Abstract
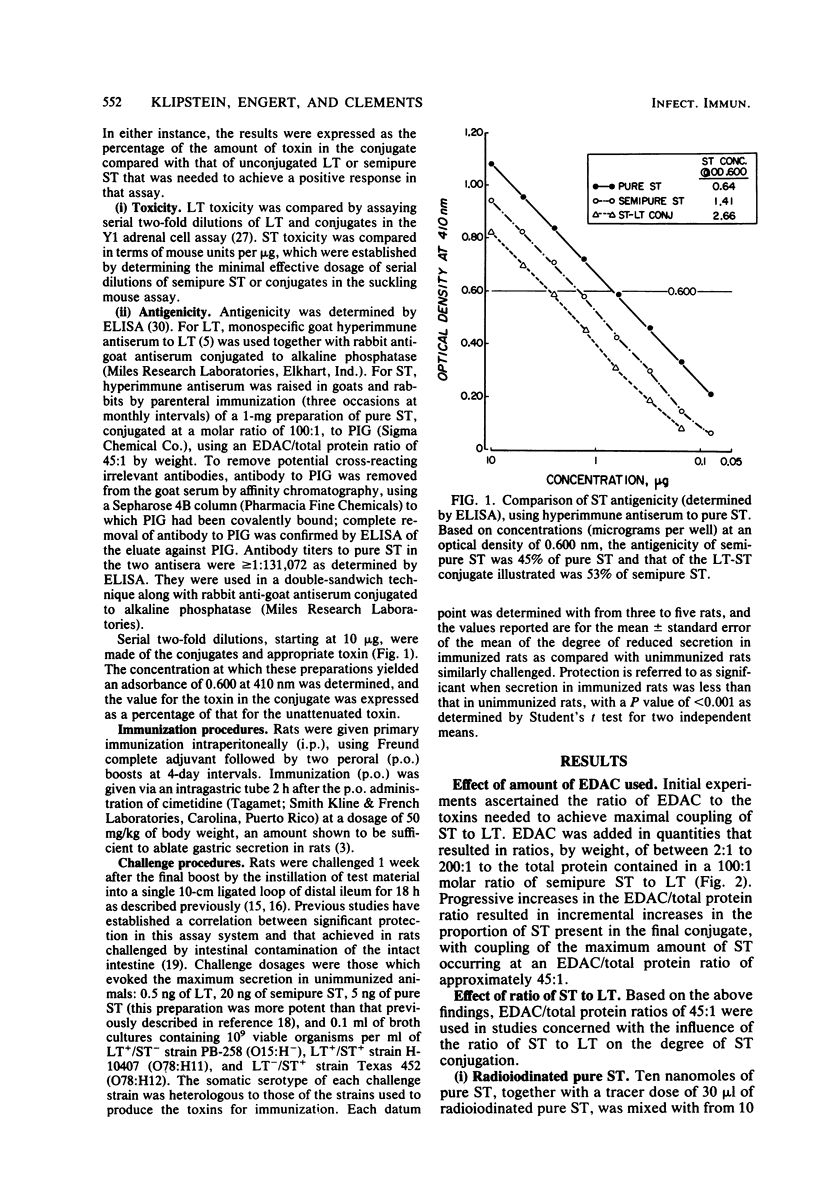
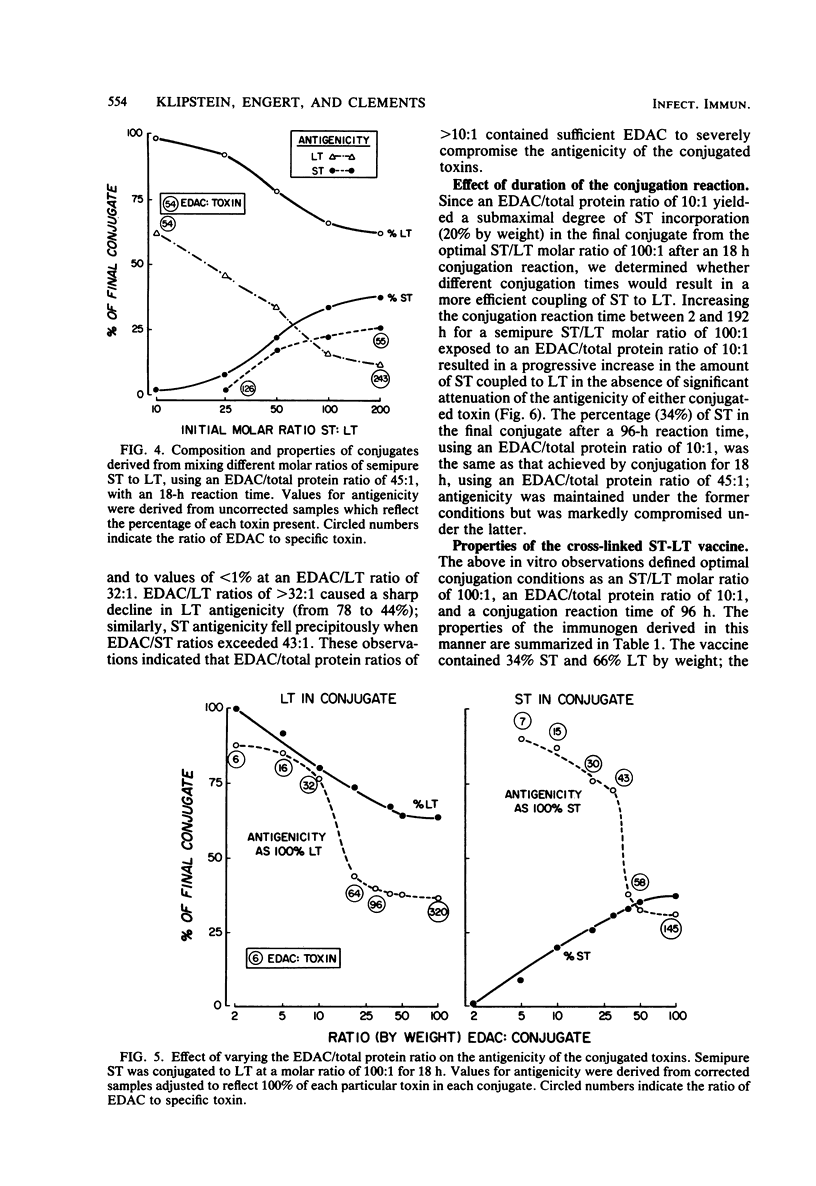
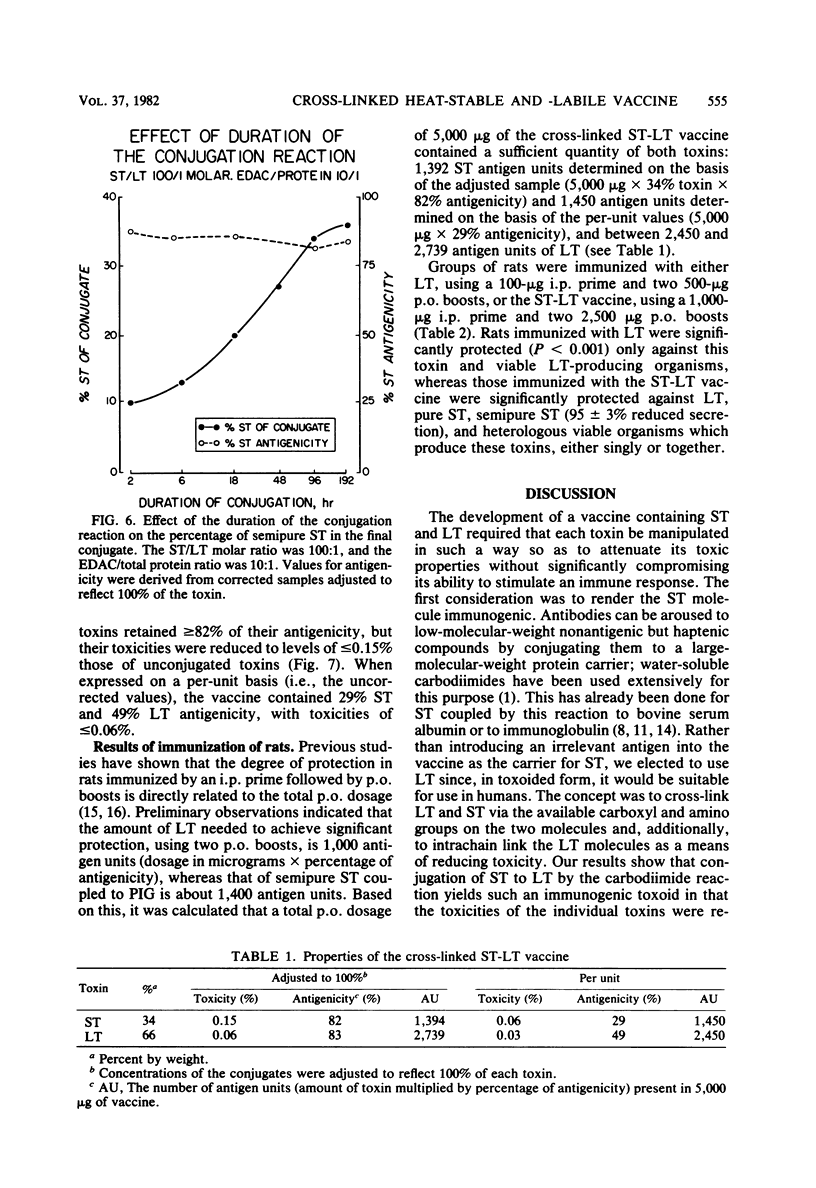
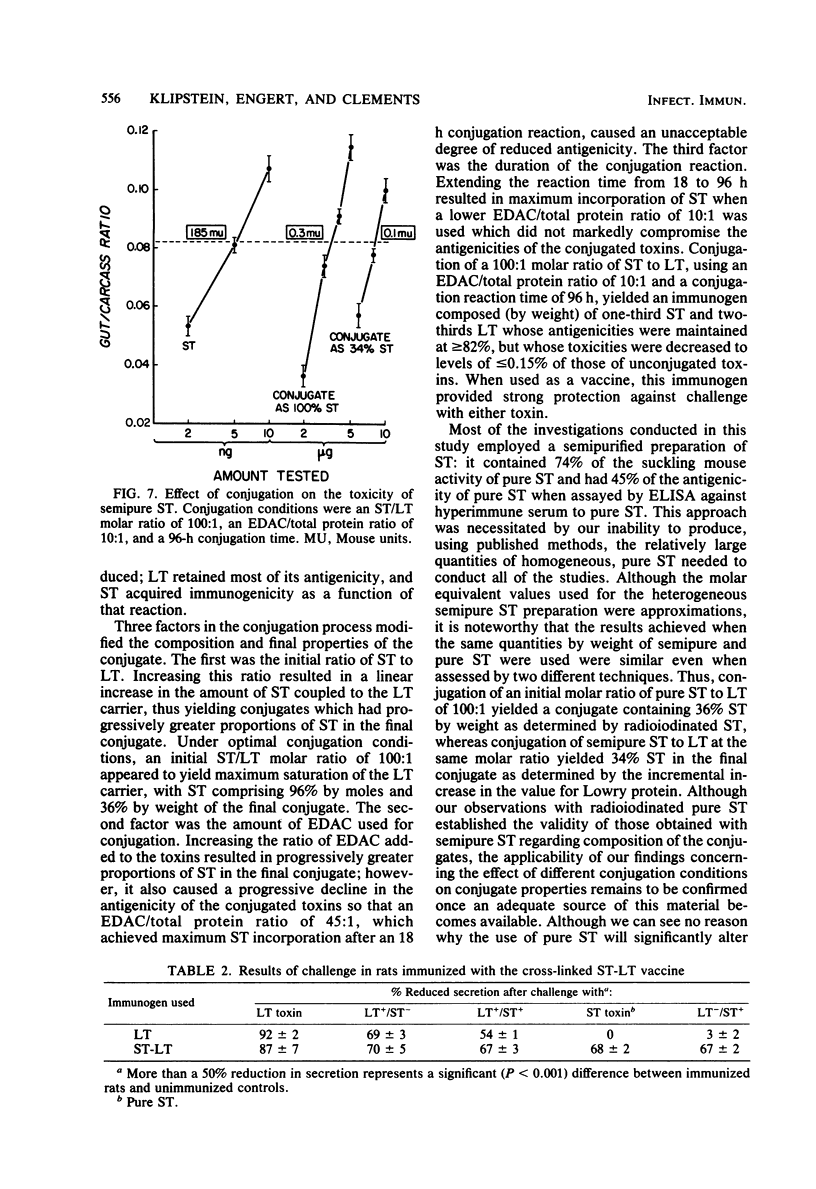
A vaccine of cross-linked heat-stable (ST) and heat-labile (LT) toxins that protects against heterologous serotypes of strains of Escherichia coli which produce either the LT or ST enterotoxin was developed by conjugating ST to LT by the carbodiimide reaction. Three interrelated factors were found to affect the composition and properties of the final conjugate: (i) the amount of carbodiimide added to the toxins, (ii) the initial ratio of ST to LT, and (iii) the duration of the conjugation reaction. Optimal conjugation conditions were identified as a carbodiimide-to-toxin ratio of 10:1 by weight, an initial molar ratio of ST to LT of 100:1, and a conjugation reaction time of 96 h. This approach yielded a conjugate that contained 96% by moles and 36% by weight pure ST, determined with radioiodinated pure ST, and 34% by weight semi-pure ST, determined by the Lowry protein method. The retained antigenicities of the conjugated toxins, as determined by enzyme-linked immunosorbent assays, was greater than or equal to 82%, and their toxicities, as determined by the Y1 adrenal cell assay for LT and by the suckling mouse assay for ST, were reduced to less than or equal to 0.15%. Immunization of rats with this cross-linked ST-LT vaccine provided strong protection against challenge with either the LT or the ST toxin or with viable heterologous strains which produce these toxins, either singly or together. These observations indicate that conjugation of ST to LT results in a unique new immunogen in that ST acquires immunogenicity as a function of the reaction, LT retains most of its antigenicity, and the toxic properties of each individual toxin are greatly reduced.