Abstract
Survivors of severe sepsis exhibit increased morbidity and mortality in response to secondary infections. Although bacterial secondary infections have been widely studied, there remains a paucity of data concerning viral infections post-sepsis. In an experimental mouse model of severe sepsis (cecal ligation and puncture, CLP) followed by respiratory syncytial virus (RSV) infection, exacerbated immunopathology was observed in the lungs of CLP mice compared to RSV-infected sham surgery mice. This virus-associated immunopathology was evidenced by increased mucus production in the lungs of RSV-infected CLP mice and correlated with increased IL-17 production in the lungs. RSV infected CLP mice exhibited increased levels of Th2 cytokines and reduced IFNγ in the lungs and lymph nodes compared to RSV-infected sham mice. In addition, CD4 T cells from CLP mice produced increased IL-17 in vitro irrespective of the presence of exogenous cytokines or blocking antibodies. This increased IL-17 production correlated wth increased STAT3 transcription factor binding to the IL-17 promoter in CD4 T cells from CLP mice. Further, in vivo neutralization of IL-17 prior to RSV infection led to a significant reduction in virus induced mucus production and Th2 cytokines. Taken together, these data provide evidence that post septic CD4+T cells are primed toward IL-17 production via increased STAT3-mediated gene transcription, which may contribute to the immunopathology of a secondary viral infection.
Keywords: Inflammation, infection, mucus, cytokines, lymphocytes
INTRODUCTION
Morbidity and mortality associated with severe sepsis occurs during two distinct phases of the septic response. During the acute phase of the disease, unchecked production of proinflammatory mediators by immune cells results in a “cytokine storm” which can result in multi-organ dysfunction and death (1). Additionally, survivors of severe sepsis go on to exhibit profound immune suppression, resulting in increased morbidity (2–4). Following sepsis, lymphocytes exhibit numerous deficiencies in activation and effector function, including poor proliferative responses and aberrant T-helper cytokine production (5, 6). In addition, modulations in peripheral regulatory T cell populations have been observed in both humans and animal models post-sepsis (7, 8), and this increase in suppressive lymphocytes correlates in animal models with increased susceptibility to solid tumors (9) and non-lethal bacterial challenge (10). Experimental models of severe sepsis utilize “two-hit” models to investigate the underlying mechanisms of post-septic immunosuppression (11), often using infectious agents that require the innate immune system for protection and clearance (e.g. Aspergillus fumigatus) (12). While these models are useful for investigating innate immune dysfunction post-sepsis, they are not as robust for studying lymphocyte responses post-sepsis, as these infectious models are often controlled with relatively little involvement of the adaptive immune system. Therefore, the development of novel “two-hit” models that target adaptive immunity post-sepsis in vivo are needed for identifying mechanisms governing lymphocyte dysfunction in the context of post-septic immunosuppression.
Respiratory syncytial virus (RSV) is a negative-sense single-strand RNA virus that is a significant human health concern, especially for infants and immunocompromised patients (13, 14). The pathology of RSV infection is unique among respiratory viral pathogens in that it displays a biphasic T-helper cytokine profile, with TH1 type cytokines (such as IFNγ) predominating during the early phase of the infection, and a shift towards TH2 (such as IL-13) (15) and Th17 (IL-17) cytokines at later time points (16). While the shift from TH1 to TH2-type inflammation may play a role in the correlation between RSV infection during infancy and increased susceptibility to asthmatic responses later in life (17), the Th17 responses may drive the chronicity of the primary RSV disease and exacerbate an existing allergic condition (16).
Based on the unique nature of RSV immune responses, and the fact that RSV is both a ubiquitous pathogen and a concern for immunocompromised patients, we tested whether survivors of severe sepsis (who are themselves immunocompromised) exhibit modulations in their ability to respond to airway infection with RSV. The present studies were aimed at identifying the possible deleterious outcomes for secondary viral infection in survivors of severe sepsis as well as identifying possible lymphocyte dysfunction following sepsis in vivo. The results of this study indicate that post-septic animals exhibit increased susceptibility to RSV infection, evidenced by increased viral burden in the lungs and enhanced production of proinflammatory cytokines (such as IL-17) leading to deleterious immunopathology. In addition, CD4+ T cells from post-septic mice produced increased levels of IL-17. Additionally, blockade of IL-17 in post-septic animals in vivo reduced the immunopathology seen following RSV infection. Taken together, these results suggest that as a consequence of severe sepsis, overproduction of IL-17 by CD4+ T cells can participate in viral-induced immunopathology through inhibiting viral clearance and promoting mucus production in the airways.
MATERIALS AND METHODS
Mice
6–8 week old female Balb/c mice were purchased from The Jackson Laboratories. All mice were maintained in specific pathogen free facilities in the Unit for Laboratory Animal Medicine at the University of Michigan and all experiments were approved by the University Committee of Use and Care of Animals (UCUCA).
Cecal Ligation and Puncture and RSV infection
CLP surgery was performed on mice as described previously (5). Briefly, a midline incision was performed on anethesized mice. For CLP. the cecum was ligated and punctured seven times with a 21-gauge needle. For sham surgery mice, the cecum was manipulated without ligation or puncture. Both sham surgery and CLP mice were treated with the antibiotic INVANZ (Ertapenem, Merck & Co., Inc., Whitehouse Station, NJ) administrated at 75 mg/kg via intraperitoneal injection beginning at 6 hours after surgery and re-injected every 24 hours until day 3 (day -11) after surgery. The average mortality rate for mice subjected to CLP in this study was 40–60% by day 4 after surgery. 14 days after the surgery, mice were infected with RSV (Day 0) intratracheally by tongue pull at 1 × 10^5 plaque-forming units (PFU)(16). The experimental groups are identified in the text and figures as follows: “SNR” – sham surgery, no RSV; “SR” – sham surgery followed by RSV challenge; “CNR” – CLP surgery, no RSV; “CR” – CLP surgery followed by RSV challenge.
Histology and RT-PCR
For histology, right lobes of the lung from infected mice were removed, fixed in 10% formalin, and stained as indicated. For RT-PCR, total RNA was extracted from the tissue using TRIzol (Invitrogen, Carlsbad, CA) and reverse transcribed to cDNA. Murine primers for IL4, IL13, IFNγ, IL17, and GAPDH were purchased from Applied Biosytems (Carlsbad, CA). Primers and probes for Muc5ac, Gob5, RSV-F, RSV-N and RSV-G were determined using primer/probe detection sets (PE Biosystems, Foster City, CA) and purchased from Sigma-Aldrich. Fold expression was calculated using the delta-delta Ct method with GAPDH serving as a housekeeping gene.
CD4 T cell skewing and Protein Assays
Spleens from from sham or CLP mice were isolated at day 14 following surgery, and CD4+ T cells were purified using magnetic beads (Miltenyi Biotech, Cambridge, MA). The cells were then plated and skewed as follows, Th0: anti-CD3 (Clone 145-2C11, BD Biosciences, San Diego, CA) (2ug/ml) and anti-CD28 (2ug/ml) (Clone 37.51, BD Biosciences); Th1: anti-CD3, anti-CD28, anti-IL-4 (10ug/ml) (eBioscience, San Diego, CA) and rIL12 (10ng/ml) (R&D Systems, Minneapolis, MN); Th2: anti-CD3, anti- CD28, anti-IL-12 (10ug/ml) (eBioscience), anti-IFNγ (10ug/ml) (eBioscience) and rIL4 (10ng/ml) (R&D Systems); Th17: anti-CD3, anti-CD28, rTGFβ (2ng/ml) (R&D Systems), rIL6 (20ng/ml) (R&D Systems), anti-IL-4 (10ug/ml) and anti-IFNγ (10ug/ml). Four days later the cells were rested for three days and then restimulated for another 48 hours. Supernatants were collected and analyzed for cytokine expression and quantified using a bead-based (Luminex) cytokine assay (Bio-Rad Laboratories, Hercules, CA). For lymph node restimulations, single cell suspensions of lymph nodes isolated from individual mice at the indicated timpoints following experimental surgery and/or RSV infection were seeded in a 96-well plate and restimulated with RSV. Forty-eight hours later the supernatants were harvested and analyzed for cytokines as described above.
