Abstract
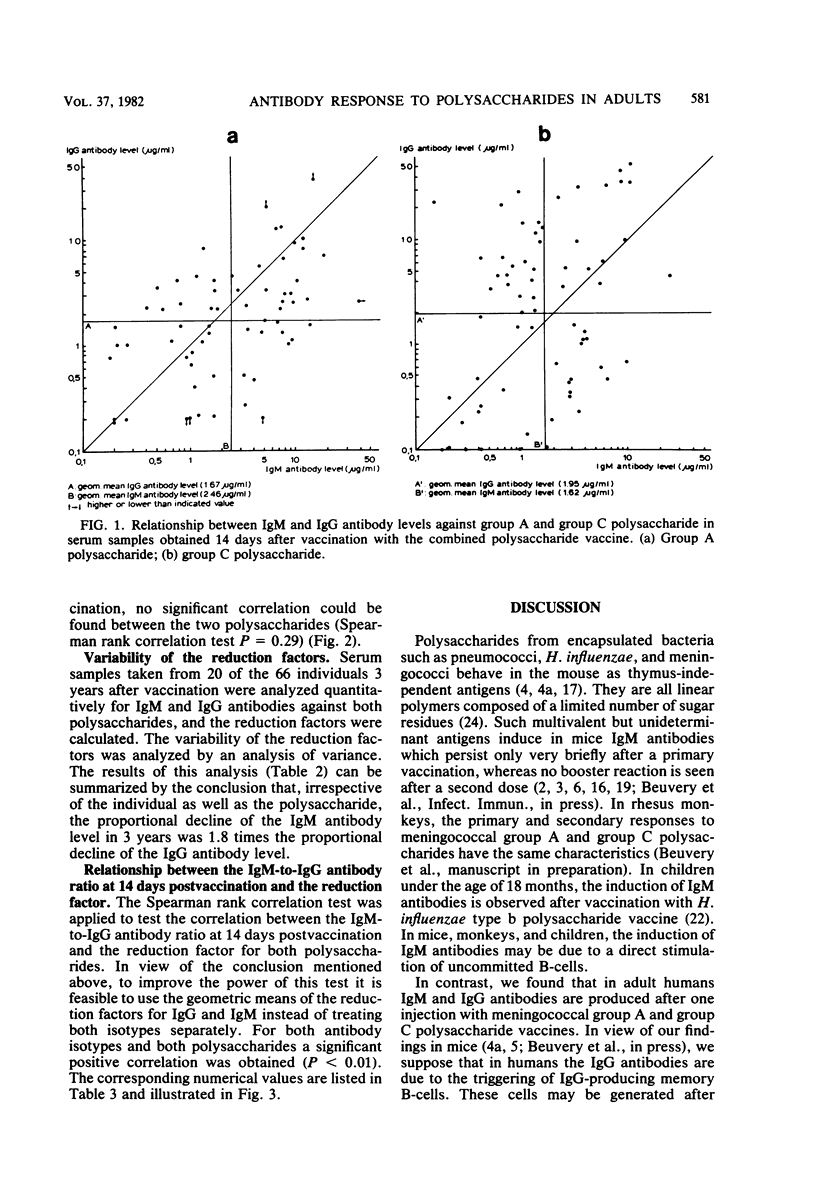
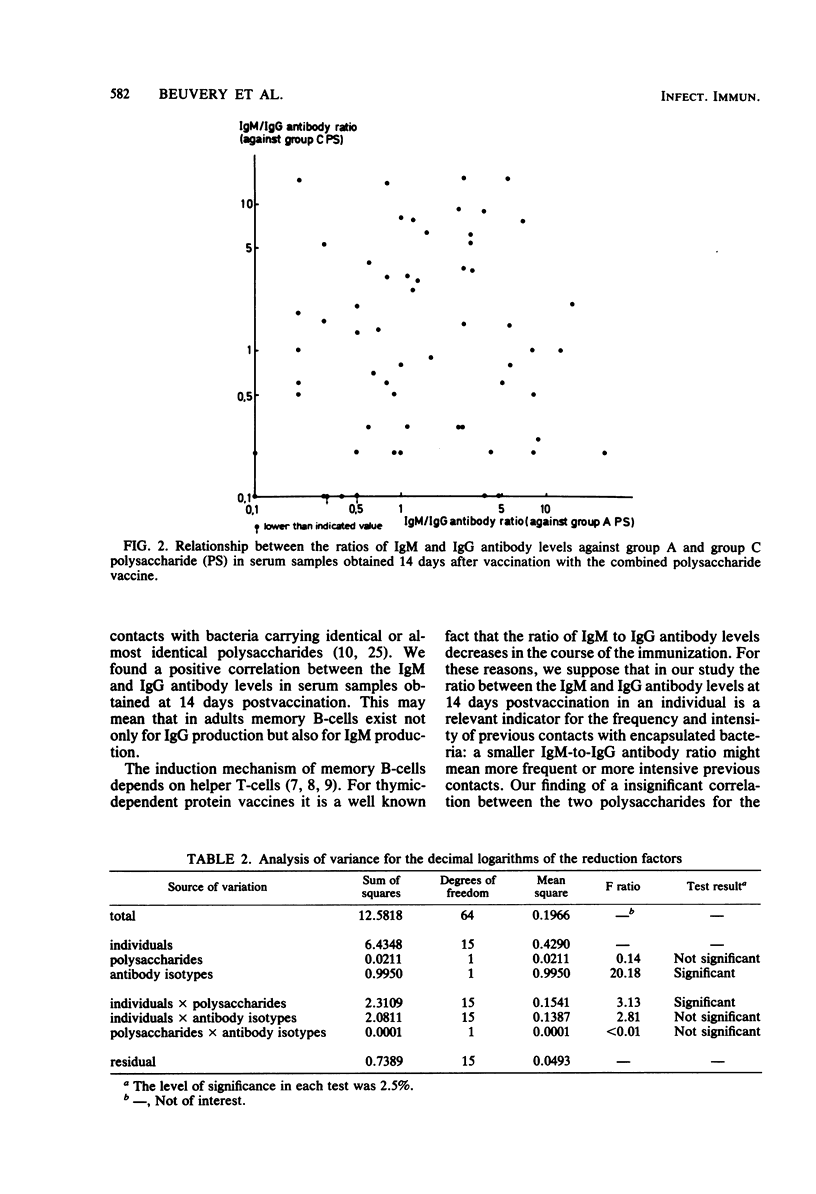
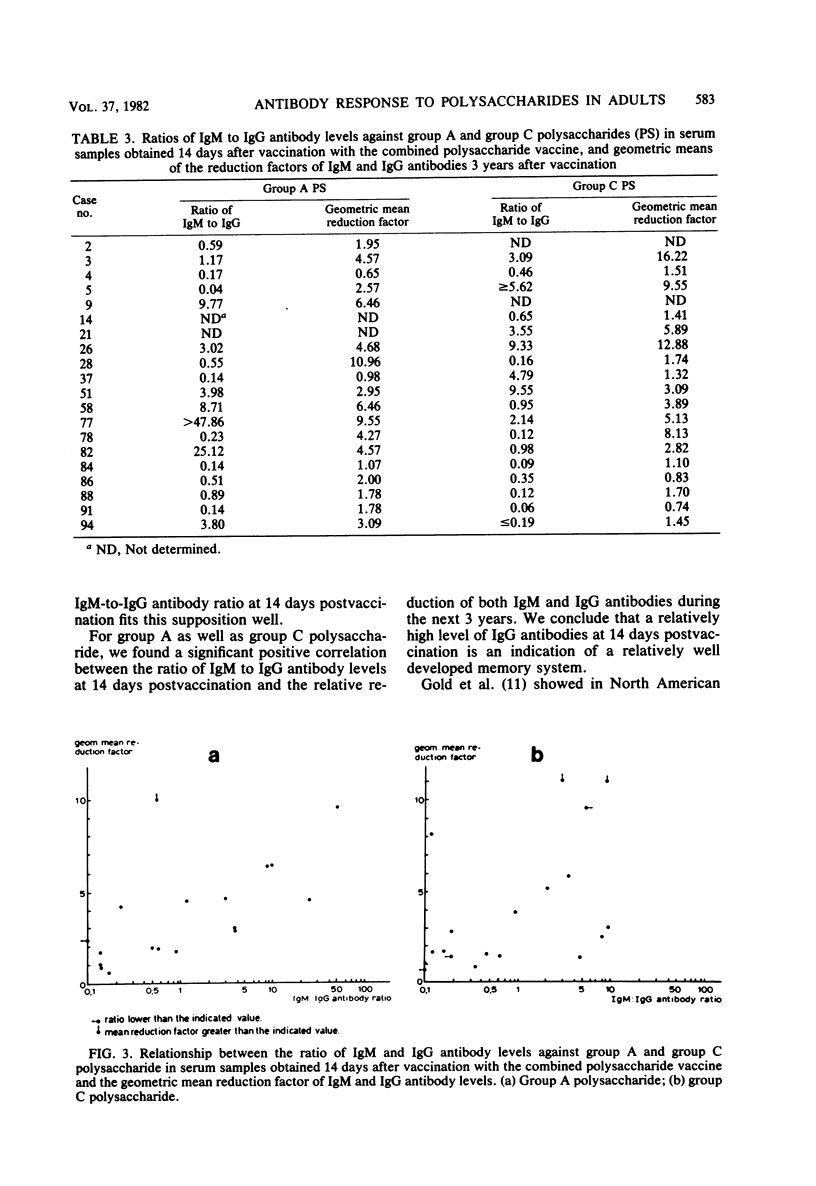
Adult volunteers were injected with a combined meningococcal group A and group C polysaccharide vaccine. Immunoglobulin M (IgM) and IgG antibody levels against both polysaccharides were measured in serum samples taken 14 days as well as 3 years after vaccination. For both group A and group C polysaccharides, the IgM and IgG antibody levels at 14 days postvaccination were positively related. The IgM-to-IgG antibody ratio at 14 days postvaccination was an indicator for the persistence of both IgM and IgG antibodies during the next 3 years; a high ratio meant a short persistence, whereas a low ratio was associated with a long persistence.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H. Regulatory role of T cells in IgG antibody formation and immune memory to type III Pneumococcal polysaccharide. J Immunol. 1974 Dec;113(6):1909–1920. [PubMed] [Google Scholar]
- Braley-Mullen H. Secondary IgG responses to type 3 pneumococcal polysaccharide. III. T cell requirement for development of B memory cells. Eur J Immunol. 1977 Nov;7(11):775–781. doi: 10.1002/eji.1830071106. [DOI] [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Role of T lymphocytes in the humoral immune response. I. Proliferation of B lymphocytes in thymus-deprived mice. J Immunol. 1974 Nov;113(5):1438–1445. [PubMed] [Google Scholar]
- Dresser D. W., Popham A. M. The influence of T cells on the initiation and expression of immunological memory. Immunology. 1979 Oct;38(2):265–274. [PMC free article] [PubMed] [Google Scholar]
