Abstract
Individuals with dysfunctional bladders may benefit from devices that track the bladder state. Recordings from pelvic and sacral nerve cuffs can detect bladder contractions, however they often have low signal quality and are susceptible to interference from non-bladder signals. Microelectrode recordings from sacral dorsal root ganglia (DRG) neurons may provide an alternate source for obtaining high quality sensory signals for bladder pressure monitoring. In this study, penetrating microelectrode arrays were inserted in the S1 and S2 DRG in two cats to record afferent spiking activity at different bladder pressures. Multivariate linear regression models were used to estimate bladder pressure from the spiking activity of DRG neurons. The best estimates were obtained with populations of 5–10 units primarily from the S2 DRG, with root mean square errors of 3.0–3.2 cm H2O (correlation coefficients of 0.5–0.9). This work demonstrates the feasibility of monitoring bladder pressure from DRG recordings.
I. Introduction
Individuals with dysfunctional bladders may benefit from a device that monitors the bladder state and provides a warning when the bladder needs emptying [1]. Measurement interfaces such as ultrasound devices, catheters, or strain gauges may provide good signal quality over short time frames but are not long-term solutions [1], [2]. Several studies that tested direct interfaces with the nervous system demonstrated an ability to detect bladder contractions from pelvic nerve [3], pudendal nerve [4] and sacral root [5]–[7] whole-nerve recordings. However, these approaches must compensate for small signals and interference from non-bladder signals before they become widely used [1].
Dorsal root ganglia (DRG) contain the cell bodies of afferent fibers that project into the spinal cord. Penetrating microelectrodes inserted into DRG allow extracellular recording of action potentials from individual neurons with a high signal to noise ratio. Moreover, high density arrays of microelectrodes allow large numbers of isolated neurons to be recorded simultaneously.
We implanted microelectrode arrays into sacral DRG (S1 and S2) to record neural activity from sensory afferents innervating the bladder. The goal of this study was to determine if bladder afferent neural signals could be recorded and used to estimate bladder pressure. This approach is an extension of previous studies showing that limb kinematics can be estimated from the firing rates of lumbar DRG neurons innervating the muscles and skin of the hind limb [8], [9].
II. Methods
A. Experimental Setup
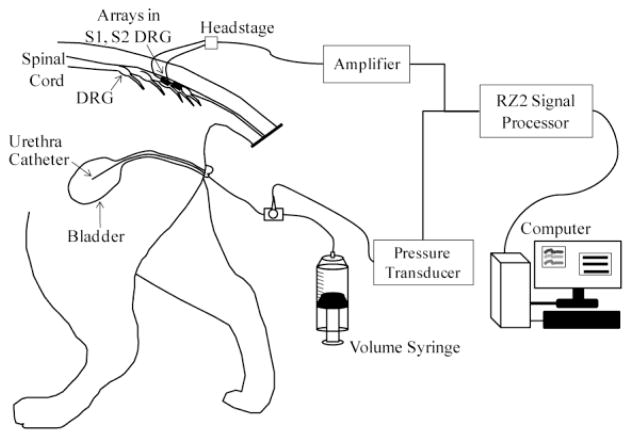
Two intact adult male cats were used in this study, with all procedures approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Each animal was sedated with ketamine (10 mg/kg) before continuous anesthesia with isoflurane (1.5–2.5%). A tracheotomy was performed and the animal was connected to a ventilator. Vitals were monitored and maintained within normal physiological ranges. An intravenous line was inserted in one or both forelimbs for fluid infusion. A polypropylene catheter (3.5 Fr, Sovereign) was inserted intraurethrally to the bladder for controlling the bladder volume and measuring the bladder pressure (Fig. 1). A stop cock on the end of the urethra catheter was used to select pressure monitoring with a pressure transducer (DTXPlus DT-4812, Beckton Dickinson) and transducer amplifier (TA-100, CWE) or volume control with a refillable syringe. A laminectomy was performed to expose the S1–S2 sacral DRG. Penetrating microelectrode arrays (90 channel MultiPort arrays, Blackrock Microsystems) were inserted in the S1 (4×10 array) and S2 (5×10 array) DRG on the left side of each cat with a pneumatic inserter (Blackrock Microsystems). In the first experiment, before the laminectomy, a decerebration was performed anterior to the superior colliculus and a low level of isoflurane (1.0–1.3%) was maintained throughout the experiment. In the second experiment, after completion of the laminectomy, the animal was transitioned off of isoflurane to α-chloralose (70 mg/kg initial dose; 20 mg/kg maintenance doses). Variations in the anesthetic regimen are not expected to affect the sensitivity of the afferents examined in this study.