Flow cytometry
Collagenase-digested (Sigma-Aldrich, St. Louis, MO) lung lobes from mice were processed into single cell suspensions by passing tissues through sterile 40-mm filters, then ammonium chloride lysis buffer was used to eliminate erythrocytes. Cells were stained with the following fluorescent antibodies in flow cytometry buffer (phosphate buffered saline, 1% w/v bovine serum albumin, 0.05% w/v sodium azide): Purified anti-CD16/32 (Fc Block) (BD Biosciences, San Jose, CA), Pacific Blue-CD3e (eBioscience), PE-Cy7-CD4 (eBioscience), PE-CD8a (BioLegend, San Diego, CA), PerCP-Cy5.5-CD49b (pan-NK) (BioLegend) and FITC-TCRγ/δ (BioLegend). For intracellular cytokine analysis, bead purified splenic CD4+ T cells were cultured in 24-well plates containing plate-bound anti-CD3 (5 µg/ml) and soluble anti-CD28 (2.5 µg/ml). After overnight incubation and in the presence of Golgi-Plug (BD Biosciences) for the last 2 hours, the cells were stained using the indicated flurophore-conjugated antibodies, in addition to Fc block (as indicated above): PE-IL17a (Biolegend), PerCP-Cy5.5-CD4 (BioLegend) and APC-Cy7 TCRβ (BioLegend). Cells were fixed in 4% paraformaldehyde and analyzed using a LSR II (BD Biosciences). Flow cytometry data was analyzed using FlowJo 9.0.1 (Tree Star, Ashland, OR, USA).
RSV and Viral Plaque Assay
RSV A strain (line 19) was derived from a clinical isolate at the University of Michigan(16). Supernatants from whole lung dispersions were serially diluted and viral plaques were determined using a RSV-specific polyclonal antibody (Millipore, Billerica, MA), as described previously(16).
Neutralization of IL-17
Neutralizing rabbit anti-mouse IL-17 antibody was generated in our laboratory as previously described (18). Animals were pre-treated via intraperitoneal injection with 2.5 mg per mouse of purified polyclonal anti-mouse IL-17 or an equivalent dose of control IgG antibody (Cab) 2.5 hours before RSV infection on day 0 and then every other day until day 6.
Statistical analysis
Significance was calculated utilizing repeated measures ANOVA when necessary, followed by post-hoc Bonferroni tests for significance between experimental groups. For single-group analysis, two-tailed Student's t tests were used to determine significance. In all cases, p values equal to or less than p=0.05 were considered statistically significant. Data analysis was performed with GraphPad Prism v5.0a for Macintosh (GraphPad software, San Diego, CA).
RESULTS
Increased mucus production in CLP RSV lungs
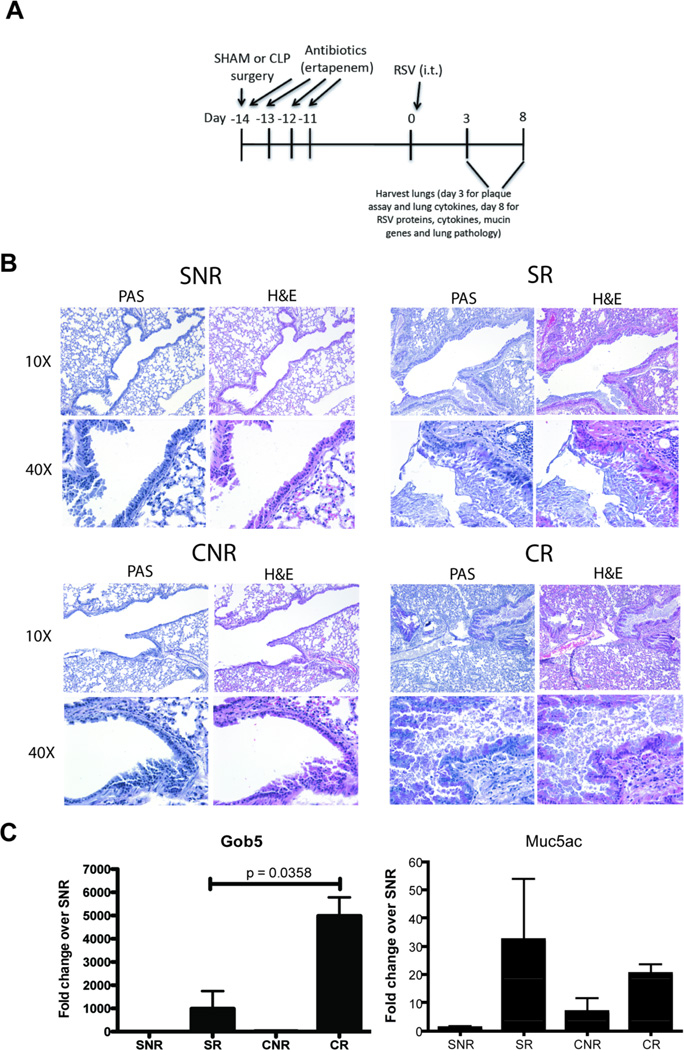
To assess the ability of post-septic mice to respond to a viral infection of the airways, sham and CLP mice were infected with RSV at day 14 post-surgery (Fig. 1A). Uninfected animals (both sham and CLP) were used as controls for the analysis of lung inflammation; as exposure to inactivated RSV does not result in lung inflammation in mice, inactivated RSV control groups were not utilized(19). At day 8 post-infection, mice were sacrificed and lungs were subjected to histological analysis. There was no significant difference in inflammatory infiltrate and mucus production in both sham and CLP lungs without RSV challenge (Fig. 1B). In response to RSV infection, sham mice exhibited extensive peribronchial inflammation, along with evidence of mucus production by airway epithelial cells, particularly in the larger conducting airways (Fig. 1B). In comparison, CLP mice infected with RSV exhibited significantly increased peribronchial inflammation as compared to sham RSV mice (Fig. 1B), with evidence of mucus plugging in both small and large conducting airways (Fig. 1B).
Fig. 1. Secondary RSV infection in post septic mice induces hyper mucus production.
A) Schematic of RSV infection in Sham or CLP. B) Sham or CLP mice were infected with RSV or left uninfected and 8 days post infection mice were euthanized and their lungs were harvested and stained with H&E or PAS. B) RNA was extracted from the lungs and mucin associated genes were analyzed by RT-PCR. Data is representative of three independent experiments, n=3–5 mice/group. The data is expressed as mean ± S.E.
Following RSV infection, robust increases in Gob5 and Muc5ac mRNA were observed in lungs of sham infected (SR) animals (Fig. 1C). In comparison, CLP infected (CR) animals exhibited greater and significant increases in the expression of Gob5 as compared to SR animals (Fig. 1C). No significant differences were observed in the expression level of Muc5ac in the lungs of either SR or CR animals (Fig. 1C).
