Abstract
Patterned microstimulation of muscle and cutaneous afferent neurons may provide tactile and proprioceptive feedback to users of advanced prosthetic limbs. However, it is unclear what types of stimulation patterns will be effective, and the parameter space for creating these patterns is prohibitively large to explore systematically using only psychophysics paradigms. In this study, we used an array of microelectrodes in primary somatosensory cortex (S1) of an isoflurane anesthetized cat to measure responses in a population of neurons evoked by various patterns of primary afferent microstimulation delivered to the L6 and L7 dorsal root ganglia (DRG). Each pattern consisted of a 300 ms train of microstimulation pulses having a fixed amplitude, pulse rate, and location in the array of DRG electrodes. Evoked responses were detectable on many S1 channels at the lowest amplitude tested (5 μA) and pulse rate (10 pulses per second). Increasing the pulse rate lowered the threshold amplitude for evoking a response on some S1 channels. Location effects were also observed. Adjacent stimulation sites evoked discriminable responses at low but not high (20 μA) amplitudes. In summary, we observed interactions between stimulation pulse rate, pulse amplitude, and location. Such interactions must be considered when designing stimulation patterns for transmitting sensory feedback by primary afferent microstimulation.
I. Introduction
Acceptance and usefulness of modern prosthetics is limited by their lack of sensory feedback [1,2]. To overcome this limitation, patterned microstimulation of primary afferent neurons is being explored as a way to transmit sensory information into the central nervous system (CNS). Recent work in amputee patients has already shown that electrical stimulation with intrafascicular electrodes in peripheral nerves evokes painless sensations of touch and joint movement that were perceived to originate in the phantom limb [3].
The dorsal root ganglia (DRG) provide a compact target for accessing large populations of somatosensory fibers with high density arrays of microelectrodes. Previous studies have shown that penetrating microelectrodes in the DRG can provide selective activation of various types of muscle and cutaneous afferents [4]. A challenging problem is how to create effective patterns of stimulation in the array of inputs provided by the electrodes. The amplitude and rate of stimulation can be varied independently on each electrode, resulting in an extremely large parameter space for creating feedback patterns.
The goal of this study was to examine how variations in the basic parameters that define a multichannel pattern affect both the threshold for evoking a response and the range over which the response is readily distinguished from other inputs. Various patterns of primary afferent microstimulation (PAMS) were applied via penetrating microelectrodes in the lumbar DRG of anesthetized cats. We quantified the response to stimulation using the firing rates of neurons recorded on an array of microelectrodes in primary somatosensory cortex (S1).
Although it is not possible to know what type of sensation (if any) is represented by each S1 response, our goal was to examine the extent to which these responses differ across variations in the stimulus parameters. Stimuli that evoke similar responses in S1 are presumed to carry similar information. However, if variation in a particular stimulus parameter (e.g., pulse rate) leads to a large modulation in the cortical response, then variation of that parameter is viewed as an effective mode of conveying information to the brain (e.g., see [5]).
This paper presents some initial results regarding threshold and discriminability as a function of the following stimulus parameters: pulse amplitude, rate, and electrode location. Interactions between these parameters are observed in determining stimulus threshold. High classification accuracy between independently activated stimulus locations suggests they can be used as separate pathways for providing feedback. Results also demonstrate interesting interactions in the neural response evoked by multichannel stimulation.
II. Methods
Results shown are from a single experiment. Experimental procedures were performed in accordance with the University of Pittsburgh IACUC.
A. Experimental Procedures
Isoflurane (1–2%) was used to maintain the cat in a surgical anesthetic plane, and after some preliminary surgery to expose the DRG, the cat was placed in a stereotaxic frame. Vitals were monitored continuously and kept within normal ranges. Electrode arrays (Blackrock Microsystems) were placed in the L6 and L7 DRGs as well as hindlimb area of S1 cortex (post-cruciate gyrus). Stimulation was conducted on 30 channels, 14 in L6 and 16 in L7 (see Fig. 1A), one or two at a time using an MS-16 stimulus isolator (TDT). Cortical recordings (48 channels) were sampled at 25 kHz using an RZ system (TDT) and manually thresholded to determine times of multi-unit spiking activity.
