Abstract
The endogenous bacteria have been hypothesized to play a significant role in the pathophysiology of critical illness, although their role in sepsis is poorly understood. The purpose of this study was to determine how commensal bacteria alter the host response to sepsis. Conventional and germ free (GF) C57Bl/6 mice were subjected to Pseudomonas aeruginosa pneumonia. All GF mice died within two days while 44% of conventional mice survived for 7 days (p=0.001). Diluting the dose of bacteria 10-fold in GF mice led to similar survival in GF and conventional mice. When animals with similar mortality were assayed for intestinal integrity, GF mice had lower levels of intestinal epithelial apoptosis but similar levels of proliferation and intestinal permeability. GF mice had significantly lower levels of TNF and IL-1β in BAL fluid compared to conventional mice without changes in systemic cytokine production. Under conventional conditions, sepsis unmasks lymphocyte control of intestinal epithelial apoptosis, since sepsis induces a greater increase in gut apoptosis in Rag-1−/− mice than wild type (WT) mice. However, in a separate set of experiments, gut apoptosis was similar between septic GF Rag-1−/− mice and septic GF WT mice. These data demonstrate that the endogenous bacteria play a protective role in mediating mortality from pneumonia-induced sepsis, potentially mediated through altered intestinal apoptosis and the local pro-inflammatory response. Additionally, sepsis-induced lymphocyte-dependent increases in gut epithelial apoptosis appear to be mediated by the endogenous bacteria.
Keywords: Sepsis, pneumonia, apoptosis, germ-free, endogenous bacteria, commensal microflora, intestine, gut
INTRODUCTION
Sepsis is the leading cause of death among critically ill patients, killing over 200,000 people annually in the United States (1). The intestine is hypothesized to play a critical role in the pathophysiology of sepsis and is frequently characterized as the “motor” of the systemic inflammatory response (2–4). Alterations in the integrity of the gut induced by sepsis include increased intestinal epithelial apoptosis, decreased proliferation, decreased villus length, and increased intestinal permeability (5–9).
The adult human intestine has over 100 trillion bacteria (10). While only a few thousand bacteria in peripheral tissues can cause a robust inflammatory response, the commensal bacteria do not induce a spontaneous pathological inflammatory response (11;12). This is due to the fact that the specialized intestinal environment has minimal microbial recognition, leading to the development of mutualism, which, in turn, is dependent upon cooperation between the innate and adaptive immune systems (13;14).
The role of the endogenous bacteria in critical illness is complex (15). This has been studied by eliminating commensal bacteria in germ-free (GF) mice that entirely lack an endogenous microflora or in hosts given oral antibiotics to markedly decrease (but not eliminate) intestinal bacterial content. Alternatively, hosts have been given probiotics to modify the intestinal microenvironment to favor growth of beneficial species (16;17).
A number of studies support the notion that commensal bacteria are beneficial in critical illness. GF mice subjected to Klebsiella pneumoniae pneumonia have significantly higher mortality than conventional mice, mediated in an IL-10 dependent manner (18). Mice receiving antibiotic pretreatment have worsened survival following Escherichia coli pneumonia associated with a 15-fold increase in bacteremia (19). Additionally, ventilated patients given enteral probiotics have a decrease in ventilator associated pneumonia compared to control patients (20), while patients with traumatic brain injury have decreased nosocomial infections, shorter ICU stays, and attenuations in abnormalities in Th1/Th2 cytokine ratio following probiotic therapy (21).
In contrast, a number of studies suggest the gut's microflora may be detrimental in critical illness. GF mice have better survival than conventional animals when subjected to hemorrhagic shock (22). Similarly, while intestinal ischemia/reperfusion causes 100% lethality in conventional animals, GF animals have 100% survival when subjected to the same insult, associated with an abrogation of the systemic inflammatory response (23). Additionally, extensive experience with selective decontamination of the digestive tract designed to decrease intraluminal gut bacteria demonstrates a slight decrease in mortality in selected patient populations (24;25).
The mechanisms through which commensal bacteria impact clinically relevant outcomes are multifactorial and include modulation of intestinal epithelial apoptosis and permeability (26;27). The adaptive immune system also plays an essential role in controlling intestinal integrity by controlling whether intestinal epithelial cells proliferate or differentiate under basal conditions (28) and partially attenuating sepsis-induced gut epithelial apoptosis (29).
This study sought to determine the role of commensal bacteria in mediating mortality, gut epithelial integrity and inflammation following Pseudomonas aeruginosa pneumonia, the most common cause of Gram-negative nosocomial pneumonia. Further, it sought to determine if the endogenous bacteria modulate lymphocyte control of sepsis-induced gut epithelial apoptosis.
MATERIALS AND METHODS
Animals
Six to ten week old GF C57Bl/6 and Rag-1−/− mice were bred and maintained in plastic gnotobiotic isolators as previously described (30). Age matched conventional C57Bl/6 mice were bred and maintained in a specific pathogen-free environment. Regardless of whether animals were GF or conventional, all mice had free access to food and water and were maintained on a 12 hour light-dark schedule. Animal studies were conducted in accordance with the National Institutes of Health guidelines for the use of laboratory animals and were approved by the Washington University Animal Studies Committee and Emory University Animal Studies Committee. Of note, all GF animal surgeries were performed in the gnotobiotic facility at Washington University. However, analysis of tissue and blood samples was performed at both this institution and Emory University, following the relocation of the senior author of the manuscript.