Increased viral burden in CLP RSV lungs
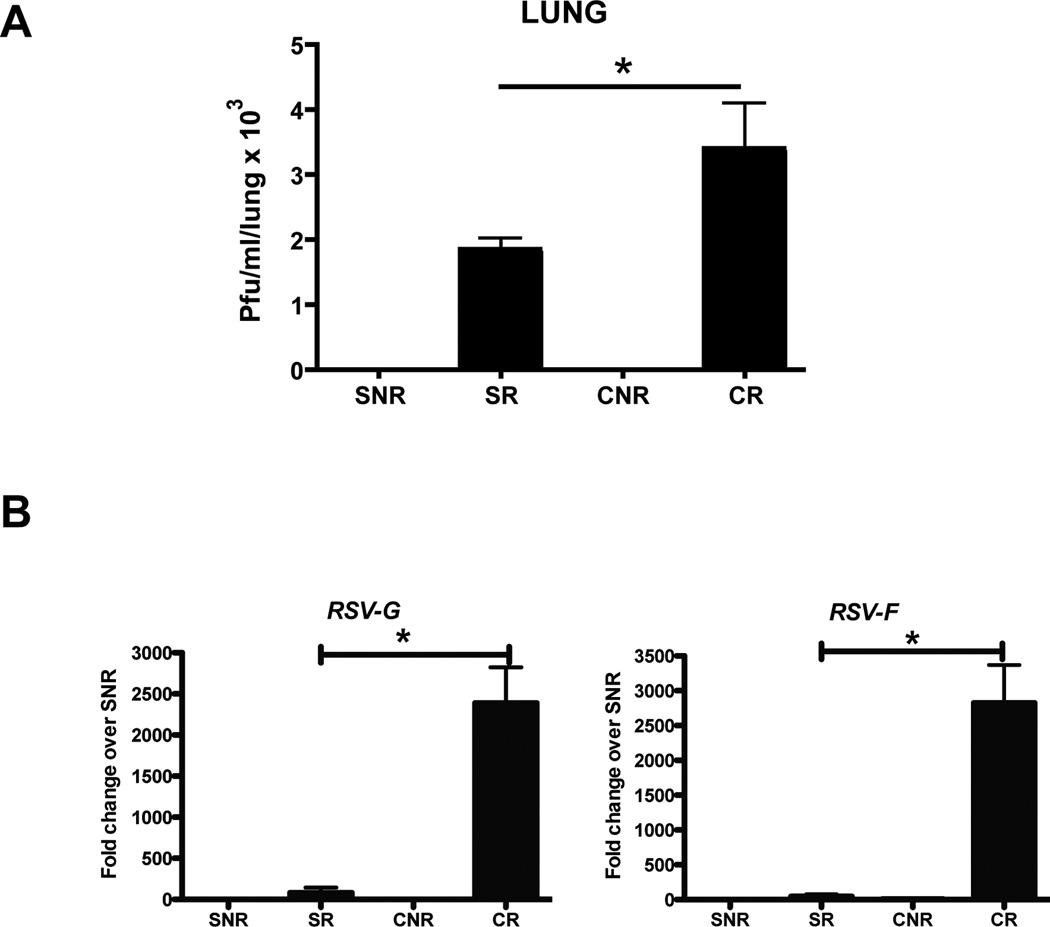
To confirm that the increase in mucus production seen in CLP RSV lungs was due to an increase in viral burden, lung homogenates from sham and CLP animals were analyzed via plaque assay. At day 3 following infection, both SR and CR mice exhibited productive viral replication in the lungs, as indicated by plaque formation in assays of lung homogenates (Fig. 2A). CR lungs exhibited increased plaque formation as compared to SR lungs (Fig. 2A). Additionally, lungs from CR animals exhibited increased expression of RSV surface protein-encoding genes (G and F) as compared to SR lungs (Fig. 2B).
Fig. 2. Increased viral burden in the lungs of RSV infected post-septic mice.
Sham or CLP mice infected with or without RSV were euthanized 3 days post infection. A) The lungs were disrupted to assess the viral burden using a plaque assay. B) RNA from 8 day post infected mice was analyzed for RSV proteins (G and F). The data is expressed as mean ± S.E and * represents p < 0.04.
Increased IL-4 production by lymph node cells from CLP RSV mice
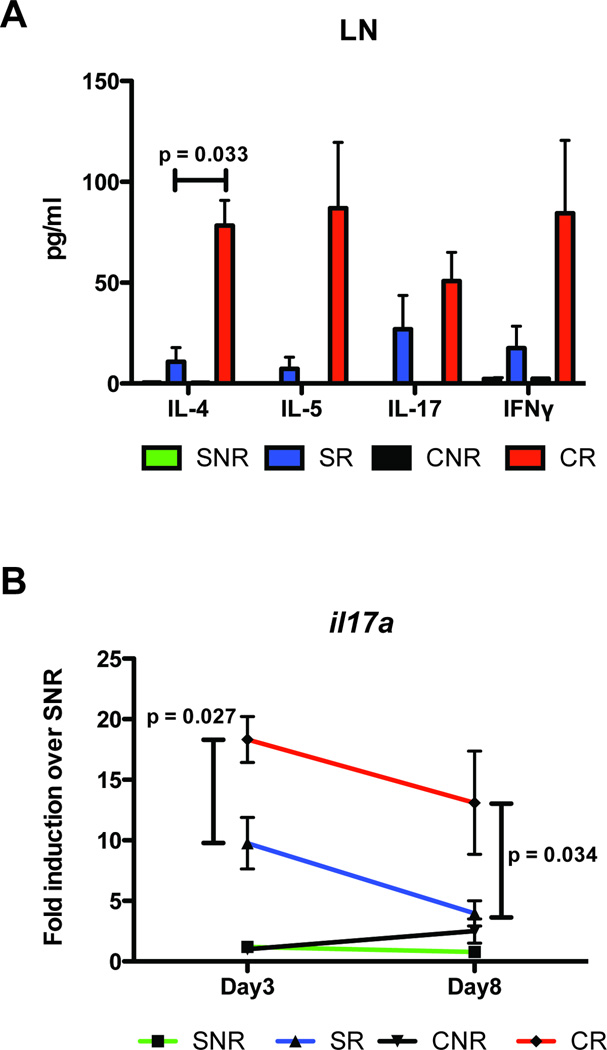
Total lymph node cells from sham and CLP mice (either unchallenged or RSV-challenged) were restimulated in vitro with RSV for analysis of cytokine production in response to viral antigen stimulus. Supernatants from in vitro restimulated lymph node cultures were analyzed via multiplex bead assay (Bioplex) for the production of various TH1, TH2 and TH17-related cytokines (Fig. 3A). While the production of IL-5, IL-17 and IFNγ appeared increased in CR lymph node cultures as compared to SR, and IL-5 in particular, these differences were not statistically significant do to variances in cytokine production by CR lymph node cells (p=0.0572 for CR vs. SR IL-5) (Fig. 3A). However, CR lymph node cultures did exhibit a significant increase in IL-4 production as compared to SR lymph node cultures following RSV challenge in vitro (Fig. 3A).
Fig. 3. Post septic mice exhibit increased pulmonary Th2 cytokines to secondary viral infection.
A) Mediastinal lymph nodes from Sham and CLP mice infected with RSV were collected on day 8 post infection, individually plated in 96-well plates, and restimulated ex vivo with RSV for an additional 48 hours. Supernatants were then collected and cytokines were analyzed by bead-based multiple cytokine analysis (Luminex). B) Sham or CLP mice were infected with RSV or left uninfected. On day 3 day and day 8 post RSV infection, mice were euthanized and lungs harvested for RNA expression of IL-17 by RT-PCR. The data is expressed as mean ± S.E. Each experiment was repeated twice, n=3–5 mice/group.