Fig. 1.
Experimental setup. A catheter was inserted intraurethrally to the bladder, for bladder pressure monitoring and volume control. Blackrock MultiPort arrays (40- and 50-channels) were inserted in the S1 and S2 DRG for recording neural activity.
B. Data Collection
Neural data from the microelectrode arrays and the analog bladder pressure were recorded using a biopotential processor for data sampling and storage (RZ2, TDT). Neural signals were band-pass filtered (300–3000 Hz) and sampled at 25 kHz, with thresholding performed online (OpenEx, TDT) to extract spike snippets for offline analysis. The bladder pressure was sampled at 100 Hz and filtered offline (4 Hz low-pass).
Neural signals from the S1 and S2 DRG were recorded while the bladder volume was controlled. First, constant bladder volume trials were performed. Starting at 0 mL and continuing in 5–10 mL increments until the bladder leaked around the urethra catheter, 1-minute constant bladder volume trials were recorded. Additional trials were recorded, at near full bladder volumes, during distension evoked contractions.
C. Bladder Pressure Estimation
Offline analyses were performed to identify neural units that responded to changes in the bladder pressure. Spikes on each electrode channel were manually sorted in OpenSorter (TDT) and saved on a local storage drive. To identify bladder afferent units, a simple linear regression was performed between the averaged spike count in each constant bladder volume trial and the corresponding average bladder pressure. Units that showed a similar change in their firing rate across the tested bladder pressure range as reported for individual pelvic [10], [11] and sacral root axons [12] were identified as bladder units.
In trials with a bladder pressure range of at least 10 cm H2O, a second regression model was created for estimating the bladder pressure. All DRG units were included in this model. Firing rates were calculated at 10 ms intervals, using a previously described linear filter [9]. A 1.5 s Gaussian kernel was used in the filter, because bladder pressure variations occur slowly and bladder units often fire less than once per second at low bladder pressures [12]. Data from the first and second halves of individual trials were parsed to form training and test periods (30–60 s for each). The linear correlation coefficients (ρ) between each individual unit and the bladder pressure were calculated for the training period. All units were ranked by their unsigned ρ value, in descending order. Next, we used data from the training period to identify coefficients in the following multivariate linear regression model:
| (1) |
In (1), πi refers to an estimate of the bladder pressure (p) at time i, based on a weighted sum of the firing rates (ri,j) of N neurons at that instant. The coefficients (ax) are determined from a least-squares fit. The fitted model was then used to estimate bladder pressure in the test period data. The regression fit and estimate was performed using population sizes (N) ranging from one to the total number of identified units, starting with the first unit in the list of neurons rank-ordered by their correlation (ρ) with bladder pressure.
The root mean square error (RMSE) between each regression period and the measured bladder pressure was calculated by (2).
| (2) |
In (2), pi and πi refer to the measured and fitted/estimated bladder pressure at time i across the t data points. The correlation coefficient (ρ) between the estimated bladder pressure and measured bladder pressure during the test period was also calculated.
III. Results
In both experiments, bladder units were identified from sacral DRG recordings and accurate estimates of the bladder pressure were obtained using only a few DRG units (average RMSE of 3.0–3.2 cm H2O).