Sepsis Model
All mice were given intratracheal injections of P. aeruginosa (ATCC 27853) or 0.9% NaCl (31–33) via midline cervical incision while under isoflurane anesthesia. A 20μ-l solution of bacteria at 0.1 A600nm diluted in 0.9% NaCl(2–4 × 106 CFU) was introduced into the trachea with a 29-gauge syringe to induce sepsis in conventional animals. A 10-fold dilution of the same bacterial solution was given to induce sepsis in all GF mice (both WT and Rag-1−/−). Sham mice were injected with an identical volume of 0.9% NaCl. All animals were then held vertically for 10 seconds to enhance delivery of the bacteria into the lungs. After closing the skin, mice received a 1ml subcutaneous injection of 0.9% NaCl to replace insensible fluid losses. All animals were sacrificed 24 hours after intratracheal injection for tissue harvest or were followed seven days for survival. Conventional mice underwent induction of sepsis in a dedicated animal surgery suite, and GF mice underwent induction of sepsis through a sterile isolator in a gnotobiotic facility using gloves attached to the isolator to maintain environmental conditions.
Intestinal epithelial apoptosis
Apoptotic cells in the intestinal epithelium were quantified using two complementary techniques: H&E-staining and active caspase-3 staining. Apoptotic cells were identified on H&E-stained sections by morphologic criteria where cells with characteristic nuclear condensation and fragmentation were considered to be apoptotic. For active caspase-3 staining, jejunal sections were deparaffinized, rehydrated, and incubated in 3% hydrogen peroxide for 10 minutes. Slides were then immersed in Antigen Decloaker (Biocare Medical, Concord, CA), heated in a pressure cooker for 45 minutes to facilitate antigen retrieval, blocked with 20% goat serum (Vector Laboratories, Burlingame, CA), and incubated with rabbit polyclonal anti-active caspase-3 (1:100; Cell Signaling Technology, Beverly, MA) overnight at 4°C. Sections were then incubated with goat anti-rabbit biotinylated secondary antibody (1:200; Vector Laboratories) for 30 minutes at room temperature, followed by Vectastain Elite ABC reagent (Vector Laboratories) for 30 minutes at room temperature and developed with diaminobenzidine which was counterstained with hematoxylin. Apoptotic cells were quantified in 100 contiguous well-oriented crypt-villus units per animal.
Intestinal villus length
Villus length was measured on H&E-stained sections as the distance in μm from the crypt neck to the villus tip in 12 well-oriented jejunal villi per animal using Image J software (National Institutes of Health, Bethesda, MA).
Intestinal proliferation
Mice received an intraperitoneal injection of 5-bromo-2'deoxyuridine (BrdU) (5 mg/mL diluted in 0.9% saline; Sigma, St. Louis, MO) 90 min prior to sacrifice to label cells in S-phase. Intestinal sections were deparaffinized, rehydrated, incubated in 1% hydrogen peroxide, immersed in Antigen Decloaker and heated in a pressure cooker for 45 min as outlined above. Sections were then blocked for 10 min with Protein Block (Dako, Carpinteria, CA) and incubated with rat monoclonal anti-BrdU (1:500; Accurate Chemical & Scientific, Westbury, NY) overnight at 4° C. After being incubated at room temperature with goat anti-rat secondary antibody (1:500; Accurate Chemical & Scientific) for 30 min, sections were incubated with streptavidin-horseradish peroxidase (1:500; Dako) for 60 min and developed with diaminobenzidine, followed by counterstaining with hematoxylin. Jejunal S-phase cells were quantified in 100 contiguous crypts.
Intestinal Permeability
Intestinal permeability was measured in vivo to fluorescein isothiocyanate conjugated-dextran (FD-4, 22mg/ml, molecular mass 4.4 kDa) (9;34). Mice were gavaged with 0.5 ml of FD-4 19 hours following induction of pneumonia or sham pneumonia. At time of sacrifice (five hours after gavage), blood was collected and centrifuged at 3000 rpm at 4°C for 20 min. Plasma (50 μl) was then diluted with an equal amount of sterile phosphate-buffered saline (pH 7.4), and the concentration of FD-4 was determined using fluorospectrometry (NanoDrop 3300, Thermo Scientific, Wilmington, DE) using an excitation wavelength of 470 nm and an emission wavelength of 515 nm with serially diluted samples as standards. All samples and standards were run in triplicate.
