Abstract
African Americans represent a high risk population for salt-sensitive hypertension and heart disease but the underlying mechanism remains unclear. Corin is a cardiac protease that regulates blood pressure by activating natriuretic peptides. A corin gene variant (T555I/Q568P) was identified in African Americans with hypertension and cardiac hypertrophy. In this study, we test the hypothesis that the corin variant contributes to the hypertensive and cardiac hypertrophic phenotype in vivo. Transgenic mice were generated to express wild-type or T555I/Q568P variant corin in the heart under the control of α-myosin heavy chain promoter. The mice were crossed into a corin knockout background to create KO/TgWT and KO/TgV mice that expressed WT or variant corin, respectively, in the heart. Functional studies showed that KO/TgV mice had significantly higher levels of pro-atrial natriuretic peptide in the heart compared with that in control KO/TgWT mice, indicating that the corin variant was defective in processing natriuretic peptides in vivo. By radiotelemetry, corin KO/TgV mice were found to have hypertension that was sensitive to dietary salt loading. The mice also developed cardiac hypertrophy at 12–14 months of age when fed a normal salt diet or at a younger age when fed a high salt diet. The phenotype of salt-sensitive hypertension and cardiac hypertrophy in KO/TgV mice closely resembles the pathological findings in African Americans who carry the corin variant. The results indicate that corin defects may represent an important mechanism in salt-sensitive hypertension and cardiac hypertrophy in African Americans.
Keywords: cardiac hypertrophy, corin, natriuretic peptide, mouse models, salt-sensitive hypertension
Hypertension is a major risk factor for cardiovascular disease such as stroke and myocardial infarction. The prevalence of hypertension is particularly high in African Americans, but the underlying mechanism is unclear.1, 2 Environmental, socioeconomic and genetic factors may all contribute to the disease.3–6 Genome-wide linkage analyses indicate several chromosomal loci that may influence blood pressure in African Americans.7, 8 Genetic variants in enzymes in epinephrine synthesis and the renin-angiotensin-aldosterone system also are associated with hypertension in this population.9–11
Natriuretic peptides are important for maintaining salt-water balance and normal blood pressure.12 Corin is a serine protease highly expressed in cardiac myocytes.13, 14 It activates natriuretic peptides, thereby regulating blood pressure and cardiac function.15, 16 In mice, corin deficiency prevented atrial natriuretic peptide (ANP) activation and caused salt-sensitive hypertension.17, 18 Corin-deficient mice had cardiac hypertrophy and poor cardiac function.17, 19, 20
Single nucleotide polymorphisms (SNPs) (T555I/Q568P) in the CORIN gene were identified in African Americans with hypertension and cardiac hypertrophy.21 These SNPs are located in exon 12 of a minor CORIN allele that is more common in African Americans than Caucasians (~12% vs. <0.2% with one or two copies of the allele).21, 22 In patients with heart failure, individuals with this minor CORIN allele had impaired natriuretic peptide processing and worse clinical outcomes compared with those without this allele.23 Biochemical studies showed that recombinant corin variant T555I/Q568P had a reduced biological activity, indicating that the SNPs may alter corin protein structure and function.24 The results suggested that corin variant T555I/Q568P may contribute to hypertension and cardiac hypertrophy in African Americans.
To test this hypothesis, we generated transgenic (Tg) mice expressing the corin variant in a corin null background and examined corin variant function in vivo and its effect on blood pressure and cardiac morphology. Here we report that the Tg mice had impaired ANP processing in the heart and developed hypertension and cardiac hypertrophy, a phenotype similar to that in African Americans with the CORIN variant allele. Our results indicate that defects in the corin-ANP pathway may be an important contributing factor in hypertension and heart disease in humans, especially in African Americans.
Methods
Generation of Tg mice
Plasmid encoding mouse corin variant T623I/Q636P, corresponding to human corin variant T555I/Q568P, was made by mutagenesis. To generate Tg mice with heart specific corin expression, corin wild-type (WT) and variant cDNAs were inserted into a plasmid driven by the mouse α-myosin heavy chain (MHC) promoter (Supplemental Figure S1A).25 The plasmids were used for pronuclear microinjection to produce Tg mice, which were crossed with corin knockout (KO) mice to generate KO/Tg mice expressing WT or variant corin in the heart in a null background. Heterozygous mice with one null allele and one WT or variant transgene allele were studied. The animal procedures were approved by the IACUC of the Cleveland Clinic. Detailed methods for making Tg mice are described in Online Supplemental Methods.