Increased IL-17 mRNA in lungs of CLP RSV mice
Lungs from SR and CR mice were recovered at day 3 and day 8 post-infection with RSV and were analyzed via real-time PCR for the expression of pro-inflammatory cytokine genes. At day 3 post-infection, lungs from CR infected mice exhibited decreased expression of IFNγ mRNA as compared to SR lungs (Data not shown). However, this difference was no longer observed in lungs from mice at day 8 post-infection (Data not shown). CR lungs exhibited significant increases in IL-17 mRNA production at both day 3 and day 8 post infection as compared to SR lungs (Fig. 3B). Lungs were also analyzed for upregulation of IL-17 associated cytokines, such as the TH17-instructing cytokines IL-6 and TGFβ. While both cytokines were observed in the lungs of RSV-infected animals, there was no significant difference in fold expression between SR and CR groups (day 3, fold expression over SNR; IL-6: 4.31 ± 1.7 for SR vs. 4.42 ± 1.7 for CR, p>0.05; TGFβ: 1.87 ± 0.6 for SR, 8.97 ± 5.0 for CR, p>0.05).
No significant differences in total number of T & NK cells in lungs of sham or CLP mice following RSV infection
One possible explanation for the increase in proinflammatory cytokine production in the lungs of CLP RSV mice (concomitant with increased mucus production) is an increase in immune cell recruitment to the lungs following CLP, in particular T cells and NK cells, both of which are involved with IFN-γ and IL-17 production. To determine if the increase in lung cytokine production was due to increased cell recruitment, lungs from sham and CLP mice were harvested at 2 and 4 days following RSV infection, and analyzed via flow cytometry for the presence of CD4+ and CD8+ T cells, γδ T cells and pan natural killer (NK) cells. While these timepoints are relatively early in the context of RSV pathogenesis, and do not necessarily represent the peak of leukocyte infiltration into the airways (20), these timepoints were chosen in an attempt to correlate the increase in proinflammatory cytokine production in the lungs with increased cell recruitment. Total cell counts were obtained by multiplying the total viable cell count obtained from digested/dispersed lungs by the percentages obtained by flow cytometry.
At day 2 post-infection, the predominant cell type in both sham and CLP RSV lungs was CD8+ T cells (Table 1). However, there were no significant differences observed in the total number of CD8+ T cells between sham and CLP RSV-infected lungs. In addition, no significant differences were observed in total numbers of CD4+ T cells between sham and CLP RSV-infected lungs (Table 1). Total numbers of γδ T and NK cells were also similar between sham and CLP lungs, with no differences apparent in response to RSV infection as shown in Table 1). At day 4 post-infection, CD4+ T cells were the predominant cell type in the lungs of both sham and CLP RSV lungs; however, as with the day 2 analyses, there were no significant differences observed in total numbers of these cells between sham and CLP RSV lungs. Similar results were observed for CD8+ T cells between sham and CLP RSV lungs (Table 1). In contrast to day 2, the total number of γδ T cells and natural killer (NK) cells had decreased and increased, respectively; however, these differences were not statistically significant between sham and CLP RSV lungs (Table 1).
Table 1.
Lymphocyte distribution in the lungs of Sham and CLP mice following secondary RSV infection
| Days Following RSV infection |
Cell type and Number (Mean ± SEM) |
Cell type and Number (Mean ± SEM) |
Cell type and Number (Mean ± SEM) |
Cell type and Number (Mean ± SEM) |
|---|---|---|---|---|
| CD4+ T cells | CD8+ T cells | Υ/δ T cells | Pan NK cells | |
| × 10^5 | × 10^5 | × 10^4 | × 10^4 | |
| Day 2 | ||||
| Sham No RSV | 2.18 ± 1.3 | 8.84 ± 5.1 | 1.81 ± 1.0 | 6.00 ± 3.5 |
| Sham RSV | 3.01 ± 1.7 | 12.90 ± 7.4 | 1.83 ± 1.0 | 5.07 ± 2.92 |
| CLP No RSV | 2.86 ± 1.6 | 12.68 ± 7.3 | 2.00 ± 1.2 | 3.54 ± 2.0 |
| CLP RSV | 2.61 ± 1.1 | 12.57 ± 5.6 | 2.49 ± 1.1 | 4.59 ± 2.1 |
| Day 4 | ||||
| Sham No RSV | 8.19 ± 4.7 | 3.47 ± 2.0 | 0.16 ± 0.09 | 14.51 ± 8.4 |
| Sham RSV | 4.99 ± 2.5 | 2.56 ± 1.3 | 0.26 ± 0.13 | 16.41 ± 8.2 |
| CLP No RSV | 11.99 ± 6.9 | 5.33 ± 3.1 | 0.12 ± 0.07 | 19.25 ± 11.1 |
| CLP RSV | 6.96 ± 3.11 | 3.88 ± 1.7 | 0.24 ± 0.11 | 15.98 ± 7.1 |
Splenic CD4+ T cells from CLP animals produced increased IL-17 as compared to sham in response to in vitro skewing with exogenous cytokines
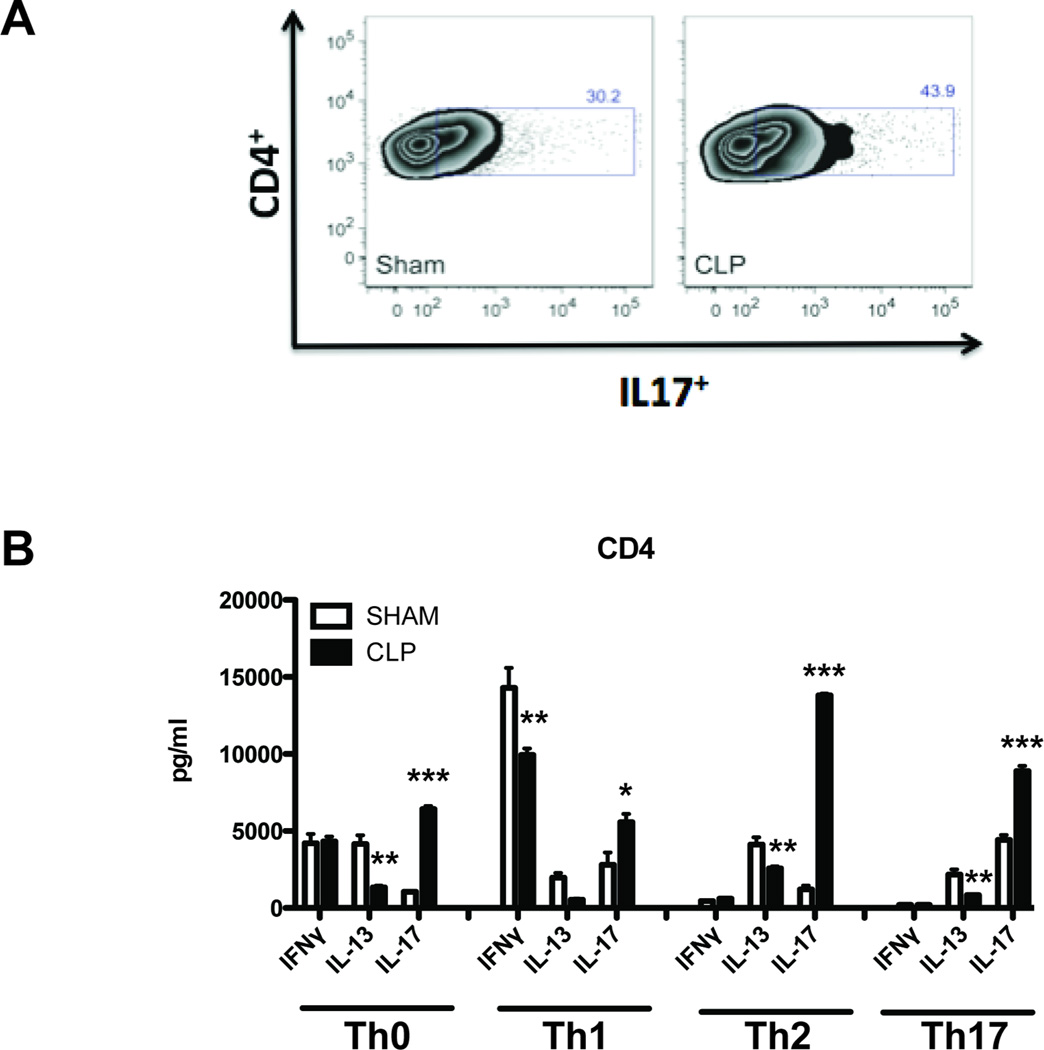
Based on the flow cytometry data, the increased production of IL-17 in the lungs of CLP RSV lungs was not due to increased numbers of lung lymphocytes. These results suggest that post-septic lymphocytes may produce more IL-17 in inflammatory responses as compared to lymphocytes from sham mice. Previous studies indicate that post-septic CD4+ T cells produce more IL-17 in response to both polyclonal stimulus in vitro (6) and in response to antigen stimulation in vivo (5), suggesting that the IL-17 production potential of post-septic CD4+ T cells would be observable in these cells prior to viral infection with RSV. Therefore, to determine if CD4+ T cells were the possible source of the increased IL-17 in lungs of CLP-RSV mice, splenic CD4+ T cells from sham and CLP mice (not infected with RSV) were restimulated with polyclonal stimulus in vitro. Splenic CD4+ T cells were utilized as a proxy for lung-resident CD4+ T cells, as naïve lungs (in this case, SNR and CNR lungs) contain relatively few CD4+ T cells as compared to peripheral immune organs (e.g. spleen and lymph nodes). Splenic CD4+ T cells from CLP exhibited increased IL-17 production, as evidenced by an increased number of IL-17+ cells following in vitro re-stimulation (43.03% ± 0.6% for CLP vs. 32.25 ± 1.1% for sham, p < 0.001) (Fig. 4A). In addition, CLP-T cells exhibited increased production of IL-17 per cell as evidenced by mean fluorescence intensity (877.4 ± 13 for CLP vs. 627.3 ± 43 for sham, p < 0.001).