Western Blot Analysis
Frozen segments of jejunum were homogenized in 5× volume of ice-cold homogenization buffer and centrifuged at 10,000 rpm at 4 °C for 5 minutes (8;29). The supernatant was collected, and total protein concentration was determined via the Bradford protein assay. Protein samples of 40μg and equal volume of 2× Laemmli buffer were heated at 95°C for 5 minutes. Samples were run on polyacrylamide gels (Bio-Rad) and then transferred to Immuno-Blot polyvinylidene difluoride membrane for 2 hours at 80V. Membranes were then blocked in 5% nonfat milk in Tris-buffered saline with 0.1% Tween 20 (Sigma) at room temperature for 60 minutes and incubated overnight with primary antibody in 4°C. The following primary antibodies were used: rabbit anti-β-actin; rabbit anti-Bax, rabbit anti-Bcl-xL, rabbit anti-Bcl-2, rabbit anti-Bid, (Cell Signaling Technology) mouse anti-TNF-R1, and rabbit anti-Fas, (Santa Cruz Biotechnology, Santa Cruz, CA). The next day, membranes were washed and incubated for 60 minutes at room temperature with horseradish peroxidase-conjugated goat anti-rabbit or horse anti-mouse immunoglobulin G (Cell Signaling Technology). Finally, membranes were developed with chemiluminescent system (Pierce, Rockford, IL) and proteins were detected after exposure to x-ray film.
Cytokines
Bronchoalveolar lavage (BAL) fluid was obtained by cannulating the trachea with a 22-gauge angiocatheter, and lavaging the lungs with 1 ml of PBS. Both BAL and blood samples (collected at the time of sacrifice) from each mouse were centrifuged for 5 minutes at 6000g, and cytokine concentrations were evaluated using a multiplex cytokine assay (Bio-Rad, Hercules, CA) according to manufacturer's instructions. All samples were run in duplicate.
Bacterial cultures
Blood and BAL fluid was serially diluted in sterile 0.9% saline and cultured on sheep blood agar plates. After incubating overnight at 37°C, colony counts were enumerated.
Statistics
Data were analyzed using the statistical software program Prism 4.0 (GraphPad, San Diego, CA) and are presented as mean ± SEM. Survival studies were analyzed using the Log-Rank test. All other data were tested for Gaussian distribution using the Shapiro-Wilk normality test. If data were found to have Gaussian distributions, comparisons were performed using the Student's t-test. If data did not have Gaussian distributions, two way comparisons were performed using the Mann Whitney test. A p value of <0.05 was considered to be statistically significant.
RESULTS
Sham GF mice have similar intestinal integrity as sham conventional mice
Since GF mice have baseline differences in multiple organs compared to conventional mice (35;36), intestinal integrity was first assayed in animals subjected to sham surgery. No detectable differences were observed between sham GF and conventional mice in intestinal apoptosis, proliferation, or permeability (data not shown). In contrast, sham GF mice had longer villi compared to sham conventional mice (417±18 μm vs. 341±10 μm, p=0.013). Neither GF nor conventional mice had bacteria detectable in their BAL fluid or blood 24 hours following sham surgery. BAL and blood levels of TNF, IL-6, IL-10, IL-1β, IL-13, and IFN-γ were low in all sham mice, regardless of whether they had endogenous bacteria (data not shown). Both sham GF and conventional mice had 100% 7-day survival following sham surgery with intratracheal injection of 0.9% saline.
Septic GF mice have higher mortality than septic conventional mice
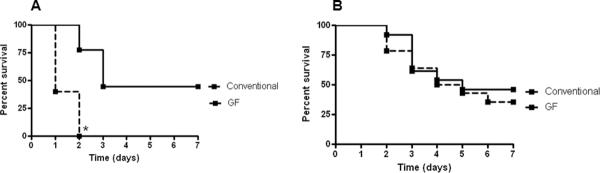
When given the identical dosage of P. aeruginosa, all GF mice died within two days, while 44% of conventional mice survived for 7 days (p=0.001, Figure 1A). For further analysis, a choice was required between a) injecting an identical amount of bacteria into all animals with the knowledge that mortality was markedly disparate between GF and conventional mice which would lead to an obligate survivor bias in GF mice due to the fact that most of them die within one day of induction of pneumonia or b) matching mortality by using a lower concentration of bacteria in GF mice. The decision was made that matching mortality (despite a different quantity of bacteria injected) rather than matching bacterial dose (with a markedly different mortality) would be a more meaningful comparison. Therefore, an additional survival curve was generated using a 10-fold dilution of P. aeruginosa in GF mice. Utilizing these different doses, 7-day mortality was similar in both groups (64% GF vs. 54% conventional, p=0.59, Figure 1B). Subsequent experiments were performed using the matched mortality model.
FIG. 1. Mortality in GF and conventional mice subjected to P. aeruginosa.
When given the identical dosage of bacteria, all GF mice died within two days, while 44% of conventional mice survived for 7 days (n=5–9, p=0.001, A). GF mice were then given a 10-fold dilution of P. aeruginosa compared to conventional mice, yielding similar 7-day mortality between the two groups of animals (n=13–14, p=0.59, B).
