT) and slowest and fastest 10% RTs were extracted from each PVT. Total sleep time, sleep efficiency and sleep onset latency on the night before each circadian phase assessment were derived from the wrist actigraphy recordings with Actiware 5.59 software (medium sensitivity, Respironics, USA). The performance variables were analyzed in ANOVAs with within subjects factors Condition (closer to or further from phase angle of 6 hours), Time (rating before bed or after wake), and between subjects factor Order (first or second circadian phase assessment). The actigraphy variables were analyzed in an ANOVA with factors Condition and Order. The Order factor was subsequently removed from all analyses as it was not significant. Statistical significance for all analyses was determined with two-tailed tests at p<0.05.
1.3 Results
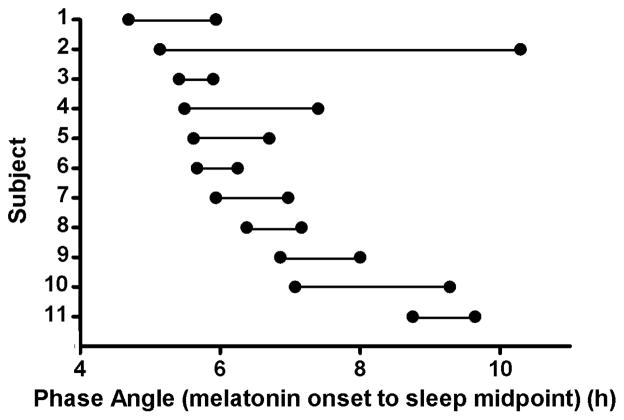
Each subject’s phase angle was calculated and classified as closer to or further from a circadian phase angle of 6 hours. Figure 1 illustrates the phase angles for each subject at the two phase assessments. On average the difference in circadian phase angle between the two assessments was only 1.1 hours (6.3 versus 7.4 hours). One subject showed a large change of 5.2 hours in phase angle.
Figure 1.
The individual circadian phase angles (melatonin onset to midpoint of sleep) in the sample. Two phase angles were calculated for each subject, represented by two dots connected by a horizontal line.
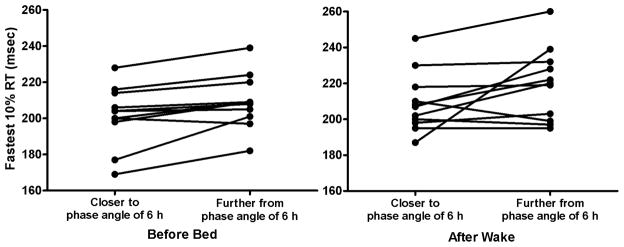
There was a significant or near significant effect of Condition in the PVT variables (mean RT p=0.042, median RT p=0.04, slowest 10% RT p=0.051, fastest 10% RT p=0.024), reflecting a significant slowing in performance when subjects were further way from a circadian phase angle of 6 hours (Table 1). There were no significant effects of Time or Condition by Time interaction. These results remained significant even after removal of the subject with a phase angle difference of 5.2 hours. Figure 2 shows the individual changes in the optimum response of fastest 10% RT from a phase angle closer to 6 versus a phase angle further from 6 for before bedtime and after wake. Ten of eleven subjects had a slower reaction time when further from the circadian phase angle of 6 hours before bedtime, whereas eight of eleven subjects showed this effect after wake. There was no significant effect of Condition (p>0.05) in any of the actigraphic sleep variables.
Table 1.
Mean and standard deviation of PVT variables (msec) closer to or further from a phase angle of 6 hours.
| Before Bedtime | After Wake Time | |||
|---|---|---|---|---|
| Closer to phase angle of 6 hours | Further from phase angle of 6 hours | Closer to phase angle of 6 hours | Further from phase angle of 6 hours | |
| Mean reaction time* | 261.3 (32.8) | 296.6 (70.1) | 280.7 (31.8) | 313.3 (75.8) |
| Median reaction time* | 250.5 (29.5) | 268.2 (34.0) | 261.8 (28.9) | 290.9 (51.5) |
| Slowest 10% reaction time† | 392.2 (105.8) | 546.6 (363.5) | 439.9 (103.8) | 541.4 (302.2) |
| Fastest 10% reaction time* | 201.5 (16.7) | 209.4 (14.8) | 209.1 (16.6) | 219.5 (20.1) |
p<0.05,
p=0.051 Main effect of Condition (circadian phase)
Figure 2.
The individual changes in the fastest 10% reaction time from closer to a phase angle of 6 hours to further from a phase angle of 6 hours, before bed (left) and after wake (right). Each line connecting two dots represents the change in optimum 10% fastest reaction time per individual.