Fig. 4. Skewed CD4 T cells from post septic mice produce increased levels of IL-17.
CD4 T cells were MACS enriched from the spleens of sham treated or post septic (CLP) mice on day 14 and assayed for cytokine production in vitro. (A) Cells were re-stimulated with anti CD3 and anti CD28 in the presence of golgi-plug overnight in vitro and stained intracellularly for IL-17 levels and analyzed by flow cytometry. (B) Cells were seeded into 96 well plates at a density of 1 × 106 cells per well. The cells were either un-skewed (Th0) or skewed towards Th1, Th2 and Th17 for 48 hours. The supernatants were then collected and analyzed for Th1, Th2 and Th17 cytokines by bead-based multiple cytokine analysis (Luminex).
To determine if the modulations in in vivo cytokine expression in CLP mice were related to modulations in CD4+ T cell effector function, CD4+ T cells from the spleens of sham and CLP mice (not infected with RSV) were isolated and skewed in vitro using polyclonal stimulus and exogenous cytokines, to determine if the increase in IL-17 production observed in CLP CD4+ T cells could occur in the context of opposing T-helper polarizing conditions (i.e. TH1 and TH2). CD4+ T cells from both sham and CLP mice produced similar amounts of IFNγ (Fig.4B) and IL-2 (data not shown) in response to TH0 re-stimulation conditions. Additionally, both sham and CLP TH0 cells produced low levels of IL-4 and IL-5 (data not shown). However, CLP TH0 cells produced significantly less IL-13 and more IL-17 than sham TH0 cells (Fig. 4B). This increase in IL-17 production by CLP T cells was also observed in TH1 cultures (Fig. 4B), and was concomitant with a decrease in IFNγ production as compared to sham T cells (Fig. 4B). This increase in IL-17 production by CLP T cells was also seen in TH2 cultures, along with a decrease in IL-13 production, as compared to sham T cells (Fig. 4B). A similar pattern was observed in TH17 cultures, with CLP T cells producing increased IL-17 and decreased IL-13 as compared to sham T cells (Fig. 4B).
Splenic CD4+ T cells from post-septic animals exhibit increased binding of STAT3 to regions of the IL-17 promoter
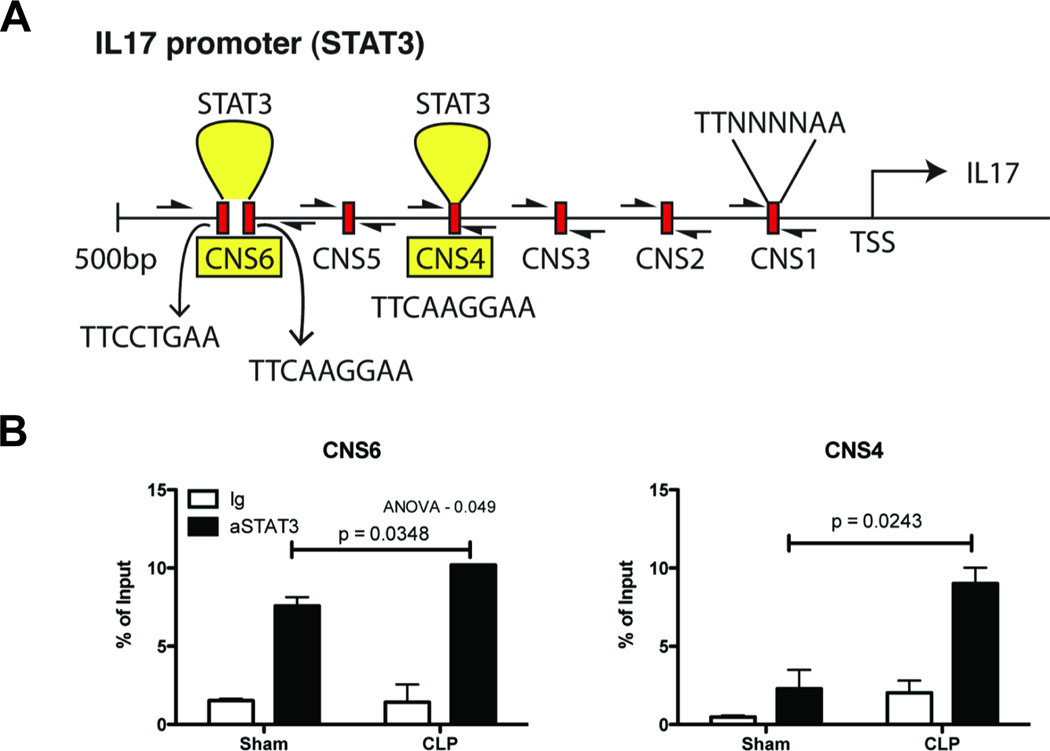
The increased production of IL-17 by CLP T cells suggested a modulation in IL-17 gene regulation in these cells. To test this hypothesis, splenic CD4+ T cells from sham and CLP mice were analyzed ex vivo for transcription factor binding to the promoter region of IL-17 via chromatin Immunoprecipitation (ChIP) assay. As the transcription factor RORγt is essential for TH17 lineage commitment, initial ChIP assays were performed to identify RORγt binding to the IL-17 promoter; however, there was no observed increase in RORγt binding to the IL-17 promoter in CLP T cells (data not shown). IL-17 production in CD4+ T cells can also be induced in a STAT3-dependent manner; to test this, ChIP assays were performed with αSTAT3 antibodies and primers directed to putative STAT3 binding sites within 500 base pairs of the IL-17a transcription start site (Fig. 5A). Increased STAT3 binding was observed in CLP T cells as compared to sham, at two putative STAT3 binding sites proximal to the IL-17a transcription start site (Fig. 5B).
Fig. 5. CD4+ T cells from post septic mice exhibit increased STAT3 binding to the IL-17 promoter.