Septic GF mice have decreased intestinal epithelial apoptosis compared to septic conventional mice
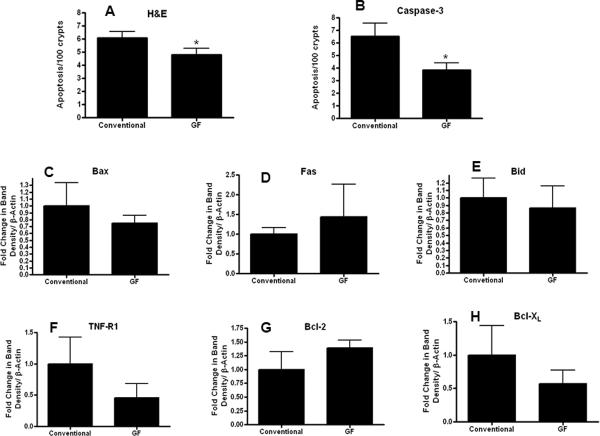
To assess intestinal integrity, gut epithelial apoptosis, proliferation, villus length and permeability were compared in GF and conventional mice subjected to P. aeruginosa pneumonia. Gut apoptosis was decreased in septic GF mice compared to septic conventional mice by both H&E (Figure 2A) and active caspase-3 staining (Figure 2B). This was not associated with statistically significant differences of pro-apoptotic proteins Bax, Fas, Bid, and TNF-R1 or anti-apoptotic proteins Bcl-2, Bcl-XL between septic GF and septic conventional mice as measured by Western blots (Figure 2C–H).
FIG. 2. Intestinal epithelial apoptosis in septic GF and conventional mice.
Gut apoptosis was decreased in GF mice compared to conventional mice (n=11–15) following P. aeruginosa pneumonia by both H&E (p=0.02, A) and active caspase-3 staining (p=0.048, B). No statistically significant differences were detected in protein levels between groups in Bax (n=7–8), Fas (n=3–4), Bid (n=5–6), TNF-R1 (n=8–9), Bcl-2 (n=6), or Bcl-XL (n=4–6, panels C–H respectively). Protein densities were normalized to β-actin and are shown as fold difference from conventional mice (assigned the value of 1 in arbitrary units).
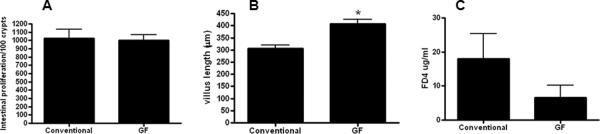
No differences in intestinal proliferation were detected between the groups (Figure 3A). Villus length was longer in septic GF mice compared to septic conventional mice (Figure 3B). This difference appeared to be related to basal differences between GF and conventional mice rather than an effect of sepsis in light of the fact the ratio of GF/conventional length was similar in both sham and septic animals. Intestinal permeability was also similar between septic GF and conventional mice (Figure 3C).
FIG. 3. Intestinal integrity in septic GF and conventional mice.
Proliferation was similar in GF mice compared to conventional mice (n=12–13) following P. aeruginosa pneumonia (p=0.71, A). Villus length was longer in GF mice compared to conventional mice (n=15–17, p=0.0002, B). However, this mimicked a difference seen in sham GF and conventional mice, and the ratio of villus length in GF/conventional mice was similar in both septic and sham mice. Intestinal permeability as measured by the amount of FD-4 in the plasma was also similar between GF and conventional mice (n=7–13, p=0.13, C).
Septic GF mice have decreased pulmonary but not systemic cytokines compared to septic conventional mice
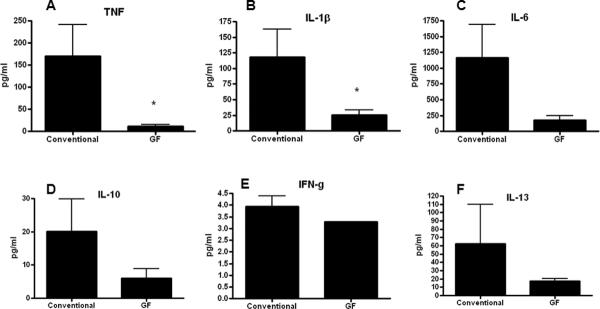
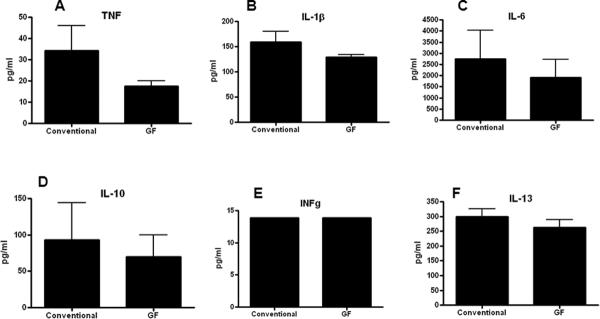
In order to determine if P. aeruginosa pneumonia differentially altered cytokine production in GF and conventional mice, BAL and serum was analyzed 24 hours after the onset of sepsis. GF mice had significantly lower levels of TNF and IL-1β in BAL fluid as well as a trend towards lower levels of all cytokines measured except IFN-γ (Figure 4). In contrast, no differences were noted in any systemic cytokine between GF and conventional mice (Figure 5). Of note, low levels of bacteria were detectable in the lungs (800 cfu/ml) but not blood of GF mice, 24 hours after the onset of pneumonia. This differs from conventional mice where higher levels of P. aeruginosa can be cultured from the lungs 24 hours after induction of pneumonia and mice are bacteremic (5;33).
FIG. 4. BAL cytokine levels in septic GF and conventional mice.
GF mice had significantly lower levels of TNF (p=0.008, A) and IL-1β (p=0.007, B). No statistically significant differences were detected in IL-6, IL-10, IFN-γ, and IL-13 levels (C–F, n=10–11 for all cytokines measured).
FIG. 5. Serum cytokine levels in septic GF and conventional mice.