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauminger S., Wilchek M. The use of carbodiimides in the preparation of immunizing conjugates. Methods Enzymol. 1980;70(A):151–159. doi: 10.1016/s0076-6879(80)70046-0. [DOI] [PubMed] [Google Scholar]
- Black R. E., Merson M. H., Huq I., Alim A. R., Yunus M. Incidence and severity of rotavirus and Escherichia coli diarrhoea in rural Bangladesh. Implications for vaccine development. Lancet. 1981 Jan 17;1(8212):141–143. doi: 10.1016/s0140-6736(81)90719-4. [DOI] [PubMed] [Google Scholar]
- Brimblecombe R. W., Duncan W. A., Durant G. J., Emmett J. C., Ganellin C. R., Leslie G. B., Parsons M. E. Characterization and development of cimetidine as a histamine H2-receptor antagonist. Gastroenterology. 1978 Feb;74(2 Pt 2):339–347. [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979 Jun;24(3):760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Yancey R. J., Finkelstein R. A. Properties of homogeneous heat-labile enterotoxin from Escherichia coli. Infect Immun. 1980 Jul;29(1):91–97. doi: 10.1128/iai.29.1.91-97.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneke C. F., Thorne G. M., Gorbach S. L. Serotypes of attachment pili of enterotoxigenic Escherichia coli isolated from humans. Infect Immun. 1981 Jun;32(3):1254–1260. doi: 10.1128/iai.32.3.1254-1260.