- Glode M. P., Robbins J. B., Liu T. Y., Gotschlich E. C., Orskov I., Orskov F. Cross-antigenicity and immunogenicity between capsular polysaccharides of group C Neisseria meningitidis and of Escherichia coli K92. J Infect Dis. 1977 Jan;135(1):94–104. doi: 10.1093/infdis/135.1.94. [DOI] [PubMed] [Google Scholar]
- Gold R., Lepow M. L., Goldschneider I., Draper T. L., Gotschlich E. C. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J Clin Invest. 1975 Dec;56(6):1536–1547. doi: 10.1172/JCI108235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969 Jun 1;129(6):1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIDELBERGER M., DILAPI M. M., SIEGEL M., WALTER A. W. Persistence of antibodies in human subjects injected with pneumococcal polysaccharides. J Immunol. 1950 Nov;65(5):535–541. [PubMed] [Google Scholar]
- Klaus G. G., Humphrey J. H. The immunological properties of haptens coupled to thymus-independent carrier molecules. I. The characteristics of the immune response to dinitrophenyl-lysine-substituted pneumococcal polysaccharide (SIII) and levan. Eur J Immunol. 1974 May;4(5):370–377. doi: 10.1002/eji.1830040513. [DOI] [PubMed] [Google Scholar]
- Lee C. J., Malik F. G., Robbins J. B. The regulation of the immune response of mice to Haemophilus influenzae type b capsular polysaccharide. Immunology. 1978 Jan;34(1):149–156. [PMC free article] [PubMed] [Google Scholar]
- Lepow M. L., Goldschneider I., Gold R., Randolph M., Gotschlich E. C. Persistence of antibody following immunization of children with groups A and C meningococcal polysaccharide vaccines. Pediatrics. 1977 Nov;60(5):673–680. [PubMed] [Google Scholar]