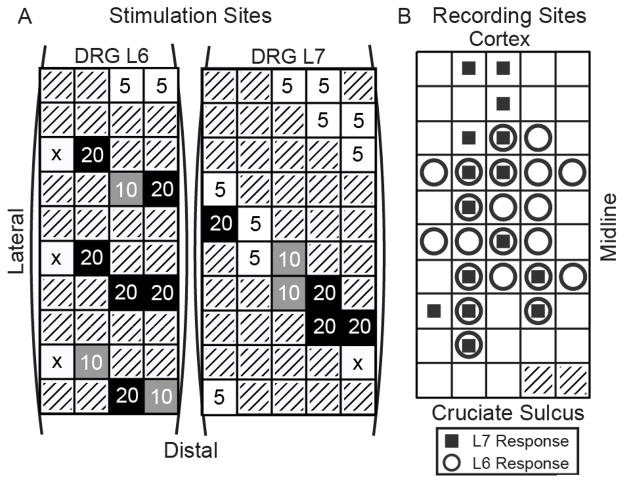
Fig. 1.
A: Spatial layout of the stimulus amplitude threshold (μA) for evoking a significant cortical response (vs. baseline) on each tested stimulus channel for the L6 and L7 DRGs. Threshold at any stimulus rate counted as being at threshold for the reported stimulus amplitude. An ‘x’ indicates that no stimulus amplitude/rate pair was sufficient to elicit a cortical response. Gray boxes represent disconnected channels. B: Spatial pattern of responses in S1 to 5 μA stimulation. Circles and squares indicate that a response was elicited by at least one electrode in the L6 or L7 DRG, respectively.
Stimulation pulses were biphasic with a 200 μs cathodic phase followed by a half amplitude 400 μs anodic phase with a distant return electrode. Discrete stimulation patterns were applied to 1 of 30 electrodes at a time and consisted of a 300 ms train of pulses having a fixed amplitude and pulse rate, followed by a 700 ms quiescent period without stimulation. A total of 360 different patterns were tested (3 intensities [5, 10, 20 μA] and 4 pulse rates [10, 100, 300, and 1000 pulses per second; pps] at 30 different electrode locations) with 10 repetitions for each pattern. Single repetitions of each pattern were tested in random order.
We also tested a limited number of 2-channel stimulation patterns using the same stimulus pulse rates and amplitudes as described above. In each 2-channel trial, the same stimulus pattern was applied synchronously to both electrodes in the pair. The S1 response to 2-channel stimulation was compared to the responses evoked by single channel stimulation on each electrode in the pair. This test was done to examine interactions in the neural responses evoked by the inputs applied at two different locations.
B. Data Analysis
The cortical response was evaluated as the spike count in a 50 ms bin starting 10 ms after the onset of a stimulation train. We used a Naive Bayes classifier with leave-one-out cross validation to determine differences in the S1 responses evoked by different stimulus patterns. Differences were considered significant if they exceeded a 99% confidence interval on chance (> 78% classification accuracy over the 20 total repetitions, 10 from each pattern). The cortical population was used for classification with the exception of Fig. 1B in which single cortical channels were used.
III. Results
A. Thresholds for evoking S1 responses
Fig. 1A shows the electrode locations that were tested in each DRG. The numbers indicate the lowest stimulation amplitude at each site that evoked a significant response in S1 as compared to baseline. Over 30% of channels (11 of 30) evoked a response at the lowest amplitude (5 μA; at least one pulse rate). A similar percentage of channels (10 of 30) required much higher currents (20 μA) to evoke a response. A small number of channels did not evoke a response at any of the levels tested, although those electrodes may have been outside the ganglia. Note that the differences are unlikely to reflect variability in the thresholds for recruiting neurons in the DRG [4], but may instead reflect differences in the pattern of connectivity from the DRG to S1. Also note that some clustering of thresholds is apparent in both arrays, which may indicate a certain level of somatotopic organization of sensory fibers within each DRG [6].
Of the 48 channels in S1, 26 showed significant responses vs. baseline from stimulation at 5 μA on at least one stimulus channel (see Fig. 1B). Eleven of these channels were responsive only to stimulation in L6, 5 were responsive only to L7, and 10 channels recorded responses evoked by stimulation in both locations.