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz J. C., Robertson D. C. Immunological properties of Escherichia coli heat-stable enterotoxins: development of a radioimmunoassay specific for heat-stable enterotoxins with suckling mouse activity. Infect Immun. 1981 Jul;33(1):193–198. doi: 10.1128/iai.33.1.193-198.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Drake K. W., Luttrell M. Development of a radioimmunoassay for Escherichia coli heat-stable enterotoxin: comparison with the suckling mouse bioassay. Infect Immun. 1981 Jul;33(1):186–192. doi: 10.1128/iai.33.1.186-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun. 1976 Jul;14(1):95–99. doi: 10.1128/iai.14.1.95-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Rouse J. D., Hughes J. M., Rowe B. Turista among members of the Yale Glee Club in Latin America. Am J Trop Med Hyg. 1980 Sep;29(5):895–900. doi: 10.4269/ajtmh.1980.29.895. [DOI] [PubMed] [Google Scholar]
- Hunter R. Standardization of the chloramine-T method of protein iodination. Proc Soc Exp Biol Med. 1970 Mar;133(3):989–992. doi: 10.3181/00379727-133-34611. [DOI] [PubMed] [Google Scholar]
- Kauffman P. E. Production and evaluation of antibody to the heat-stable enterotoxin from a human strain of enterotoxigenic Escherichia coli. Appl Environ Microbiol. 1981 Oct;42(4):611–614. doi: 10.1128/aem.42.4.611-614.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Clements J. D. Immunization of rats with heat-labile enterotoxin provides uniform protection against heterologous serotypes of enterotoxigenic Escherichia coli. Infect Immun. 