Western Blotting and ELISA
To analyze corin protein in hearts, tissues were homogenized in a buffer containing 50 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 1% Triton X-100 (vol/vol), and a protease inhibitor cocktail (1:100 dilution, Sigma). Proteins were analyzed by SDS-PAGE and Western blotting with a polyclonal antibody (Berlex Biosciences).26 Cardiac pro-ANP expression was analyzed by Western blotting with a polyclonal antibody (Santa Cruz). Plasma levels of N-terminal (NT)-pro-ANP were measured by ELISA (Alpco Diagnostics).
Heart Membrane Fractions and Pro-ANP Processing Assay
Cell membrane fractions from hearts were prepared by ultracentrifugation.26 Cell membrane pellets were resuspended in an NP-40 buffer and protein concentrations were determined by a Bradford method (Bio-Rad). Recombinant pro-ANP from transfected HEK293 cells was added to the heart membranes and incubated at 37°C over time. Pro-ANP conversion to ANP was analyzed by immunoprecipitation and Western blotting.27, 28 Detailed methods are described in Online Supplemental Methods.
Blood Pressure Measurement
Blood pressure was monitored continuously by radiotelemetry in conscious and unrestrained mice.25 Detailed methods for radiotelemetry are described in Online Supplemental Methods.
Effects of Dietary Salt on Blood Pressure
Mice were fed normal (0.3% NaCl), or high (4% and 8% NaCl) salt diets (Harlan Teklad) for three weeks. Blood pressure was monitored by radiotelemetry before, during and after different salt diets.
Histological Analysis of Hearts
Hearts were isolated, weighed and fixed with 10% formalin. Longitudinal and transversal sections (5 μm in thickness) were stained with hematoxylin and eosin (H&E). Computer-assisted measurement (Measure IT, Olympus) at a high magnification (×400) was used to determine the diameter of ~100 individual cardiac myocytes in 5 randomly selected fields in left ventricular (LV) sections. The analysis was done in a blind fashion.
Statistical Analysis
Data were analyzed using the Prism software (Graph-Pad) and presented as means ± SD. Comparisons between two groups were made using the Student’s t test. Three or more groups were compared using ANOVA followed by post hoc least significant difference. A P value of <0.05 was considered to be statistically significant.
Results
Generation of Corin Tg Mice
To test corin variant function in vivo, we generated Tg mice with cardiac-specific expression of the corin variant and WT control. Transgene copy numbers in founder lines were determined by Southern blotting (Supplemental Figure S1B). WT and corin variant founders with similar transgene copy numbers were selected to cross with corin KO mice to create KO/Tg mice expressing WT or corin variant (V) in the heart in a null background (Supplemental Figure S1C). The tissue specificity of Tg corin expression was verified by RT-PCR (Supplemental Figure S1D). Similar levels of heart-specific corin protein expression were confirmed by Western analysis (Supplemental Figure S1E and F).
Impaired Pro-ANP Processing in Corin KO/TgV Mice
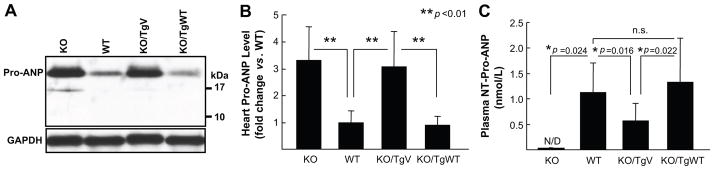
Previously, corin variant T555I/Q568P was found to have a reduced pro-ANP processing activity in cell-based assays.24 To determine if the variant had an impaired activity in vivo, pro-ANP levels in hearts from KO/TgWT and KO/TgV mice were analyzed by Western blotting. An ~20-kDa band, representing pro-ANP, was found to be stronger in intensity in corin KO and KO/TgV mice compared to that in WT and KO/TgWT mice (Figure 1A). Quantitatively, pro-ANP levels in WT and KO/TgWT mice were comparable (p>0.05, n=6), whereas the levels in corin KO and KO/TgV mice were ~3-fold higher than that of WT or KO/TgWT mice (p<0.01, n=6) (Figure 1B). On Western blots, ANP was not detected, indicating that it was secreted from the heart once activated from pro-ANP.
Figure 1. Pro-ANP expression and processing.
(A) Western analysis of pro-ANP in hearts from corin KO, WT, KO/TgWT and KO/TgV mice. (B) Quantitative data of Western blots from 6 independent experiments. (C) Plasma levels of NT-pro-ANP in corin KO, WT, KO/TgWT and KO/TgV mice. n≥8 per group. N/D, not detectable; n.s., not significant.