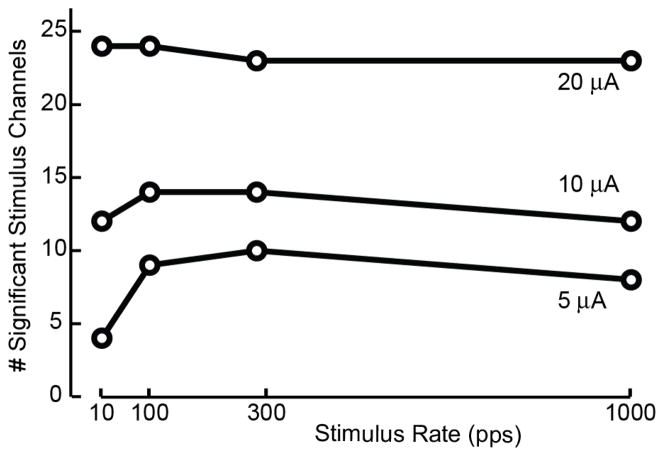
Fig. 2 illustrates the interaction between stimulation pulse rate and amplitude on the threshold for evoking a cortical response vs. baseline. With 5 μA stimulation applied at 10 pps, only 4 DRG electrodes evoked a significant response in S1. More than twice as many stimulation channels evoked a response at 5 μA when higher pulse rates were used. A similar but smaller effect was observed for 10 μA stimulation pulses, whereas no effect was observed at 20 μA. Although higher amplitude stimulation was most effective in surpassing the threshold needed to evoke an S1 response, it is clear that high pulse rate stimulation is also effective at facilitating stronger responses in S1.
Fig. 2.
Interaction between stimulation pulse rate and amplitude on threshold for evoking a response in S1. Number of stimulus channels that evoked a discriminable response in cortex as a function of stimulus pulse rate for 5, 10, and 20 μA pulses.
B. Effects of stimulation location
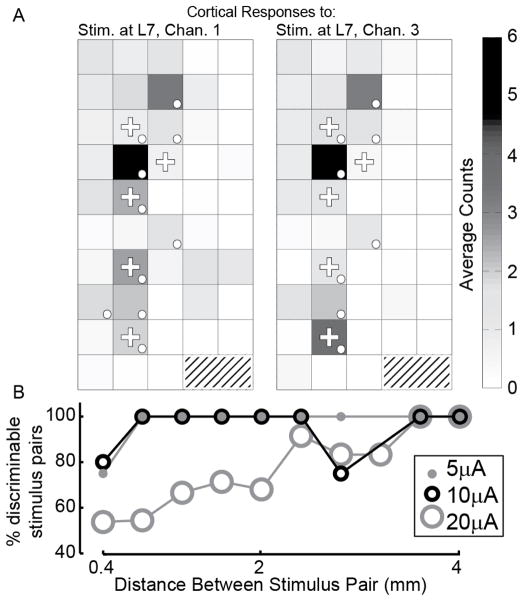
We examined how the discriminability of the S1 response varied with the relative location of two stimulation sites in the DRG arrays. Fig. 3A shows examples of the neural response averaged for stimulus patterns (10 repetitions each) that were applied at channels 1 and 3 (566 μm apart) in the L7 DRG. The averaged responses appear qualitatively similar but were discriminated reliably by a Naive Bayes classifier with 90% accuracy (18/20).
Fig. 3.
Discriminability of S1 responses evoked by stimulation at different locations, pulse rates, and amplitudes. A: Examples of the average cortical response to stimulation on channels 1 (left) and 3 (right) in the L7 DRG at 5 μA and 100 pps. Shown is the average spike count observed 10 – 60 ms following stimulus onset. Dots denote channels showing a significant response; plus symbols (+) indicate channels used for classification. Although they have similar average responses, they can be classified with 90% accuracy. B: Percentage of channel pairs that evoked discriminable S1 responses as a function of the separation distance between the 2 stimulation sites (rate = 100 pps). Only stimulus electrodes that evoked significant responses vs. baseline were included. Pairings were kept within arrays. Distances have been grouped to nearest 0.4 mm.
Fig. 3B shows the percentage of stimulation pairs that evoked discriminable responses in S1 as a function of the distance separating the two stimulation sites (rate = 100 pps in all cases). At 5 and 10 μA, the cortical response was sufficient to discriminate between the stimulus pairs at most distances, with a slight decrease for adjacent stimulus sites as well as one outlier pair separated by 2.8 mm at 10 μA. At 20 μA, the recruitment of a much larger number of primary afferent neurons resulted in S1 response patterns that were difficult to distinguish from each other when coming from nearby stimulus electrodes. For discriminable stimulus pairs, and across all distances, the average classification accuracies were 97%, 93%, and 90%, for stimulation amplitudes 5, 10, and 20 μA, respectively.