1981 Jun;32(3):1100–1104. doi: 10.1128/iai.32.3.1100-1104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Clements J. D. Protection in rats immunized with Escherichia coli heat-stable enterotoxin. Infect Immun. 1981 Nov;34(2):637–639. doi: 10.1128/iai.34.2.637-639.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Protective effect of immunization of rats with holotoxin or B subunit of Escherichia coli heat-labile enterotoxin. Infect Immun. 1981 Jan;31(1):144–150. doi: 10.1128/iai.31.1.144-150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Respective contributions to protection of primary and booster immunization with Escherichia coli heat-labile enterotoxin in rats. Infect Immun. 1981 Jan;31(1):252–260. doi: 10.1128/iai.31.1.252-260.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Short H. B. Protective effect of immunization with heat-labile enterotoxin in gnotobiotic rats monocontaminated with enterotoxigenic Escherichia coli. Infect Immun. 1980 Apr;28(1):163–170. doi: 10.1128/iai.28.1.163-170.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler E. M. Neonatal enteric colibacillosis of pigs and current research on immunization. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):588–591. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine M. M., Rennels M. B., Daya V., Hughes T. P. Hemagglutination and colonization factors in enterotoxigenic and enteropathogenic Escherichia coli that cause diarrhea. J Infect Dis. 1980 Jun;141(6):733–737. doi: 10.1093/infdis/141.6.733. [DOI] [PubMed] [Google Scholar]