A. Bladder Units
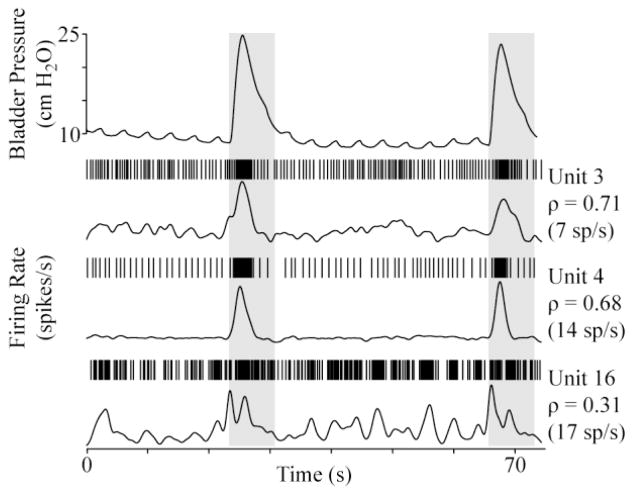
Six DRG units in each cat were identified as bladder units, with a majority in S2 (4 in first cat; 6 in second cat). The bladder units were quiet when the bladder was empty and had mean firing rates between 2 and 8 spikes per second (15 spikes per second max) at pressures above 30 cm H2O. An additional 8 and 27 units (30 total in S2) were weakly correlated to bladder pressure and 108 and 156 other units were identified in the two experiments that did not respond to changes in bladder pressure. Fig. 2 shows the firing rates of two bladder units exhibiting a high correlation with bladder pressure and a third unit that was more weakly correlated during two small bladder contractions.
Fig. 2.
Example DRG unit responses during brief bladder contractions at a constant bladder volume (20 mL). For each unit, a raster of the spike times (top) and the firing rate (bottom) are given. The correlation coefficient (ρ) between each unit and the bladder pressure and the maximum firing rate are also given. The unit number corresponds to their rank-order by descending ρ for the trial.
B. Bladder Pressure Estimation
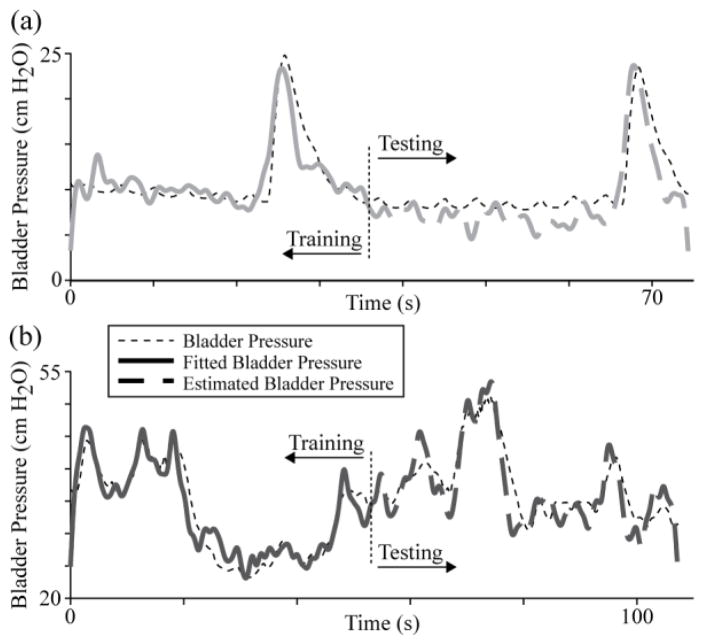
Four trials in the first experiment (1-1 through 1–4; bladder volumes of 10, 20, 60 and 50 mL) and two in the second experiment (2-1 and 2-2; bladder volumes of 5 and 35 mL) had a sufficient bladder pressure range to use with the regression model. Fig. 3 gives example regression model training and testing results for two trials (1–2, 1–4). In these examples, only five DRG units were used, leading to regression testing period RMSE of 2.2 and 2.8 cm H2O (ρ = 0.86 and 0.88), respectively.
Fig. 3.
Example bladder pressure regression trials at (a) lower (trial 1–2) and (b) higher (trial 1–4) bladder pressure ranges. In each, 5 units with the highest correlation coefficient values were used in the regression model to estimate the bladder pressure in the testing period after the vertical dotted line. a) 20 mL, training RMSE = 1.9 cm H2O; testing RMSE = 2.2 cm H2O (ρ = 0.86). b) 50 mL, training RMSE = 2.3 cm H2O; testing RMSE = 2.8 cm H2O (ρ = 0.88).