No statistically significant differences were noted in any systemic cytokine levels (A–F, n=12–13 for all cytokines measured).
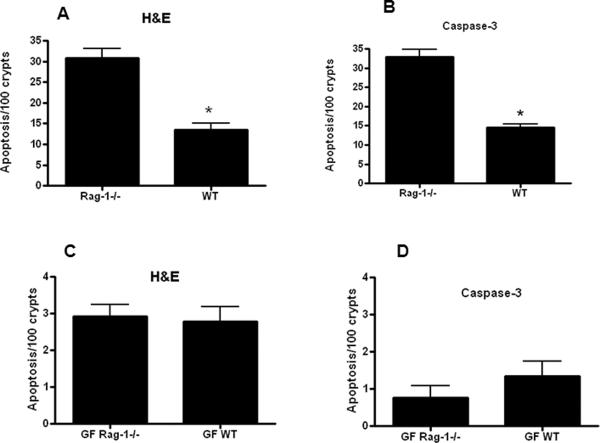
Lymphocyte control of sepsis-induced apoptosis is dependent upon endogenous bacteria
Rag-1−/− mice lack an adaptive immune system, and conventional unmanipulated Rag-1−/− mice have similar levels of gut epithelial apoptosis as conventional unmanipulated WT mice (29). However, sepsis unmasks a lymphocyte-dependent increase in gut apoptosis since intestinal epithelial apoptosis is higher in conventional septic Rag-1−/− mice than septic WT mice (Figure 6A, B). In contrast, no upregulation in gut apoptosis is seen in GF septic Rag-1−/− mice, which have a similar low level of apoptosis as seen in GF septic WT mice (Figure 6C, D). Systemic and BAL cytokine levels were similar in both GF septic Rag-1−/− mice and GF septic WT mice (data not shown).
FIG. 6. Intestinal epithelial apoptosis in septic GF and conventional mice, in the presence and absence of lymphocytes.
Gut apoptosis was higher in conventional Rag-1−/− mice than conventional WT mice (n=8–10) following P. aeruginosa pneumonia by both H&E (p=0.0003, A) and active caspase-3 staining (p=0.0006, B). In contrast, gut apoptosis was similar in GF septic Rag-1−/− mice and GF septic WT mice (n=9–13, p=0.78 and 0.24 respectively, C, D).
DISCUSSION
While exogenous bacteria causing infection are a primary focus of therapy in septic patients, the gut's endogenous bacteria may also play a critical role in the pathophysiology of critical illness. This study demonstrates that the commensal bacteria are beneficial in sepsis, as all GF mice died rapidly when subjected to a model of P. aeruginosa pneumonia that, in conventional mice, mimics the mortality seen in clinical studies of septic shock. This survival difference was associated with alterations in intestinal apoptosis and the local pro-inflammatory response. Additionally, the commensal bacteria mediate lymphocyte regulation of sepsis-induced gut epithelial apoptosis, as the augmentation in intestinal apoptosis seen in conventional septic Rag-1−/− mice compared to conventional septic WT mice is abrogated in GF mice.
Existing literature on the role of the endogenous bacteria in critical illness is conflicting, with some studies demonstrating a benefit (18–21) and others demonstrating harm (22–25). There are a number of reasons why unraveling the role of the endogenous bacteria in critical illness may be difficult. The gut flora is dramatically altered in critical illness in patients, and this is exacerbated by antibiotic usage and changes in intestinal motility, which can lead to differences in type and virulence of bacteria (37;38). Next, there is no fully-accepted method in which to alter the endogenous bacteria. In patients, this can be done by administration of probiotics, but the type of bacteria to administer as well as the optimal dose and timing are not yet understood in any detail. In contrast, selective decontamination of the gut decreases but does not eliminate gut flora and also includes administration of systemic antibiotics in addition to oral antibiotics. Animal studies that upregulate or downregulate intestinal bacteria face similar issues as studies in patients. GF mice have the advantage of totally eliminating the endogenous bacteria so a comparison can be made between mice with commensal flora and those with a lifelong deficiency. However, they have the limitation of chronic immune alterations as well as the fact that the endogenous flora are not a single organism, but a complex ecosystem of trillions of bacteria including numerous different types of microorganisms.
Within this context, this study adds to the existing literature on the importance of the endogenous bacteria in a clinically relevant model of sepsis. Our findings that mortality is strikingly higher in GF mice following P. aeruginosa pneumonia mimic those recently published by Fagundes et al. in K. pneumoniae pneumonia (18). However, the pathophysiology of the infections appears to be, at least somewhat different. Mice with P. aeruginosa pneumonia have decreased TNF and IL-1β levels in BAL fluid whereas mice with K. pneumoniae pneumonia have a marked increase in pulmonary IL-10 levels. Further, we identified minimal or no bacteria in either lungs or blood of GF mice, which was significantly less than bacterial concentrations in conventional infected mice. This pattern is precisely the opposite of GF mice given K. pneumoniae pneumonia, which have a significantly higher bacterial burden in GF mice than conventional mice. It should be noted, however, that there were significant differences in study design in these two studies of GF mice with different species of pathogenic Gram-negative pneumonia. Whereas both studies sacrificed mice 24 hours after onset of pneumonia, we chose to study a different model of P. aeruginosa pneumonia where conventional and GF mice had similar mortalities (approximately 50%), whereas the study using K. pneumoniae pneumonia had 100% survival in conventional mice and 25% survival in GF mice at this timepoint. Since our model had matched mortality but a smaller injury (with less bacteria injected in GF mice) as opposed to matched injury with widely varying mortality, it is difficult to know if the discrepancies seen are due to differences in bacteria studied, the differences in severity of insult/number of bacteria injected or differences in mortality.