A) Putative STAT3 binding sequences on Il17 promoter (CNS 1–6) with 0.5kb upstream of the transcription start site was analyzed by ChIP assay. (B) Chromatin was immunoprecipitated-using control Ig or STAT3 antibody and DNA was quantified using CNS 1–6 specific primers. The data is expressed as mean ± S.E. * p = 0.05, ** p <0.04, *** p <0.001. All experiments were repeated twice.
In vivo blockade of IL-17 reduced mucus production and viral burden in lungs of CLP mice following RSV infection
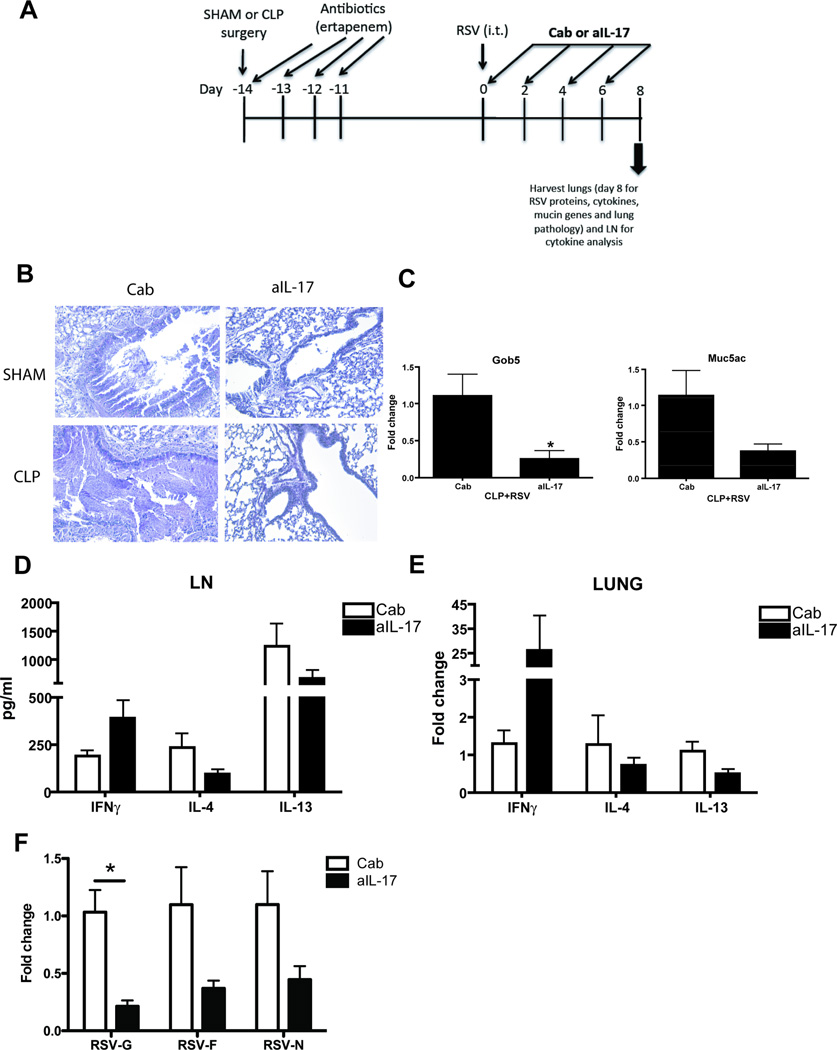
To determine if the increase in IL-17 production observed both in RSV-infected lungs and in activated CD4+ T cells from spleens of CLP mice was involved with the increased mucus production and viral burden observed in these mice, blocking antibodies to IL-17 were used in the CR infection model. SR and CR animals were treated with αIL-17 antibody via intraperitoneal injection at the time of viral infection, as well as two and four days later (Fig. 6A). Two days after the final antibody treatment, mice were sacrificed and lungs were analyzed for mucus production, mucus-associated gene expression, and expression of proinflammatory cytokine genes and viral gene products. In addition, lymph nodes from SR and CR mice were re-stimulated in vitro with RSV for analysis of cytokine expression. Treatment with αIL-17 had a significant effect on mucus production and lung inflammation, with a reduction in inflammatory infiltrates and PAS+ epithelial cells (Fig. 6B). These histological changes were also associated with reductions in expression of the mucus-associated genes Gob5 and Muc5ac, with αIL-17 treatment reducing their expression by half in CR lungs (Fig. 6C).
Fig. 6. Neutralizing IL-17 in post septic mice reduces viral load and reverses RSV induced pulmonary pathology.
A) Schematic of treatment with neutralizing antibody to IL-17 in RSV infected post septic mice. B) Sham or post septic (CLP) mice were treated with control antibody (Cab) or neutralizing antibody to IL-17, 2.5 hours prior to RSV infection on day 0. The mice were treated with 3 more doses of Cab or anti-IL-17 on days 2, 4 and 6 before they were euthanized and their lungs were harvested for PAS staining for mucus, and C) RNA analysis of mucus associated genes – Gob5 and Muc5ac. D) The LN from CLP mice infected with RSV and treated with Cab or anti-IL-17 were further re-stimulated with RSV for 48 hours. The supernatants were collected and cytokine analysis was performed using the bioplex-200 system. Lungs from CLP mice infected with RSV and treated with Cab or anti-IL-17 were analyzed for RNA expression of E) cytokines IFNγ, IL-4 and IL-13 and F) RSV-G, RSV-F and RSV-N proteins. Data is representative of two independent experiments, n=3–7 mice/group. The data is expressed as mean ± S.E. and * p < 0.05 was considered significant.
To determine if the reduction in lung inflammation was due to changes in proinflammatory cytokine expression in the lungs of CR mice receiving αIL-17, lymph nodes were analyzed for protein (Fig. 6D) expression following RSV re-stimulation, and lungs were analyzed for cytokine mRNA expression (Fig. 6E), as well as viral gene expression (Fig. 6F). Treatment of CLP+RSV mice with αIL-17 in vivo had a slight effect on the production of IFNγ (increased), IL-4 and IL-13 (decreased) in lymph node cells; however these differences were not statistically significant (Fig. 6D). In contrast, αIL-17 treatment had a dramatic effect on expression of IFNγ mRNA in whole lung tissue, with a significant increase as compared to Control ab (Cab)-treated CLP+RSV lungs (Fig. 6E). No significant differences were observed in the expression of IL-4 or IL-13 mRNA in the lungs of CLP+RSV αIL-17 treated mice as compared to Cab treatment (Fig. 6E). In addition, αIL-17 treatment resulted in a significant decrease in the expression of the RSV-G gene in CLP+RSV lungs, as compared to Cab treatment (Fig. 6F). Marked decreases in RSV-F and RSV-N were also observed in CLP+RSV αIL-17 treated animals. (Fig. 6F)
DISCUSSION
Survivors of severe sepsis remain susceptible to secondary and nosocomial infections, especially in regards to opportunistic infections of the lung. The results of this study indicate that following sepsis, animals are more susceptible to airway infection with RSV. The increase in IL-17 production in post-septic mice in the context of RSV infection appears to play a role in the increased viral burden and mucus production seen in the airways. IL-17a mRNA levels are significantly increased in post-septic mice at early timepoints following RSV infection, and remain elevated throughout the progression of the infection. Additionally, IL-17 production is only observed in restimulated lymph node cultures from RSV-infected animals, and IL-17a mRNA levels remain at “wild-type” levels in post-septic lungs in the absence of RSV infection, suggesting that viral infection stimulates increased IL-17 production in post-septic animals. In addition, in vivo neutralization of IL-17 results in a reduction in the severity of both disease parameters. Taken together, these results indicate that survivors of severe sepsis develop a propensity to produce IL-17 in response to inflammatory stimuli, and that this increased IL-17 production in response to secondary infection can result in deleterious immunopathology in certain contexts, i.e. viral infections of the lung.