By ELISA, plasma NT-pro-ANP was measured. The levels in WT mice were 1.13 ± 0.58 nmol/L (n=11) but undetectable in KO mice (n=8) (Figure 1C). In corin KO/TgV mice, the levels were significantly lower than that of WT and KO/TgWT mice (0.57 ± 0.35 vs. 1.13 ± 0.58 and 1.33 ± 0.87 nmol/L, respectively; both p values<0.05, n=8–10). There was no significant difference between WT and KO/TgWT mice (Figure 1C). The results indicated that detected plasma NT-pro-ANP represented cleaved N-terminal pro-ANP fragments and that pro-ANP processing was impaired in corin KO/TgV mice.
Pro-ANP Processing by Heart Membranes
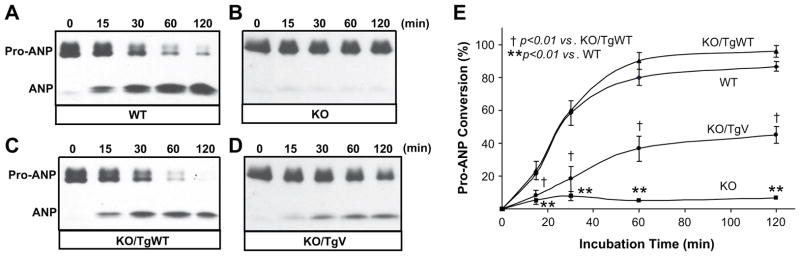
Corin is a membrane protein.28, 29 To determine corin activity in hearts, we prepared heart membrane fractions from Tg mice and measured pro-ANP processing activity. The activity was detected in a time-dependent manner in WT but not KO mice (Figure 2A and B). A similar activity was observed in KO/TgWT mice (Figure 2C). The activity was significantly reduced in KO/TgV mice (Figure 2D and E).
Figure 2. Pro-ANP processing activity in Tg mouse hearts.
(A–D) Pro-ANP processing activity was assayed using membrane fractions from corin WT, KO, KO/TgWT and KO/TgV mouse hearts. Conversion of pro-ANP to ANP was analyzed by immunoprecipitation and Western blotting. (E) Quantitative data of Western blots from 3 independent experiments.
Hypertension in Corin KO/TgV Mice
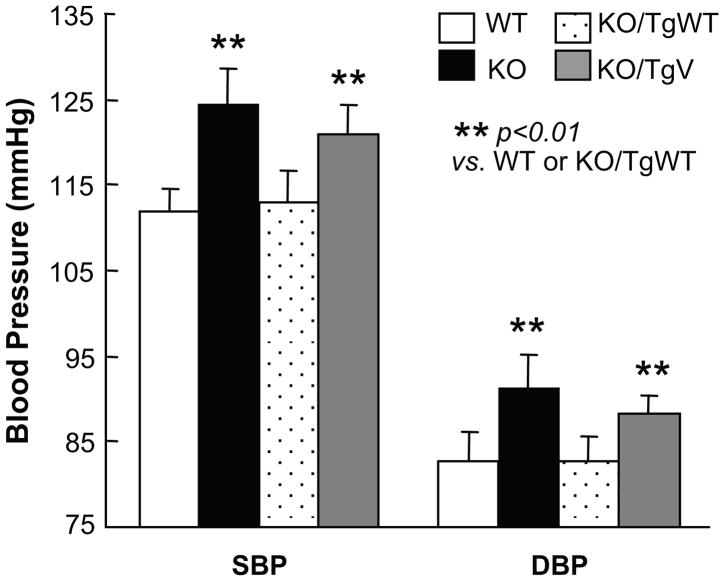
Corin variant T555I/Q568P was associated with hypertension and cardiac hypertrophy in African Americans.21, 30 We measured blood pressure in corin KO/TgWT and KO/TgV mice. On a normal salt (0.3% NaCl) diet, corin KO mice had higher blood pressures than WT mice [systolic (SBP) 124 ± 4 vs. 112 ± 3 mmHg, p<0.01; diastolic (DBP) 91 ± 4 vs. 83 ± 3 mmHg, p<0.01] (Figure 3). In KO/TgWT mice, both SBP and DBP were restored to normal levels (SBP 113 ± 4 vs. 112 ± 3 mmHg in WT; DBP 83 ± 3 vs. 83 ± 3 mmHg in WT, both p values >0.05) (Figure 3). In KO/TgV mice, blood pressures remained high (SBP 121 ± 4 mmHg; DBP 88 ± 2 mmHg, p<0.01 vs. WT or KO/TgWT) (Figure 3).