Survival in critically ill GF mice is drastically different depending on whether the injury is initiated via pneumonia or noninfectious inflammation. While mortality is markedly increased in GF septic animals, it is improved or abolished in GF mice subjected to either hemorrhagic shock or SMA occlusion (22, 23). The reason for this discrepancy is not entirely clear. It may relate to differences in acuity of insults as both shock and SMA occlusion represent single, time-limited insults while sepsis is an ongoing process. What may be adaptive in responding to a short-term insult may prove to be maladaptive when the insult is ongoing and the host response fails to turn itself off. It may also relate to either differences in production of toxic substances or receptor signaling that mediates the host response. The intestine generates toxic lymph in preclinical shock models which has been shown to increase mortality. There is also increased production of reactive oxygen species in ischemia/reperfusion injuries. While there is assuredly overlap, bacteria in sepsis may signal via pathogen associated molecular pattern molecules while shock and ischemia/reperfusion are noninfectious insults that may signal through endogenous danger signals mediated by danger associated molecular pattern molecules. Ultimately, the differences in mortality in GF mice between sepsis and noninfectious models of critical illness must involve crosstalk between the endogenous bacteria and the host response in some fashion. The microflora must be able to modulate a beneficial response to an infectious insult but contribute to a negative response to a severe, acute noninfectious insult.
The gut has been hypothesized to be the “motor” of the systemic inflammatory response syndrome, and it has long been established that there is crosstalk between the three main elements of the gut – epithelium, commensal bacteria, and immune cells (2). It was therefore reasonable to examine whether eliminating the microflora in GF mice affected integrity in the surrounding intestinal epithelium. Of all elements of gut integrity examined, apoptosis was the only one that was different, with lower levels of apoptosis present in septic GF mice than conventional GF mice. This was counterintuitive as gut epithelial apoptosis is associated with increased mortality in sepsis (5;8;9), and we do not have a clear explanation for this unexpected finding. It is important to note, however, that our results demonstrate that the endogenous bacteria not only impact gut epithelial apoptosis, they impact lymphocyte control of sepsis-induced gut epithelial apoptosis. Under basal conditions in conventional mice, unmanipulated Rag-1−/− mice have similar levels of gut apoptosis as unmanipulated WT mice. Sepsis induces an upregulation in gut apoptosis in WT mice, and this is further augmented in Rag-1−/− mice. We have previously demonstrated that sepsis unmasks lymphocyte control of gut apoptosis in a model of fecal peritonitis (29) and extend that observation herein by demonstrating similar findings in P. aeruginosa pneumonia. In contrast, gut epithelial apoptosis is similar in GF septic Rag-1−/− mice and GF septic WT mice. Taken together this suggests that following sepsis a) the endogenous bacteria have a pro-apoptotic effect on the gut epithelium, b) lymphocytes have an anti-apoptotic effect on the gut epithelium, and c) the endogenous bacteria help mediate the anti-apoptotic effect the lymphocytes have on the gut epithelium since this is nullified in the absence of the endogenous bacteria.
This study has a number of limitations. First the decision was made to match mortality rather than using similar doses of bacteria in GF and conventional mice subjected to pneumonia. The rationale behind this was that most GF mice were dead within 24 hours, and there was significant concern that matching injury would lead to a survivor bias in mice since only animals that survived could be assayed at 24 hours. Further, we were concerned that the differential mortality between GF and conventional mice was so profound that the differences in host response secondary to the fact that one group of mice was destined to die shortly after the experiments were concluded might have overwhelmed any differences resulting from the lack of endogenous bacteria. While we have used a similar strategy in aged mice (39), it is important to note that figures 2–5 represent mice given different injuries with similar mortalities. All observed differences as well as parameters that were not different could have resulted from the decreased initial bacterial burden in GF mice. To address this, in part, additional experiments were performed on conventional mice receiving either 2–4 × 106 CFU or 2–4 × 105 CFU of bacteria. Both BAL and systemic cytokine levels were as likely to be higher as lower in animals that received the lower dose of bacteria (data not shown), suggesting that the differences seen in GF mice are not exclusively due to bacterial load. Additionally, even though WT and Rag-1−/−GF mice received the same dose of bacteria, we did not examine mortality in Rag-1−/−GF mice so do not know if they had similar survival following sepsis as WT GF mice had. Furthermore, all studies with GF mice have the inherent limitation of the fact that they have a number of baseline differences. Although GF mice are a well-accepted method of studying the endogenous bacteria, it is impossible to attribute all observed phenotypes following sepsis simply to the absence of the endogenous bacteria since this does not take basal differences into account. Finally, the study design was limited to a single timepoint (24 hours) and assaying limited tissues (gut, blood, BAL cytokines). A more detailed timecourse or analysis of other tissues may have led to additional insights that were missed by our study design.