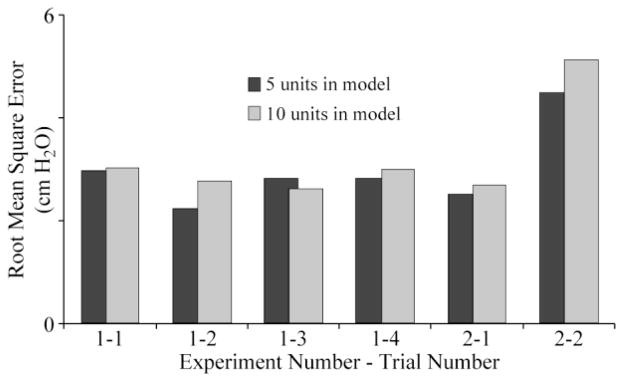
Across each bladder pressure trial, the best regression testing results were obtained for population sizes of 5–10 units. As the population size in the model for each trial was increased during the training period, the RMSE rapidly decreased while the ρ approached one. However, once more than 10 units were used in the training model, the accuracy of the bladder pressure estimate decreased as more non-bladder units were included. Fig. 4 gives RMSE values for the regression model testing period for population sizes of five and ten units. Across the six trials, the average RMSE was 3.0 ± 0.8 cm H2O for five units, 3.2 ± 1.0 cm H2O for ten units, 3.5 ± 0.8 cm H2O for fifteen units, and 3.8 ± 1.2 cm H2O for twenty units. An opposite trend was observed for the correlation coefficient ρ: 0.72 ± 0.16, 0.66 ± 0.24, 0.61 ± 0.21, and 0.56 ± 0.22, for the same population sizes.
Fig. 4.
Regression root mean square error values for bladder pressure estimation trials with 5 or 10 units in model. The average RMSE for 5 (3.0 ± 0.8 cm H2O) and 10 units (3.2 ± 1.0 cm H2O) across these trials was not different.
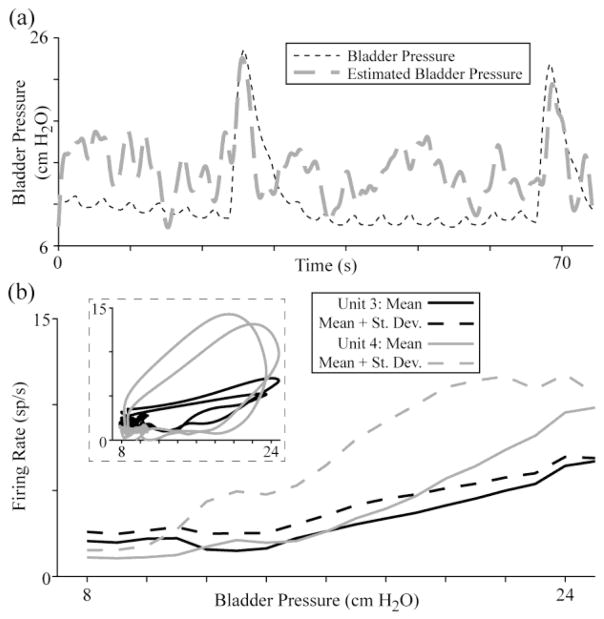
Bladder pressure estimates for an entire trial based on a previous trial were less accurate. Fig. 5(a) gives the pressure estimate for trial 1–2 based on the regression model from trial 1-1 using ten units. While the accuracy of this estimate is lower than for the trial 1–2 regression model used on itself (RMSE of 4.4 cm H2O vs. 2.8 cm H2O), the bladder contractions are still evident. As seen in Fig. 5(b), not all units responded in a linear fashion to changes in the bladder pressure, which was partially a result of hysteresis in the firing rate for some units as the bladder contracted and relaxed.
Fig. 5.