Figure 3. Hypertension in corin KO/TgV mice.
SBP and DBP in Tg mice on a normal salt (0.3% NaCl) diet were measured by radiotelemetry. Data are mean ± SD from at least 6 mice per group. **p<0.01 vs. WT or KO/TgWT mice by two-way ANOVA.
Salt-sensitive Hypertension in Corin KO/TgV Mice
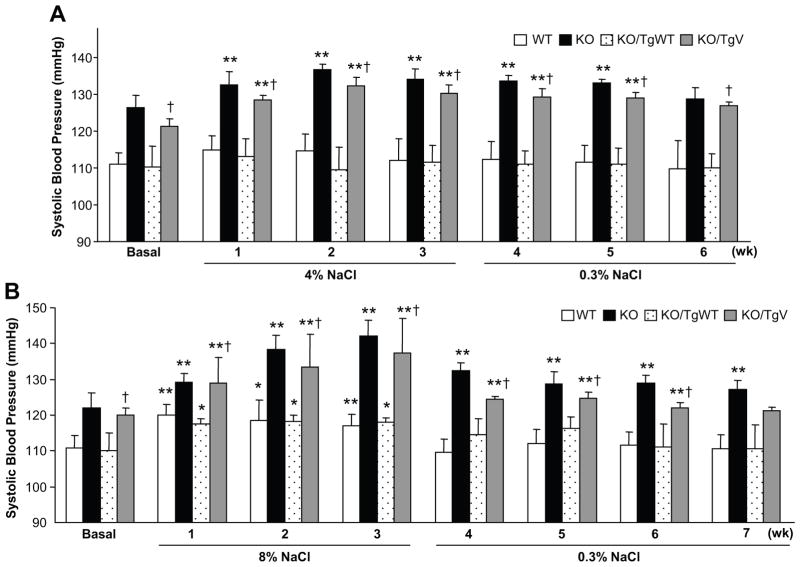
We next tested effects of high-salt diets on blood pressure. On a 4% NaCl diet, SBP in KO/TgV mice increased within a week from 121 ± 2 to 129 ± 1 mmHg (p<0.01) (Figure 4A). Similarly, DBP also increased in these mice (data not shown). Similar salt-sensitive hypertension occurred in corin KO mice (Figure 4A). In contrast, blood pressure did not increase significantly in WT or KO/TgWT mice (Figure 4A). When the mice were switched to the normal salt diet, blood pressure in KO and KO/TgV mice remained high for 2 more weeks (Figure 4A).
Figure 4. Salt-sensitive hypertension in corin KO/TgV mice.
(A) Tg mice on a 0.3% NaCl diet (basal) were switched to a 4% NaCl diet for 3 weeks (wk) and then back to the 0.3% NaCl diet. SBP data from 6–10 mice per group are shown. Corin WT and KO mice were included as controls. (B) Similar studies were conducted in the Tg mice on an 8% NaCl diet. SBP data from 4–6 mice per group are shown. *p<0.05 or **p<0.01 vs. basal of the same genotype; †p<0.01 vs. KO/TgWT of the same group by two-way ANOVA.
When the mice were fed with an 8% NaCl diet, blood pressure in KO/TgV mice increased further (SBP from 120 ± 2 to 137 ± 10 mmHg, p<0.01), which was similar to that in KO mice (Figure 4B). On this high salt diet, blood pressure in WT and KO/TgWT mice also increased (Figure 4B). When the mice were switched to the normal salt diet, blood pressure in WT and KO/TgWT mice quickly returned to normal levels whereas that in KO and KO/TgV mice remained high for 3–4 weeks (Figure 4B).
Cardiac Hypertrophy in Corin KO/TgV Mice
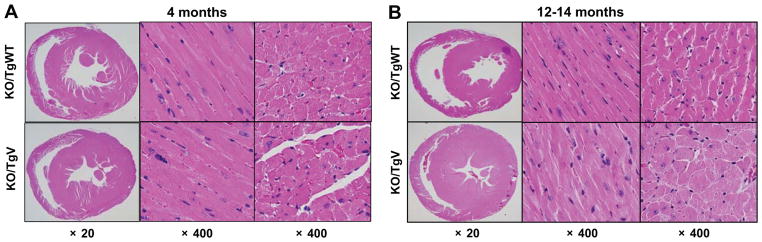
Previous studies showed that corin KO mice developed cardiac hypertrophy at ~12 months of age.17 Consistently, no apparent cardiac hypertrophy was observed in 4-month old KO/TgV mice (Figure 5A). LV wall thickness increased significantly by 12–14-months in these mice (Figure 5B). Such a change was not observed in KO/TgWT mice (Figure 5B). In 12–14-month old KO/TgV mice, LV muscle fibers were much thicker with an average diameter of 20.1 ± 1.5 μm, significantly greater than that in KO/TgWT mice (14.6 ± 1.3 μm, p<0.01) (Figure 5B). The ratio of heart weight to body weight or tibia length was significantly greater in 12–14-month old KO/TgV mice compared to that in KO/TgWT mice of similar age (Supplemental Figure S2A and B).