Despite these limitations, this study demonstrates that GF mice have a marked increase in mortality when subjected to P. aeruginosa pneumonia, associated with alterations in gut apoptosis and the local inflammatory response. Further the endogenous bacteria not only play a role in mediating sepsis-induced intestinal epithelial apoptosis, but they are also necessary for lymphocyte control of sepsis-induced gut apoptosis. Further experiments are needed to determine the cellular and molecular determinants of the crosstalk between the gut's epithelium, immune system and endogenous bacteria and how this impacts survival in sepsis.
Acknowledgments
This work was supported by funding from National Institutes of Health (GM072808, GM095442, GM66202, GM008795, DK52574)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- (1).Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- (2).Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007 Oct;28(4):384–393. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Dominguez JA, Coopersmith CM. Can we protect the gut in critical illness? The role of growth factors and other novel approaches. Crit Care Clin. 2010;26(3):549–565. doi: 10.1016/j.ccc.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15(1):1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- (5).Dominguez JA, Vithayathil PJ, Khailova L, Lawrance CP, Samocha AJ, Jung E, Leathersich AM, Dunne WM, Coopersmith CM. Epidermal Growth Factor Improves Survival and Prevents Intestinal Injury in a Murine Model of Pseudomonas Aeruginosa Pneumonia. Shock. 2011;36(4):381–389. doi: 10.1097/SHK.0b013e31822793c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Yu P, Martin CM. Increased gut permeability and bacterial translocation in Pseudomonas pneumonia-induced sepsis. Crit Care Med. 2000;28:2573–2577. doi: 10.1097/00003246-200007000-00065. [DOI] [PubMed] [Google Scholar]
- (7).Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Schreiber A, Hackam DJ. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176(5):3070–3079. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- (8).Clark JA, Clark AT, Hotchkiss RS, Buchman TG, Coopersmith CM. Epidermal growth factor treatment decreases mortality and is associated with improved gut integrity in sepsis. Shock. 2008;30(1):36–42. doi: 10.1097/shk.0b013e31815D0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Clark JA, Gan H, Samocha AJ, Fox AC, Buchman TG, Coopersmith CM. Enterocyte-specific epidermal growth factor prevents barrier dysfunction and improves mortality in murine peritonitis. Am J Physiol Gastrointest Liver Physiol. 2009;297(3):G471–G479. doi: 10.1152/ajpgi.00012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- (11).Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- (12).Mueller C, Macpherson AJ. Layers of mutualism with commensal bacteria protect us from intestinal inflammation. Gut. 2006;55(2):276–284. doi: 10.1136/gut.2004.054098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, Geuking MB, Beutler B, Tedder TF, Hardt WD, Bercik P, Verdu EF, McCoy KD, Macpherson AJ. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;25(5940):617–620. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Steele L, Mayer L, Cecilia BM. Mucosal immunology of tolerance and allergy in the gastrointestinal tract. Immunol Res. 2012 doi: 10.1007/s12026-012-8308-4. e pub March 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Wells CL, Hess DJ, Erlandsen SL. Impact of the indigenous flora in animal models of shock and sepsis. Shock. 2004;22(6):562–568. doi: 10.1097/01.shk.0000145935.24344.2d. [DOI] [PubMed] [Google Scholar]
- (16).Morrow LE, Gogineni V, Malesker MA. Probiotic, prebiotic, and synbiotic use in critically ill patients. Curr Opin Crit Care. 2012;18:186–191. doi: 10.1097/MCC.0b013e3283514b17. [DOI] [PubMed] [Google Scholar]
- (17).Schultz MJ, Haas LE. Antibiotics or probiotics as preventive measures against ventilator-associated pneumonia: a literature review. Crit Care. 2011;15(1):R18. doi: 10.1186/cc9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Fagundes CT, Amaral FA, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM, Souza DG. Transient TLR activation restores inflammatory response and ability to control pulmonary bacterial infection in germfree mice. J Immunol. 2012;188(3):1411–142. doi: 10.4049/jimmunol.1101682. [DOI] [PubMed] [Google Scholar]
- (19).Chen LW, Chen PH, Hsu CM. Commensal microflora contribute to host defense against Escherichia coli pneumonia through Toll-like receptors. Shock. 2011;36(1):67–75. doi: 10.1097/SHK.0b013e3182184ee7. [DOI] [PubMed] [Google Scholar]
- (20).Morrow LE, Kollef MH, Casale TB. Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010;182(8):1058–1064. doi: 10.1164/rccm.200912-1853OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Tan M, Zhu JC, Du J, Zhang LM, Yin HH. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15(6):R290. doi: 10.1186/cc10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Ferraro FJ, Rush BF, Jr, Simonian GT, Bruce CJ, Murphy TF, Hsieh JT, Klein K, Condon M. A comparison of survival at different degrees of hemorrhagic shock in germ-free and germ-bearing rats. Shock. 1995;4(2):117–120. doi: 10.1097/00024382-199508000-00007. [DOI] [PubMed] [Google Scholar]