- Morgan R. L., Isaacson R. E., Moon H. W., Brinton C. C., To C. C. Immunization of suckling pigs against enterotoxigenic Escherichia coli-induced diarrheal disease by vaccinating dams with purified 987 or K99 pili: protection correlates with pilus homology of vaccine and challenge. Infect Immun. 1978 Dec;22(3):771–777. doi: 10.1128/iai.22.3.771-777.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalin D. R., McLaughlin J. C., Rahaman M., Yunus M., Curlin G. Enterotoxigenic Escherichia coli and idiopathic diarrhoea in Bangladesh. Lancet. 1975 Dec 6;2(7945):1116–1119. doi: 10.1016/s0140-6736(75)91005-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. L., Koplan J. P., Wachsmuth I. K., Wells J. G., Gangarosa E. J., Guerrant R. L., Sack D. A. Epidemic diarrhea at Crater Lake from enterotoxigenic Escherichia coli. A large waterborne outbreak. Ann Intern Med. 1977 Jun;86(6):714–718. doi: 10.7326/0003-4819-86-6-714. [DOI] [PubMed] [Google Scholar]
- Sack D. A., Kaminsky D. C., Sack R. B., Wamola I. A., Orskov F., Orskov I., Slack R. C., Arthur R. R., Kapikian A. Z. Enterotoxigenic Escherichia coli diarrhea of travelers: a prospective study of American Peace Corps volunteers. Johns Hopkins Med J. 1977 Aug;141(2):63–70. [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B., Hirschhorn N., Brownlee I., Cash R. A., Woodward W. E., Sack D. A. Enterotoxigenic Escherichia-coli-associated diarrheal disease in Apache children. N Engl J Med. 1975 May 15;292(20):1041–1045. doi: 10.1056/NEJM197505152922001. [DOI] [PubMed] [Google Scholar]
- Staples S. J., Asher S. E., Giannella R. A. Purification and characterization of heat-stable enterotoxin produced by a strain of E. coli pathogenic for man. J Biol Chem. 1980 May 25;255(10):4716–4721. [PubMed] [Google Scholar]