Figure 5. Cardiac hypertrophy in corin KO/TgV mice on normal salt diet.
H&E-stained heart sections from corin KO/TgWT and KO/TgV mice at 4-months (A) or 12–14-months (B) of age on a 0.3% NaCl diet were shown at low (×20) and high (×400) magnifications.
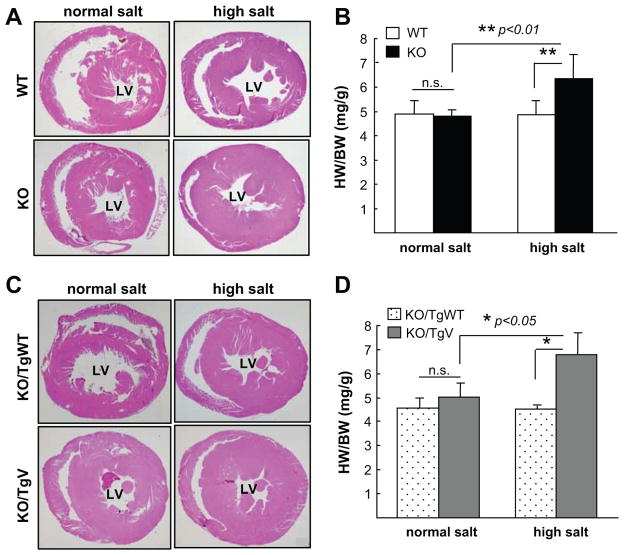
We next tested the effect of high salt diet on cardiac hypertrophy. When 4-month old WT, KO, KO/TgWT and KO/TgV mice, which did not have LV hypertrophy, were fed with an 8% NaCl diet for 3 weeks, LV wall thickness and ratio of heart weight to body weight or tibia length all increased in KO and KO/TgV mice (Figure 6A–D and Supplemental Figure S3A–B). In contrast, these changes were not observed in WT and KO/TgWT mice (Figure 6A–D and Supplemental Figure S3A–B).
Figure 6. Cardiac hypertrophy in corin KO/TgV mice on high salt diet.
H&E-stained heart sections from corin WT and KO (A) or KO/TgWT and KO/TgV (C) mice at 4-months of age on normal and 8% NaCl diets were shown at a lower (× 20) magnification. Ratios of heart weight (HW) to body weight (BW) (B, D) were calculated from 8–10 mice per group. n.s., not significant.
Discussion
Hypertension occurs in all ethnic groups but its high prevalence in African Americans is striking.1, 2 Corin is a cardiac protease that regulates blood pressure. Genetic studies have identified corin variant T555I/Q568P in African Americans who had hypertension and cardiac hypertrophy.21, 30 In biochemical studies, recombinant corin variant T555I/Q568P had an impaired natriuretic peptide processing activity,24 suggesting that genetic variations in the CORIN gene may reduce corin activity in vivo, thereby contributing to hypertension in African Americans.
Mouse models are useful tools to study human genetic variants.31, 32 To test our hypothesis, we generated KO/TgWT and KO/TgV mice that had comparable cardiac corin levels in a corin null background. We found similarly low levels of pro-ANP in hearts from WT and KO/TgWT mice, whereas the levels were much higher in corin KO and KO/TgV mice (Figure 1A and B). Consistently, comparable plasma levels of NT-pro-ANP fragments were detected in WT and KO/TgWT mice, whereas the levels were low in KO/TgV mice and undetectable in KO mice (Figure 1C). The results show that pro-ANP processing was restored in the heart in KO/TgWT but not KO/TgV mice, supporting that the corin variant was defective in vivo.