- (23).Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol. 2004;173(6):4137–4146. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
- (24).de Smet AM, Kluytmans JA, Cooper BS, Mascini EM, Benus RF, van der Werf TS, van der Hoeven JG, Pickkers P, Bogaers-Hofman D, van der Meer NJ, Bernards AT, Kuijper EJ, Joore JC, Leverstein-van Hall MA, Bindels AJ, Jansz AR, Wesselink RM, de Jongh BM, Dennesen PJ, van Asselt GJ, te Velde LF, Frenay IH, Kaasjager K, Bosch FH, van IM, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360(1):20–31. doi: 10.1056/NEJMoa0800394. [DOI] [PubMed] [Google Scholar]
- (25).Silvestri L, van Saene HK, Milanese M, Gregori D, Gullo A. Selective decontamination of the digestive tract reduces bacterial bloodstream infection and mortality in critically ill patients. Systematic review of randomized, controlled trials. J Hosp Infect. 2007;65(3):187–203. doi: 10.1016/j.jhin.2006.10.014. [DOI] [PubMed] [Google Scholar]
- (26).Yang R, Gallo DJ, Baust JJ, Watkins SK, Delude RL, Fink MP. Effect of hemorrhagic shock on gut barrier function and expression of stress-related genes in normal and gnotobiotic mice. Am J Physiol Regul Integr Comp Physiol. 2002;283(5):R1263–R1274. doi: 10.1152/ajpregu.00278.2002. [DOI] [PubMed] [Google Scholar]
- (27).Diebel LN, Liberati DM, Dulchavsky SA, Diglio CA, Brown WJ. Enterocyte apoptosis and barrier function are modulated by SIgA after exposure to bacteria and hypoxia/reoxygenation. Surgery. 2003;134(4):574–580. doi: 10.1016/s0039-6060(03)00302-7. [DOI] [PubMed] [Google Scholar]
- (28).Kerneis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277(5328):949–952. doi: 10.1126/science.277.5328.949. 1997. [DOI] [PubMed] [Google Scholar]
- (29).Stromberg PE, Woolsey CA, Clark AT, Clark JA, Turnbull IR, McConnell KW, Chang KC, Chung CS, Ayala A, Buchman TG, Hotchkiss RS, Coopersmith CM. CD4+ lymphocytes control gut epithelial apoptosis and mediate survival in sepsis. FASEB J. 2009;23(6):1817–1825. doi: 10.1096/fj.08-119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Fox AC, Breed ER, Liang Z, Clark AT, Zee-Cheng BR, Chang KC, Dominguez JA, Jung E, Dunne WM, Burd EM, Farris AB, Linehan DC, Coopersmith CM. Prevention of Lymphocyte Apoptosis in Septic Mice with Cancer Increases Mortality. J Immunol. 2011;187(4):1950–1956. doi: 10.4049/jimmunol.1003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Fox AC, Robertson CM, Belt B, Clark AT, Chang KC, Leathersich AM, Dominguez JA, Perrone EE, Dunne WM, Hotchkiss RS, Buchman TG, Linehan DC, Coopersmith CM. Cancer causes increased mortality and is associated with altered apoptosis in murine sepsis. Crit Care Med. 2010;38(3):886–893. doi: 10.1097/CCM.0b013e3181c8fdb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).McConnell KW, McDunn JE, Clark AT, Dunne WM, Dixon DJ, Turnbull IR, Dipasco PJ, Osberghaus WF, Sherman B, Martin JR, Walter MJ, Cobb JP, Buchman TG, Hotchkiss RS, Coopersmith CM. Streptococcus pneumoniae and Pseudomonas aeruginosa pneumonia induce distinct host responses. Crit Care Med. 2010;38:223–241. doi: 10.1097/CCM.0b013e3181b4a76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Wang L, Srinivasan S, Theiss AL, Merlin D, Sitaraman SV. Interleukin-6 induces keratin expression in intestinal epithelial cells: potential role of keratin-8 in interleukin-6-induced barrier function alterations. J Biol Chem. 2007;282(11):8219–8227. doi: 10.1074/jbc.M604068200. [DOI] [PubMed] [Google Scholar]
- (35).Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006;36(9):2336–2346. doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- (36).Macpherson AJ, Geuking MB, McCoy KD. Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology. 2005;115(2):153–162. doi: 10.1111/j.1365-2567.2005.02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Shimizu K, Ogura H, Hamasaki T, Goto M, Tasaki O, Asahara T, Nomoto K, Morotomi M, Matsushima A, Kuwagata Y, Sugimoto H. Altered gut flora are associated with septic complications and death in critically ill patients with systemic inflammatory response syndrome. Dig Dis Sci. 2011;56(4):1171–1177. doi: 10.1007/s10620-010-1418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Alverdy JC, Chang EB. The re-emerging role of the intestinal microflora in critical illness and inflammation: why the gut hypothesis of sepsis syndrome will not go away. J Leukoc Biol. 2008;83(3):461–466. doi: 10.1189/jlb.0607372. 2008. [DOI] [PubMed] [Google Scholar]
- (39).Turnbull IR, Clark AT, Stromberg PE, Dixon DJ, Woolsey CA, Davis CG, Hotchkiss RS, Buchman TG, Coopersmith CM. Effects of aging on the immunopathologic response to sepsis. Crit Care Med. 2003;7(3):1018–1023. doi: 10.1097/CCM.0b013e3181968f3a. [DOI] [PMC free article] [PubMed] [Google Scholar]