Example bladder pressure estimation trial using a regression model from a prior trial. (a) The RMSE for estimating trial 1–2 from a model based on trial 1-1 was 4.4 cm H2O for 10 units (shown here; ρ = 0.55) and 5.2 cm H2O for 5 units (ρ = 0.69). (b) Many bladder units have varying firing rate response curves during rapid changes in pressure and for different bladder pressure ranges. Here, the mean firing rate at each pressure value and (inset) firing rate against pressure at each time point are shown for units 3 and 4 (corresponding to trial 1–2, Fig. 2).
IV. Discussion
This study demonstrates that DRG neural activity can be used to estimate bladder pressure. In two experiments we estimated the bladder pressure in 30–60 second segments based on the activity of ten or less DRG units. The best results were obtained at low unit population counts (Fig. 4) that primarily consisted of bladder units from S2.
This approach to estimating bladder pressure compares favorably with whole-nerve cuff approaches. Both DRG single unit (Fig. 2, Fig. 3) and whole nerve approaches [3]–[7] are able to detect rapid changes in bladder pressure with reasonable accuracy. However, long-term implant studies of DRG-based interfaces are needed to develop a microelectrode array and implant procedure that enable reliable neural recordings to be obtained for several years. A DRG microelectrode interface has several advantages over whole-nerve approaches. Recording from DRG units yields a higher resolution for bladder contractions, as individual units have clear increases in firing rates (Fig. 2) as compared to low signal-to-noise ratios and/or sub-microvolt changes in whole-nerve electroneurograms [3]–[6]. A DRG interface with individual bladder units would monitor slow pressure changes with greater accuracy than whole-nerve approaches, which typically only reveal phasic activity [3], [4] or gradual changes at higher pressures [6]. Tracking bladder activity with DRG bladder units will likely have a lower false positive rate for indicating bladder contractions than whole nerve approaches which are not selective for non-bladder activity [5], as DRG units for other pelvic functions may be identified and also monitored. Applying low current levels to a nerve improves the whole-nerve signal quality [7], however this approach may still be susceptible to false positives from non-bladder activity. Furthermore, as suggested by the predominance of S2 DRG units among bladder units, a single DRG may be sufficient for monitoring bladder pressure.
The DRG units that were identified as bladder units responded to changes in bladder pressure (Fig. 2, Fig. 5) in a similar manner as individual pelvic nerve and sacral root filaments [10]–[12]. These units responded to changes in the bladder pressure, rather than bladder volume, as evidenced by the firing rate changes in Fig. 2 at a constant bladder volume. Some of these units demonstrated a hysteresis in their firing rate when the bladder contracted and relaxed (Fig. 5(b)). This is a known characteristic [10] which can make fitting a regression model challenging across lengthy time periods involving rapid pressure changes.
Although this work shows promise for monitoring the bladder pressure, further studies should be performed. Nonlinear characteristics of bladder units (Fig. 5) suggest that the inclusion of bladder pressure velocities (contraction, relaxation) and the use of splines and other advanced regression techniques [13] may improve model accuracy. This approach can also be integrated with a contraction detection algorithm and closed loop stimulation for bladder control while optimizing the firing rate kernel. Longer kernels will yield more accurate regression models while shorter kernels will lead to quicker response times.
V. Conclusion
This study establishes that linear regression of recordings from individual sacral DRG units may provide reasonably accurate estimates of bladder pressure, with several advantages over other techniques such as whole nerve cuff recordings. Further studies are needed to establish this approach as a viable alternative to whole-nerve approaches.
Acknowledgments
This work was supported in part by NIH grants 1R01-EB007749 and 1R21-NS-056136, and TATRC grant W81XWH-07-1-0716.
The authors thank members of the Rehabilitation Neural Engineering Lab for their assistance during the animal experiments and with data analysis: Ingrid Albrecht, Chris Ayers, Dennis Bourbeau, Jim Hokanson, Tyler Simpson, and Joost Wagenaar.
Contributor Information
Tim M. Bruns, Email: tmb59@pitt.edu, Department of Physical Medicine and Rehabilitation, University of Pittsburgh, Pittsburgh, PA 15213 USA (phone: 412-383-1426).