In our previous in vitro studies,24 the corin variant exhibited impaired zymogen activation and hence reduced activity. Because of lacking a suitable antibody that recognizes the activated corin protease fragment, we were unable to directly determine corin zymogen activation in mouse hearts. To circumvent this problem, we developed an assay measuring corin activity in heart membrane fractions. The results showed that corin activity was significantly lower in KO/TgV mice than that in KO/TgWT mice (Figure 2). Since corin protein levels were similar in KO/TgWT and KO/TgV mouse hearts (Supplemental Figure S1E and F), reduced corin activity in KO/TgV mouse hearts was probably due to impaired corin zymogen activation, consistent with our previous in vitro findings. Recent studies indicated that impaired corin zymogen activation and reduced corin activity may be important in the pathogenesis of heart failure in patients.26, 33, 34
Corin is essential for maintaining normal blood pressure.35 We found elevated SBP and DBP in corin KO/TgV mice (Figure 3). Moreover, blood pressure in KO/TgV mice was highly sensitive to dietary salt loading, a phenotype similar to that of corin KO mice (Figure 4). Recent studies in mice showed that corin deficiency caused sodium retention in an ENaC-dependent mechanism, which may underlie salt-sensitive hypertension.17, 18, 36, 37 African Americans are known for high prevalence of salt-sensitive hypertension.3–5 Population studies show that corin variant T555I/Q568P allele was more common in African Americans than Caucasians.21 It is possible, therefore, that the corin variant may contribute to high prevalence of salt-sensitive hypertension in African Americans.
Natriuretic peptides are shown to have a direct anti-hypertrophic function in the heart.38–40 Mice lacking either corin or ANP developed cardiac hypertrophy.17, 19, 20, 41 In African Americans, corin variant T555I/Q568P was associated with severe cardiac hypertrophy.30 In this study, we showed that mice carrying the corin variant developed significant cardiac hypertrophy either at an older age when on a normal salt diet or at a younger age when on a high salt diet (Figures 5 and 6). Thus, the results from our mouse model studies helped to establish a link between the corin variant and the cardiac phenotype in vivo.
Supplementary Material
Perspectives.
The results from this study showed that Tg mice expressing the corin variant identified in African Americans developed hypertension and cardiac hypertrophy. The phenotype mimics the clinical features in African Americans who carry the CORIN variant allele. The results provide direct experimental evidence that this CORIN allele is defective in vivo, suggesting that the corin variant may contribute to hypertension and heart disease in African Americans. Previously, SNPs in the genes coding for ANP or its receptor also were reported in patients with hypertension and cardiac hypertrophy.42, 43 Together, these data suggest that defects in the corin-ANP pathway may be an important mechanism in hypertension and cardiac hypertrophy in patients. Most recently, corin and ANP have been found to act locally in the pregnant uterus to regulate spiral artery remodeling, which is critical for preventing pregnancy-induced hypertension.25 Our findings should encourage more genetic studies to determine if additional corin gene variants or mutations may play a role in hypertensive disease in patients.
Novelty and Significance.
What Is New?
This study shows that Tg mice expressing the corin variant identified in African Americans developed salt-sensitive hypertension and cardiac hypertrophy.
The data provide direct experimental evidence that this CORIN variant allele is defective in vivo.
What Is Relevant?
African Americans are a high risk population for salt-sensitive hypertension and heart disease.
The CORIN variant gene allele is associated with African Americans with hypertension and cardiac hypertrophy but its contribution to the disease was unknown.
Summary
These data indicate that corin gene defects may be an important mechanism in salt-sensitive hypertension and cardiac hypertrophy in patients, especially in African Americans.
Acknowledgments
We thank Xiaolan Zhao of the Lerner Core Facility for DNA sequencing.
Sources of Funding
This work was supported in part by grants from the NIH (HL089298; HD064634) and the Priority Academic Program Development of Jiangsu Higher Education Institutions in China. .
Footnotes
Disclosures
None.