- Lerman S. P., Romano T. J., Mond J. J., Heidelberger M., Thorbecke G. J. Induction of primary and inhibition of secondary antibody response to hapten by hapten conjugates of type III pneumococcal polysaccharide. Cell Immunol. 1975 Feb;15(2):321–335. doi: 10.1016/0008-8749(75)90011-8. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N. Properties of the major component of a peptic digest of rabbit antibody. Science. 1960 Dec 9;132(3441):1770–1771. doi: 10.1126/science.132.3441.1770. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Sivonen A., Mäkelä H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977 Nov;60(5):730–737. [PubMed] [Google Scholar]
- Robbins J. B., Myerowitz L., Whisnant J. K., Argaman M., Schneerson R., Handzel Z. T., Gotschlich E. C. Enteric bacteria cross-reactive with Neisseria meningitidis groups A and C and Diplococcus pneumoniae types I and 3. Infect Immun. 1972 Nov;6(5):651–656. doi: 10.1128/iai.6.5.651-656.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B. Vaccines for the prevention of encapsulated bacterial diseases: current status, problems and prospects for the future. Immunochemistry. 1978 Nov;15(10-11):839–854. doi: 10.1016/0161-5890(78)90117-7. [DOI] [PubMed] [Google Scholar]
- Ruitenberg E. J., Steerenberg P. A., Brosi B. J., Buys J. Reliability of the enzyme-linked immunosorbent assay (ELISA) for the serodiagnosis of Trichinella spiralis infections in conventionally raised pigs. J Immunol Methods. 1976;10(1):67–83. doi: 10.1016/0022-1759(76)90008-9. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]