Sepsis-induced mortality can manifest in two distinct fashions: either as a result of multi-organ dysfunction following the “cytokine storm” of acute sepsis, or as a result of the complex immunosuppression that follows. Experimental models of sepsis often utilize opportunistic lung infections such as Aspergillus fumigatus (12) to model post-septic immunosuppression. Viral infections also can have an adverse effect on sepsis severity and post-septic mortality; for example, sepsis can reactivate latent cytomegalovirus (CMV) infections in both humans and animal models (21, 22), and certain studies suggest that CMV reactivation can have a deleterious effect on the survival of septic patients (23). Despite these results, there remains a paucity of experimental models utilizing secondary viral infections following sepsis. Therefore, the use of RSV in a “two-hit” sepsis model is useful, as RSV is a ubiquitous human pathogen that can cause significant pathology in immunosuppressed individuals. Additionally, the use of RSV as a secondary infectious agent is pertinent to the TH shift observed in survivors of severe sepsis (24), as immune responses to RSV are multiphasic in nature, and proceed through TH1, TH2 and TH17-type cytokine responses (16).
Recent studies in human patients and animal models have identified divergent roles for IL-17 in the pathogenesis of sepsis and sepsis-induced mortality. Originally, it was thought that IL-17 would play a prominent role in the acute phase of sepsis, primarily though its ability to modulate the development and activation of neutrophils (25), innate immune cells that play an important role in mediating the septic response. However, studies of IL-17 levels in peripheral blood of septic patients indicate little correlation between IL-17 production and the severity of the septic response (26). In contrast, modulation of IL-17 function in experimental models of sepsis can have a dramatic effect on sepsis-induced mortality. For example, blockade of IL-17 in vivo using antagonist antibody treatment increased survival in mouse models of polymicrobial sepsis (27). In addition, mice lacking the ability to signal through the IL-17 receptor (IL-17R−/−) exhibited increased susceptibility to “mild” models of surgically induced sepsis, unlike control animals that did not exhibit mortality (28). Interestingly, increased IL-17 production by post-septic CD4+ T cells has been observed in response to polyclonal stimulus in vitro (6) and in the context of both TH1 and TH2 inflammation in vivo (5). In addition, increases in IL-17 mRNA have been observed in intraepithelial γδ T cells following the onset of experimental sepsis in animal models (29). These results indicate that polymicrobial sepsis can create an environment promoting IL-17 production, and this dysregulated IL-17 production by CD4+ T cells may adversely modulate the immune response to secondary infections in post-septic patients.
Recent studies have indicated a role for IL-17 in mediating reactive airway disease phenotypes, and mucus production in particular. Members of the extended IL-17 family have been implicated in mediating allergic airway disease phenotypes; for example, IL-17E (also known as IL-25) can induce the production of many TH2 cytokines in the lung, including IL-4, -5 and -13, and can mediate eosinophil accumulation via increased eotaxin (CCL11) production (30). In addition, recent studies have implicated IL-17A as an important factor for mucus production in the airways (16, 18). Interestingly, these studies provide a mechanism whereby viral infections, often considered to generate primarily TH1-type responses, can mediate TH2-type reactive airway pathology though the upregulation of IL-17 production. This phenomenon is evidenced in the present study, whereby in vivo blockade of IL-17 results in a reduction of RSV-induced airway mucus production in sham surgery animals. These results suggest that the aberrant production of IL-17 in post-septic animals exacerbates RSV immunopathology both through limiting productive immunity (as evidenced by increased viral titers) and exacerbating deleterious responses (such as type 2 cytokines and mucus production). Further, these results suggest that IL-17 may negatively regulate productive viral immunity in the lung, especially when taken in context with previously published reports highlighting links between neutralization of IL-17 and decreased RSV viral burden(16). Future studies into the role of IL-17 in post-septic immune dysfunction may help clarify whether this increase in IL-17 is attributable to de novo generation of TH17 cells, or active suppression of other T-helper subsets via IL-17 dependent mechanisms.
In contrast, the effects of increased IL-17 production on post-septic susceptibility to RSV infection may be due to suppression of IFNγ-mediated immune responses rather than strictly a promotion of TH2 cytokines. While there remains a paucity of data implicating a role of IL-17 in suppressing TH1 responses, previous studies of RSV pathogenesis suggest that modulations in IFNγ can have a dramatic effect on the outcome of the infection(31). As post-septic CD4+ T cells exhibit deficiencies in IFNγ production, this decreased TH1 response coupled with an exacerbated TH17 response may be driving infection severity in post-septic animals. These results suggest that treatment of post-septic animals (and perhaps human patients) with exogenous IFNγ may help to protect against viral infection following sepsis. However, previous studies suggest that post-septic immunosuppression (evidenced by increased susceptibility to bacterial infection) is not resolved with the administration of IFNγ (32). In addition, IFNγ production in post-septic animals did not appear affected in this study, suggesting that the increased susceptibility to RSV infection observed in this model is not strictly due to a decrease in IFNγ.
Lymphocyte-derived cytokines appear skewed towards TH2-type responses following sepsis (24). This skewing towards TH2-type cytokine responses is also supported by the depressed IL-12 production by accessory myeloid cells (such as dendritic cells) following severe injury and/or sepsis (33). Previous studies have indicated a propensity for IL-17 production by post-septic CD4+ T cells both in vivo and in vitro (5, 6). In agreement with these studies, we show that CD4+ T cells from post-septic animals expressed increased IL-17 following in vitro skewing with exogenous cytokines, suggesting that the sepsis response may have conditioned the surviving CD4+ T cells to produce IL-17 upon secondary stimulation. Interestingly, this increase in IL-17 production appears dependent on STAT3 and not RORγt. As IL-17a is not thought to signal via IL-17 receptor-dependent STAT3 phosphorylation(34), the observed increased in STAT3 binding to the IL-17a promoter appears to be further evidence of the increased propensity of post-septic CD4+ T cells to produce IL-17. STAT3 plays an important role in initiating IL-17 gene transcription, and STAT3-dependent signals (such as IL-6 receptor signaling) play an important role in the development of IL-17 producing CD4+ T cells (35). Aberrant STAT3 signaling in post-septic T cells may participate in the increased IL-17 production observed by these cells in response to TH17-opposing cytokines (such as IFNγ and IL-4).
In the present study, increased IL-17 production by post-septic CD4+ T cells resulted in increased viral titers and increased mucus production following airway RSV infection. This increase in IL-17 has a deleterious effect on viral immunity despite being a pro-inflammatory response, as it mediates immunopathology (i.e. mucus) without providing protection against the infectious agent (i.e. RSV viral titers). The results of this study provide further evidence for the susceptibility of survivors of severe sepsis to secondary infections, and respiratory viral infections in particular. In addition, these studies provide evidence that severe sepsis can shift immune responses towards IL-17 production, in addition to TH2 responses. Importantly, these studies implicate increased IL-17 production by CD4+ T cells as an indicator of post-septic immune dysfunction, restricting the ability of the post-septic immune system to respond effectively to pulmonary viral infection.
ACKNOWLEDGEMENTS
The authors would like to thank Aaron Berlin and Andrew Rasky for technical assistance, and Judith Connett for critically editing the manuscript.