References
- 1.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 2.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 3.Campese VM. Why is salt-sensitive hypertension so common in blacks? Nephrol Dial Transplant. 1996;12:399–403. doi: 10.1093/ndt/12.3.399. [DOI] [PubMed] [Google Scholar]
- 4.Palacios C, Wigertz K, Martin BR, Jackman L, Pratt JH, Peacock M, McCabe G, Weaver CM. Sodium retention in black and white female adolescents in response to salt intake. J Clin Endocrinol Metab. 2004;89:1858–1863. doi: 10.1210/jc.2003-031446. [DOI] [PubMed] [Google Scholar]
- 5.Pratt JH, Ambrosius WT, Agarwal R, Eckert GJ, Newman S. Racial difference in the activity of the amiloride-sensitive epithelial sodium channel. Hypertension. 2002;40:903–908. doi: 10.1161/01.hyp.0000039749.75068.f4. [DOI] [PubMed] [Google Scholar]
- 6.Saha C, Eckert GJ, Ambrosius WT, Chun TY, Wagner MA, Zhao Q, Pratt JH. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension. 2005;46:481–487. doi: 10.1161/01.HYP.0000179582.42830.1d. [DOI] [PubMed] [Google Scholar]
- 7.Rice T, Cooper RS, Wu X, Bouchard C, Rankinen T, Rao DC, Jaquish CE, Fabsitz RR, Province MA. Meta-analysis of genome-wide scans for blood pressure in African American and Nigerian samples. The National Heart, Lung, and Blood Institute GeneLink Project. Am J Hypertens. 2006;19:270–274. doi: 10.1016/j.amjhyper.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Zhu X, Luke A, Cooper RS, Quertermous T, Hanis C, Mosley T, Gu CC, Tang H, Rao DC, Risch N, Weder A. Admixture mapping for hypertension loci with genome-scan markers. Nat Genet. 2005;37:177–181. doi: 10.1038/ng1510. [DOI] [PubMed] [Google Scholar]
- 9.Cui J, Zhou X, Chazaro I, DeStefano AL, Manolis AJ, Baldwin CT, Gavras H. Association of polymorphisms in the promoter region of the PNMT gene with essential hypertension in African Americans but not in whites. Am J Hypertens. 2003;16:859–863. doi: 10.1016/s0895-7061(03)01026-4. [DOI] [PubMed] [Google Scholar]
- 10.Henderson SO, Haiman CA, Mack W. Multiple Polymorphisms in the renin- angiotensin-aldosterone system (ACE, CYP11B2, AGTR1) and their contribution to hypertension in African Americans and Latinos in the multiethnic cohort. Am J Med Sci. 2004;328:266–273. doi: 10.1097/00000441-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Rotimi C, Puras A, Cooper R, McFarlane-Anderson N, Forrester T, Ogunbiyi O, Morrison L, Ward R. Polymorphisms of renin-angiotensin genes among Nigerians, Jamaicans, and African Americans. Hypertension. 1996;27:558–563. doi: 10.1161/01.hyp.27.3.558. [DOI] [PubMed] [Google Scholar]
- 12.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 13.Tran KL, Lu X, Lei M, Feng Q, Wu Q. Upregulation of corin gene expression in hypertrophic cardiomyocytes and failing myocardium. Am J Physiol Heart Circ Physiol. 2004;287:H1625–1631. doi: 10.1152/ajpheart.00298.2004. [DOI] [PubMed] [Google Scholar]
- 14.Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274:14926–14935. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q. The serine protease corin in cardiovascular biology and disease. Front Biosci. 2007;12:4179–4190. doi: 10.2741/2379. [DOI] [PubMed] [Google Scholar]
- 16.Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75:142–146. doi: 10.1038/ki.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A. 2005;102:785–790. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Shen J, Cui Y, Jiang J, Chen S, Peng J, Wu Q. Impaired sodium excretion and salt-sensitive hypertension in corin-deficient mice. Kidney Int. 2012;82:26–33. doi: 10.1038/ki.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckley CL, Stokes AJ. Corin-deficient W-sh mice poorly tolerate increased cardiac afterload. Regul Pept. 2011;172:44–50. doi: 10.1016/j.regpep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, Gurish M, Mathis D, Benoist C, Lee DM. Genetic inversion in mast cell-deficient (W(sh)) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173:1693–1701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, Leonard D, Ho SI, Wu Q, Post W, Drazner MH. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 22.Pan J, Hinzmann B, Yan W, Wu F, Morser J, Wu Q. Genomic structures of the human and murine corin genes and functional GATA elements in their promoters. J Biol Chem. 2002;277:38390–38398. doi: 10.1074/jbc.M205686200. [DOI] [PubMed] [Google Scholar]