C. 2-channel stimulation effects
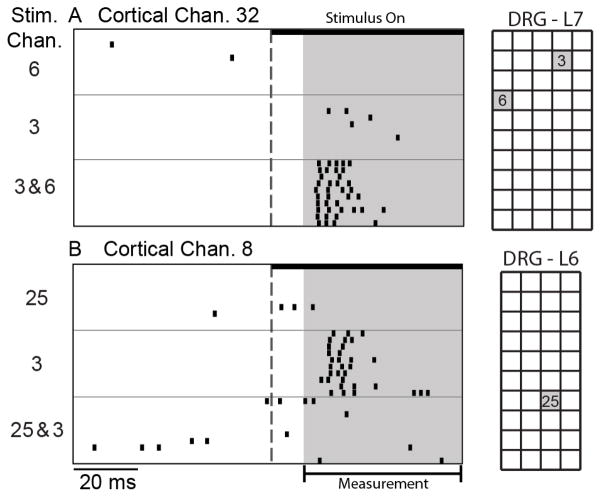
Fig. 4 shows examples of responses evoked on two S1 channels during single and 2-channel stimulation. The S1 channel in Fig. 4A did not respond to stimulation on either of the channels independently, but responded strongly when the same two channels were stimulated together. This type of response suggests a convergence of excitatory inputs activated by channels 3 and 6 (1.44 mm apart), such that the combined activation was sufficient to evoke a response in S1. In Fig. 4B, a response was evoked by stimulation on channel 3, but the response disappeared when paired with stimulation on channel 25. This pattern of responses suggests an inhibitory effect of channel 25 on the response evoked by channel 3. In total, 5.5% of the responses to 2-channel stimulation were similar to Fig. 4A and 14.8% of the responses were similar to Fig. 4B.
Fig. 4.
Two examples of S1 responses to single and 2-channel stimulation at 5μA. A: This S1 channel did not respond to stimulation at channel 6 or 3; concurrent stimulation on channels 3 and 6 evoked a vigorous response (rate = 1000 pps). B: This S1 channel responded to stimulation on channel 3, but not 25. When both channels were stimulated, the response was nearly eliminated (rate = 100 pps).
IV. Discussion
A. Providing Sensory Feedback
The primary aim of this work was to investigate means of providing sensory feedback to the nervous system through patterned microstimulation of primary afferent neurons. Although the response of most primary afferents to external stimuli is fairly well understood, details of how these afferent inputs are integrated in the CNS and their effects on higher-order neural networks are less clear. The manner in which these afferent inputs are integrated is a crucial determinant of the information conveyed by the combined activation of these inputs. Electrical microstimulation of primary afferents can yield insight into how to design stimulation patterns that are effective in conveying information to the CNS, even if they are not naturalistic.
The focus of this paper is on understanding how basic patterns of stimuli differentially activate a population of neurons in S1. We are assuming that if stimuli evoke similar cortical responses, then they convey similar information to the brain. Stimuli that evoke distinct responses, as determined by classification with machine learning algorithms, are presumably discernable by the brain as being different as well. At this point, we cannot conclude that distinct responses in S1 indicate differences in evoked percepts. Future studies will test for perceptual differences using psychophysical discrimination experiments.
B. Results: Implications for sensory neural prosthetics
One of the challenges with using multichannel microelectrode arrays to provide sensory feedback is figuring out how to encode information in the high dimensional input space that is available. The basic parameters of stimulation include pulse amplitude, pulse rate and electrode location and variations in each of these parameters affects the recruitment of neurons. Our results demonstrated that the threshold for evoking a response in S1 was highly dependent on these three parameters. Interactions among these parameters may greatly reduce the effective size of the input space. For example, interactions between stimulation amplitude and pulse-rate (Fig. 2) indicate one mode of dimensionality reduction; at high stimulation amplitudes, variations in pulse rate are less effective in evoking distinct responses. Similarly, interactions between stimulus amplitude and electrode locations (Fig. 3) indicate that the spatial resolution may be reduced as the stimulus amplitude is increased.
It is generally accepted that effective stimulation parameters will vary with stimulus location due to the recruitment of a different neural population. There may exist other dependencies between our stimulus parameters which could be used to inform stimulus design. For example, stimulus channels that primarily activate muscle spindles may require spatial summation to sufficiently activate the cortex [7]. High threshold stimulus channels may be more effective if coactivated rather than simply increasing stimulus amplitude. With more data we plan on building statistical models that characterize these dependencies.