Robert A. Gaunt, Email: rag53@pitt.edu, Department of Physical Medicine and Rehabilitation, University of Pittsburgh, Pittsburgh, PA 15213 USA.
Douglas J. Weber, Departments of Physical Medicine and Rehabilitation and Bioengineering, University of Pittsburgh, Pittsburgh, PA 15213 USA and the Pittsburgh Department of Veterans Affairs Medical Center, Pittsburgh, PA 15240 USA.
References
- 1.Mendez A, Sawan M. Chronic monitoring of bladder volume: a critical review and assessment of measurement tools. Canadian Journal of Urology. 2011;18:5504–16. [PubMed] [Google Scholar]
- 2.Haugland M, Sinkjaer T. Interfacing the body3s own sensing receptors into neural prosthesis devices. Technology and Health Care. 1999;7:393–399. [PubMed] [Google Scholar]
- 3.Jezernik S, Wen JG, Rijkhoff NJM, Djurhuus JC, Sinkjaer T. Analysis of bladder related nerve cuff electrode recordings from preganglionic pelvic nerve and sacral roots in pigs. The Journal of Urology. 2000 Apr;163:1309–14. [PubMed] [Google Scholar]
- 4.Wenzel BJ, Boggs JW, Gustafson KJ, Grill WM. Detecting the onset of hyper-reflexive bladder contractions from the electrical activity of the pudendal nerve. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2005 Sep;13:428–35. doi: 10.1109/TNSRE.2005.848355. [DOI] [PubMed] [Google Scholar]
- 5.Jezernik S, Grill WM, Sinkjaer T. Detection and inhibition of hyperreflexia-like bladder contractions in the cat by sacral nerve root recording and electrical stimulation. Neurourology and Urodynamics. 2001 Jan;20:215–30. doi: 10.1002/1520-6777(2001)20:2<215::aid-nau23>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Kurstjens GAM, Borau A, Rodríguez A, Rijkhoff NJM, Sinkjær T. Intraoperative recording of electroneurographic signals from cuff electrodes on extradural sacral roots in spinal cord injured patients. The Journal of Urology. 2005 Oct;174:1482–1487. doi: 10.1097/01.ju.0000173005.70269.9c. [DOI] [PubMed] [Google Scholar]
- 7.Saleh A, Sawan M, Elzayat EA, Corcos J, Elhilali MM. Detection of the bladder volume from the neural afferent activities in dogs: experimental results. Neurological Research. 2008 Feb;30:28–35. doi: 10.1179/016164108X268250. [DOI] [PubMed] [Google Scholar]
- 8.Weber DJ, Stein RB, Everaert DG, Prochazka A. Decoding sensory feedback from firing rates of afferent ensembles recorded in cat dorsal root ganglia in normal locomotion. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2006;14:240–243. doi: 10.1109/TNSRE.2006.875575. [DOI] [PubMed] [Google Scholar]
- 9.Weber DJ, Stein RB, Everaert DG, Prochazka A. Limb-state feedback from ensembles of simultaneously recorded dorsal root ganglion neurons. Journal of Neural Engineering. 2007;4:S168–S180. doi: 10.1088/1741-2560/4/3/S04. [DOI] [PubMed] [Google Scholar]
- 10.Winter D. Receptor characteristics and conduction velocities in bladder afferents. Journal of Psychiatric Research. 1971 Aug;8:225–35. doi: 10.1016/0022-3956(71)90021-5. [DOI] [PubMed] [Google Scholar]
- 11.Iggo A. Tension receptors in the stomach and the urinary bladder. The Journal of Physiology. 1955;128:593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Häbler H, Jänig W, Koltzenburg M. Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. The Journal of Physiology. 1993;463:449–60. doi: 10.1113/jphysiol.1993.sp019604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagenaar JB, Ventura V, Weber DJ. State-space decoding of primary afferent neuron firing rates. Journal of Neural Engineering. 2011 Jan;8:016002. doi: 10.1088/1741-2560/8/1/016002. [DOI] [PMC free article] [PubMed] [Google Scholar]