- 23.Rame JE, Tam SW, McNamara D, Worcel M, Sabolinski ML, Wu AH, Dries DL. Dysfunctional corin I555(P568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: results from the Genetic Risk Assessment in Heart Failure substudy. Circ Heart Fail. 2009;2:541–548. doi: 10.1161/CIRCHEARTFAILURE.109.866822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, Qin J, Wu Q. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103:502–508. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui Y, Wang W, Dong N, Lou J, Srinivasan DK, Cheng W, Huang X, Liu M, Fang C, Peng J, Chen S, Wu S, Liu Z, Dong L, Zhou Y, Wu Q. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484:246–250. doi: 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S, Sen S, Young D, Wang W, Moravec CS, Wu Q. Protease corin expression and activity in failing hearts. Am J Physiol Heart Circ Physiol. 2010;299:H1687–1692. doi: 10.1152/ajpheart.00399.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao X, Wang W, Chen S, Wu Q. Role of glycosylation in corin zymogen activation. J Biol Chem. 2007;282:27728–27735. doi: 10.1074/jbc.M703687200. [DOI] [PubMed] [Google Scholar]
- 28.Qi X, Jiang J, Zhu M, Wu Q. Human corin isoforms with different cytoplasmic tails that alter cell surface targeting. J Biol Chem. 2011;286:20963–20969. doi: 10.1074/jbc.M110.217570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang J, Wu S, Wang W, Chen S, Peng J, Zhang X, Wu Q. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J Biol Chem. 2011;286:10066–10072. doi: 10.1074/jbc.M110.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rame JE, Drazner MH, Post W, Peshock R, Lima J, Cooper RS, Dries DL. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49:857–864. doi: 10.1161/01.HYP.0000258566.95867.9e. [DOI] [PubMed] [Google Scholar]
- 31.Kuang SQ, Kwartler CS, Byanova KL, Pham J, Gong L, Prakash SK, Huang J, Kamm KE, Stull JT, Sweeney HL, Milewicz DM. Rare, nonsynonymous variant in the smooth muscle-specific isoform of myosin heavy chain, MYH11, R247C, alters force generation in the aorta and phenotype of smooth muscle cells. Circ Res. 2012;110:1411–1422. doi: 10.1161/CIRCRESAHA.111.261743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu F, Dollé ME, Berton TR, Kuiper RV, Capps C, Espejo A, McArthur MJ, Bedford MT, van Steeg H, de Vries A, Johnson DG. Mouse models for the p53 R72P polymorphism mimic human phenotypes. Cancer Res. 2010;70:5851–5859. doi: 10.1158/0008-5472.CAN-09-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong N, Chen S, Yang J, He L, Liu P, Zheng D, Li L, Zhou Y, Ruan C, Plow E, Wu Q. Plasma soluble corin in patients with heart failure. Circ Heart Fail. 2010;3:207–211. doi: 10.1161/CIRCHEARTFAILURE.109.903849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibebuogu UN, Gladysheva IP, Houng AK, Reed GL. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ Heart Fail. 2011;2011:114–120. doi: 10.1161/CIRCHEARTFAILURE.109.895581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Q, Kuo HC, Deng GG. Serine proteases and cardiac function. Biochim Biophys Acta. 2005;1751:82–94. doi: 10.1016/j.bbapap.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Klein JD. Corin: an ANP protease that may regulate sodium reabsorption in nephrotic syndrome. Kidney Int. 2010;78:635–637. doi: 10.1038/ki.2010.223. [DOI] [PubMed] [Google Scholar]
- 37.Polzin D, Kaminski HJ, Kastner C, Wang W, Krämer S, Gambaryan S, Russwurm M, Peters H, Wu Q, Vandewalle A, Bachmann S, Theilig F. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int. 2010;78:650–659. doi: 10.1038/ki.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calderone A, Thaik CM, Takahashi N, Chang DL, Colucci WS. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J Clin Invest. 1998;101:812–818. doi: 10.1172/JCI119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, Schneider MD, Garbers DL, Kuhn M. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest. 2003;111:1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knowles JW, Esposito G, Mao L, Hagaman JR, Fox JE, Smithies O, Rockman HA, Maeda N. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J Clin Invest. 2001;107:975–984. doi: 10.1172/JCI11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melo LG, Veress AT, Chong CK, Pang SC, Flynn TG, Sonnenberg H. Salt-sensitive hypertension in ANP knockout mice: potential role of abnormal plasma renin activity. Am J Physiol. 1998;274:R255–261. doi: 10.1152/ajpregu.1998.274.1.R255. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama T, Soma M, Takahashi Y, Rehemudula D, Kanmatsuse K, Furuya K. Functional deletion mutation of the 5′-flanking region of type A human natriuretic peptide receptor gene and its association with essential hypertension and left ventricular hypertrophy in the Japanese. Circ Res. 2000;86:841–845. doi: 10.1161/01.res.86.8.841. [DOI] [PubMed] [Google Scholar]
- 43.Rubattu S, Bigatti G, Evangelista A, Lanzani C, Stanzione R, Zagato L, Manunta P, Marchitti S, Venturelli V, Bianchi G, Volpe M, Stella P. Association of atrial natriuretic peptide and type a natriuretic peptide receptor gene polymorphisms with left ventricular mass in human essential hypertension. J Am Coll Cardiol. 2006;48:499–505. doi: 10.1016/j.jacc.2005.12.081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.