Rate/amplitude interactions suggest paradigms that model perceived intensity as a function of stimulus rate at a fixed amplitude [3] might not generalize to other amplitudes. A desire to selectively activate neurons means that lower stimulation amplitudes would be preferred. Low stimulus amplitudes, however, might limit the range of perceived intensities due to the decrease in responsiveness at high frequencies (Fig. 2). This potential tradeoff is something that our experimental model would examine.
Results such as those shown in Fig. 3 can be used to inform the design of electrode array geometries. Ochoa and Torebjörk [8] mention that artificial stimulation almost never led to natural touch, due to inappropriate or insufficient recruitment. This may be improved by maximizing the number of effective stimulus channels. In this experimental context, this would correspond to maximizing the number of discriminable stimulus channels for different arrays designs. Since we can discriminate between a majority of neighboring electrodes (Fig. 3B) at low amplitudes, this suggests the need for denser arrays, or possibly more complicated stimulation paradigms such as current steering.
With some exceptions, the majority of somatosensory stimulation feedback studies have examined the use of single channels in isolation. Thoroughly examining groups of stimuli is difficult because of the staggering number of channel combinations. Fig. 4 indicates that interactions between stimulus sites exist. Future work can elucidate to what degree these interactions are at the site of recruitment versus convergence of inputs, the dependence of these interactions on the stimulus parameters, and ultimately how multiple channels can be used to increase the amount of deliverable feedback information.
Acknowledgments
This work was supported in part by the NIH Grant 1R21-Ns-056136 and TATRC Grant W81XWH-07-1-0716.
The authors would like to thank Ingrid Albrecht and Tyler Simpson for their assistance during the animal experiment.
Contributor Information
James A. Hokanson, Email: jah104@pitt.edu, Department of Bioengineering, Univ. of Pittsburgh, Pittsburgh, PA, 15213 USA.
Christopher A. Ayers, Email: caa28@pitt.edu, Department of Bioengineering, Univ. of Pittsburgh, Pittsburgh, PA, 15213 USA.
Robert A. Gaunt, Email: rag53@pitt.edu, Department of Physical Medicine and Rehabilitation, Univ. of Pittsburgh, Pittsburgh, PA, 15260 USA.
Tim M. Bruns, Email: tmb59@pitt.edu, Department of Physical Medicine and Rehabilitation, Univ. of Pittsburgh, Pittsburgh, PA, 15260 USA.
Douglas J. Weber, Department of Physical Medicine and Rehabilitation, and the Department of Bioengineering, Univ. of Pittsburgh, Pittsburgh, PA, 15213 USA, and the Pittsburgh Department of Veterans Affairs Medical Center, Pittsburgh, PA 15240 USA.
References
- 1.Mooney V. Sensory feedback in upper-extremity amputees. Clinical Orthopaedics and Related Research. 1976:274–275. [Google Scholar]
- 2.Atkins DJ, Heard DCY, Donovan WH. Epidemiologic Overview of Individuals with Upper-Limb Loss and Their Reported Research Priorities. JPO Journal of Prosthetics and Orthotics. 1996;8:2–11. [Google Scholar]
- 3.Dhillon GS, Lawrence SM, Hutchinson DT, Horch KW. Residual function in peripheral nerve stumps of amputees: implications for neural control of artificial limbs. The Journal of hand surgery. 2004;29:605–608. doi: 10.1016/j.jhsa.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Gaunt RA, Hokanson JA, Weber DJ. Microstimulation of primary afferent neurons in the L7 dorsal root ganglia using multielectrode arrays in anesthetized cats: thresholds and recruitment properties. Neural Eng. 2009;6:55009. doi: 10.1088/1741-2560/6/5/055009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anani AB, Ikeda K, Körner LM. Human ability to discriminate various parameters in afferent electrical nerve stimulation with particular reference to prostheses sensory feedback. Medical & biological engineering & computing. 1977 Jul;15:363–73. doi: 10.1007/BF02457988. [DOI] [PubMed] [Google Scholar]
- 6.Wessels W. A rostrocaudal somatotopic organization in the brachial dorsal root ganglia of neonatal rats. Clinical neurology and neurosurgery. 1993;95:S3–11. doi: 10.1016/0303-8467(93)90027-e. [DOI] [PubMed] [Google Scholar]
- 7.Macefield VG, Gandevia SC, Burke D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. Journal of Physiology. 1990;429:113–129. doi: 10.1113/jphysiol.1990.sp018247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochoa J, Torebjork HE. Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervating the human hand. The Journal of Physiology. 1983;342:633–654. doi: 10.1113/jphysiol.1983.sp014873. [DOI] [PMC free article] [PubMed] [Google Scholar]