1.4 Discussion/Conclusion
These results suggest that subtle shifts in circadian phase angle of ~ 1 hour, akin to those seen during daylight saving time changes and recovery from weekend sleep, can affect human performance. Our results support a single previous report of decrements in memory and verbal fluency on Monday morning following a 2 hour delay in sleep on the weekend (Yang and Spielman, 2001). Most likely this group’s failure to later replicate their initial finding was because of a smaller sample size in their follow up study (n=10 vs. n=30). Interestingly, the approximate 10 msec slowing in fastest 10% RT that we observed when subjects were further from a circadian phase angle of 6 hours is remarkably similar to a 10 msec slowing in fastest 10% RT on a 5 minute PVT others observed between midnight and 1am in healthy subjects (Figure 3 in Loh et al., 2004). The significant change in performance could be due to changes in circadian phase angle, changes in sleep or both. While no significant changes in total sleep time, sleep efficiency or sleep onset latency were detected, actigraphy is often not sensitive enough to distinguish between quiet wakefulness and sleep. Nonetheless, subtle changes in circadian phase angle of about 0.5 hours have previously been shown not to significantly affect sleep, even when it is measured polysomnographically in double blind conditions (Yang et al., 2001). Our study was also conducted double blind in that neither subjects nor research staff was aware of the subjects’ circadian phase until the study was completed.
There are several limitations to our results which should be considered preliminary. The results come from a small sample derived from a retrospective analysis of all data in our laboratory that met our selection criteria (section 1.2). In our analysis we used a proposed optimal circadian phase angle of 6 hours between the melatonin onset and midpoint of sleep. While this circadian phase angle is often reported in healthy subjects (Burgess and Eastman, 2005) it may not be optimal for all individuals, especially those of extreme chronotype. Indeed, there may be differences in patient groups with depression – a phase angle difference greater than 6 is associated with more depression in winter depressives (Lewy et al., 2006), but in a small pilot study of non-seasonal depressives was associated with less depression (Emens et al., 2009a). Nonetheless, the phase angle difference provides a useful tool to capture sleep timing relative to circadian phase, as opposed to circadian phase alone. Using this metric, our preliminary results suggest that commonly occurring small shifts in circadian phase may not only affect subjective sleepiness and well being, but also cognitive performance.
Acknowledgments
We thank Elizabeth Beam, Jillian Canton, Erin Cullnan, Corinne Eckstein, Rose Diskin, Marissa Dziepak, Heather Holly, Thomas Molina, Jacqueline Munoz, Meredith Rathert, Christina Suh, and Nicole Woodrick for their assistance with data collection. We also thank Christina Suh for creating the figures. This work was made possible by grants from the National Institutes of Health (NIH) R01 NR007677 and R01 HL083971. The content is solely the responsibility of the authors and does not represent the official views of NIH. NIH had no role in study design, data collection and analysis, interpretation of the data, and in the preparation, review or approval of manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burgess HJ. Partial sleep deprivation reduces phase advances to light in humans. J Biol Rhythms. 2010;25:460–468. doi: 10.1177/0748730410385544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14:229–237. doi: 10.1111/j.1365-2869.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009a;168:259–261. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Emens J, Lewy AJ, Rough JN, Songer JB. Sub-clinical dysphoria correlates with phase delayed circadian misalignment in healthy individuals. Sleep. 2009b;32:A355–A356. [Google Scholar]
- Ferguson SA, Kennaway DJ, Baker A, Lamond N, Dawson D. Sleep and circadian rhythms in mining operators: limited evidence of adaptation to night shifts. Appl Ergon. 2012;43:695–701. doi: 10.1016/j.apergo.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. Self-assessment questionnaire to determine morningness- eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Janszky I, Ljung R. Shifts to and from daylight saving time and incidence of myocardial infarction. N Engl J Med. 2008;359:1966–1968. doi: 10.1056/NEJMc0807104. [DOI] [PubMed] [Google Scholar]
- Kantermann T, Juda M, Merrow M, Roenneberg T. The human circadian clock's seasonal adjustment is disrupted by daylight saving time. Curr Biol. 2007;17:1996–2000. doi: 10.1016/j.cub.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci USA. 2006;103:7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh S, Lamond N, Dorrian J, Roach G, Dawson D. The validity of psychomotor vigilance tasks of less than 10-minute duration. Behav Res Methods Instrum Comput. 2004;36:339–346. doi: 10.3758/bf03195580. [DOI] [PubMed] [Google Scholar]
- NationalaSleepaFoundation. [Accessed May 15, 2012];Sleep in America Poll Summary of Findings. 2011 www.sleepfoundation.org.
- Smith MR, Eastman CI. Phase delaying the human circadian clock with blue-enriched polychromatic light. Chronobiol Int. 2009;26:709–725. doi: 10.1080/07420520902927742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Wright HR, Lack LC. Sleeping-in on the weekend delays circadian phase and increases sleepiness the following week. Sleep Biol Rhythms. 2008;6:172–179. [Google Scholar]
- Wright KP, Jr, Hull JT, Hughes RJ, Ronda JM, Czeisler CA. Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cogn Neurosci. 2006;18:508–521. doi: 10.1162/jocn.2006.18.4.508. [DOI] [PubMed] [Google Scholar]
- Yang CM, Spielman A. The effect of a delayed weekend sleep pattern on sleep and morning functioning. Psychol Health. 2001;16:715–725. [Google Scholar]
- Yang CM, Spielman AJ, D'Ambrosio P, Serizawa S, Nunes J, Birnbaum J. A single dose of melatonin prevents the phase delay associated with a delayed weekend sleep pattern. Sleep. 2001;24:272–281. doi: 10.1093/sleep/24.3.272. [DOI] [PubMed] [Google Scholar]