FUNDING: This work was supported by NIH R01AI073876-03 to NWL and NIH R01HL031237-27 to SLK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med. 2008 Jul;26(6):711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 2.Perl TM, Dvorak L, Hwang T, Wenzel RP. Long-term survival and function after suspected gram-negative sepsis. JAMA. 1995 Jul 26;274(4):338–345. [PubMed] [Google Scholar]
- 3.Quartin AA, Schein RM, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997 Apr 2;277(13):1058–1063. [PubMed] [Google Scholar]
- 4.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010 May;38(5):1276–1283. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 5.Carson WF, IV, Ito T, Schaller M, Cavassani KA, Chensue SW, Kunkel SL. Dysregulated Cytokine Expression by CD4+ T cells from Post-Septic Mice Modulates both Th1 and Th2-Mediated Granulomatous Lung Inflammation. PLoS One. 2011;6(5):e20385. doi: 10.1371/journal.pone.0020385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson WF, IV, Cavassani KA, Ito T, Schaller M, Ishii M, Dou Y, Kunkel SL. Impaired CD4+ T-cell proliferation and effector function correlates with repressive histone methylation events in a mouse model of severe sepsis. Eur J Immunol. 2010 Apr;40(4):998–1010. doi: 10.1002/eji.200939739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scumpia PO, Delano MJ, Kelly KM, O'Malley KA, Efron PA, McAuliffe PF, Brusko T, Ungaro R, Barker T, Wynn JL, Atkinson MA, Reeves WH, Salzler MJ, Moldawer LL. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J Immunol. 2006 Dec 1;177(11):7943–7949. doi: 10.4049/jimmunol.177.11.7943. [DOI] [PubMed] [Google Scholar]
- 8.Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, Lepape A. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003 Jul;31(7):2068–2071. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- 9.Cavassani KA, Carson WF, IV, Moreira AP, Wen H, Schaller MA, Ishii M, Lindell DM, Dou Y, Lukacs NW, Keshamouni VG, Hogaboam CM, Kunkel SL. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood. 2010 Jun 3;115(22):4403–4411. doi: 10.1182/blood-2009-09-241083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nascimento DC, Alves-Filho JC, Sonego F, Fukada SY, Pereira MS, Benjamim C, Zamboni DS, Silva JS, Cunha FQ. Role of regulatory T cells in long-term immune dysfunction associated with severe sepsis. Crit Care Med. 2010 Aug;38(8):1718–1725. doi: 10.1097/CCM.0b013e3181e78ad0. [DOI] [PubMed] [Google Scholar]
- 11.Muenzer JT, Davis CG, Dunne BS, Unsinger J, Dunne WM, Hotchkiss RS. Pneumonia after cecal ligation and puncture: a clinically relevant "two-hit" model of sepsis. Shock. 2006 Dec;26(6):565–570. doi: 10.1097/01.shk.0000235130.82363.ed. [DOI] [PubMed] [Google Scholar]
- 12.Benjamim CF, Hogaboam CM, Lukacs NW, Kunkel SL. Septic mice are susceptible to pulmonary aspergillosis. Am J Pathol. 2003 Dec;163(6):2605–2617. doi: 10.1016/S0002-9440(10)63615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson-Caswell M, Muncie HL., Jr Respiratory syncytial virus infection in children. Am Fam Physician. 2011 Jan 15;83(2):141–146. [PubMed] [Google Scholar]
- 14.Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000 Jul;13(3):371–384. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tekkanat KK, Maassab HF, Cho DS, Lai JJ, John A, Berlin A, Kaplan MH, Lukacs NW. IL-13-induced airway hyperreactivity during respiratory syncytial virus infection is STAT6 dependent. J Immunol. 2001 Mar 1;166(5):3542–3548. doi: 10.4049/jimmunol.166.5.3542. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee S, Lindell DM, Berlin AA, Morris SB, Shanley TP, Hershenson MB, Lukacs NW. IL-17-Induced Pulmonary Pathogenesis during Respiratory Viral Infection and Exacerbation of Allergic Disease. Am J Pathol. 2011 Jul;179(1):248–258. doi: 10.1016/j.ajpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005 Jan 15;171(2):137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 18.Lukacs NW, Smit JJ, Mukherjee S, Morris SB, Nunez G, Lindell DM. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J Immunol. 2010 Aug 15;185(4):2231–2239. doi: 10.4049/jimmunol.1000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller AL, Bowlin TL, Lukacs NW. Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J Infect Dis. 2004 Apr 15;189(8):1419–1430. doi: 10.1086/382958. [DOI] [PubMed] [Google Scholar]
- 20.Smit JJ, Boon L, Lukacs NW. Respiratory virus-induced regulation of asthma-like responses in mice depends upon CD8 T cells and interferon-gamma production. Am J Pathol. 2007 Dec;171(6):1944–1951. doi: 10.2353/ajpath.2007.070578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forster MR, Trgovcich J, Zimmerman P, Chang A, Miller C, Klenerman P, Cook CH. Antiviral prevention of sepsis induced cytomegalovirus reactivation in immunocompetent mice. Antiviral Res. 2010 Mar;85(3):496–503. doi: 10.1016/j.antiviral.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Muller L, Klemm A, Weiss M, Schneider M, Suger-Wiedeck H, Durmus N, Hampl W, Mertens T. Active cytomegalovirus infection in patients with septic shock. Emerg Infect Dis. 2006 Oct;12(10):1517–1522. doi: 10.3201/eid1210.060411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalil AC, Florescu DF. Is cytomegalovirus reactivation increasing the mortality of patients with severe sepsis? Crit Care. 2011 Mar 24;15(2):138. doi: 10.1186/cc10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller AC, Rashid RM, Elamin EM. The "T" in trauma: the helper T-cell response and the role of immunomodulation in trauma and burn patients. J Trauma. 2007 Dec;63(6):1407–1417. doi: 10.1097/TA.0b013e31815b839e. [DOI] [PubMed] [Google Scholar]
- 25.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004 Oct;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Frangen TM, Bogdanski D, Schinkel C, Roetman B, Kalicke T, Muhr G, Koller M. Systemic IL-17 after severe injuries. Shock. 2008 Apr;29(4):462–467. doi: 10.1097/shk.0b013e3181598a9d. [DOI] [PubMed] [Google Scholar]
- 27.Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ferrara JL, Ward PA. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008 Jul;22(7):2198–2205. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- 28.Freitas A, Alves-Filho JC, Victoni T, Secher T, Lemos HP, Sonego F, Cunha FQ, Ryffel B. IL-17 receptor signaling is required to control polymicrobial sepsis. J Immunol. 2009 Jun 15;182(12):7846–7854. doi: 10.4049/jimmunol.0803039. [DOI] [PubMed] [Google Scholar]
- 29.Lee WY, Hu YM, Ko TL, Yeh SL, Yeh CL. Glutamine Modulates Sepsis-Induced Changes to Intestinal Intraepithelial gammadeltaT Lymphocyte Expression in Mice. Shock. 2012 Jul 6; doi: 10.1097/SHK.0b013e3182655932. [DOI] [PubMed] [Google Scholar]
- 30.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002 Jul 1;169(1):443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 31.Harker JA, Lee DC, Yamaguchi Y, Wang B, Bukreyev A, Collins PL, Tregoning JS, Openshaw PJ. Delivery of cytokines by recombinant virus in early life alters the immune response to adult lung infection. J Virol. 2010 May;84(10):5294–5302. doi: 10.1128/JVI.02503-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphey ED, Sherwood ER. Bacterial clearance and mortality are not improved by a combination of IL-10 neutralization and IFN-gamma administration in a murine model of post-CLP immunosuppression. Shock. 2006 Oct;26(4):417–424. doi: 10.1097/01.shk.0000226343.70904.4f. [DOI] [PubMed] [Google Scholar]
- 33.Wen H, Hogaboam CM, Gauldie J, Kunkel SL. Severe sepsis exacerbates cell-mediated immunity in the lung due to an altered dendritic cell cytokine profile. Am J Pathol. 2006 Jun;168(6):1940–1950. doi: 10.2353/ajpath.2006.051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009 Aug;9(8):556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007 Apr 15;178(8):4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]