Abstract
Obesity is a major risk factor for hypertension (HT). The co-presentation of HT and insulin resistance (IR) suggests a role for IR in blood pressure (BP) dysregulation. To test this hypothesis peripheral IR has been genetically subtracted in a model of obesity by crossing leptin receptor mutant mice (KdbHPTP) with mice lacking protein tyrosine phosphatase 1B (insulin desensitizer, HdbKPTP) to generate obese insulin sensitive mice (KdbKPTP). BP was recorded in lean (HdbHPTP, HdbKPTP) and obese (KdbHPTP, KdbKPTP) mice via telemetry and a frequency analysis of the recording was performed to determine BP variability. Correction of IR in obese mice normalized BP values to baseline levels (HdbHPTP: 116±2 mmHg; KdbHPTP: 129±4; KdbKPTP: 114±5mmHg) and restored BP variability by decreasing its standard deviation and the frequency of BP values over the upper autoregulatory limit of the kidneys. However, while IR-induced increases in proteinuria (vs. 53±13μg/day, HdbHPTP) were corrected in KdbKPTP (112±39 vs. 422±159 μg/day, KdbHPTP), glomerular hypertrophy was not. IR reduced plasma aldosterone levels ruling out a role for mineralocorticoids in the development of hypertension. Taken together, these data indicate that correction of IR prevents hypertension, BP variability and microalbuminuria in obese mice. While the mechanism remains to be fully determined, increases in aldosterone or sympathoactivation of the cardiovascular system seem to be less likely contributors.
Keywords: aldosterone, obese mice, db/db mice, pressure variability, Hypertension, Obesity, Insulin Resistance, Protein Tyrosine Phosphatase 1B, Blood Pressure Variability
Introduction
Hypertension is a major risk factor for cardiovascular disease affecting one third of the American population1. Although obesity has been clearly identified as a major risk factor for the development of hypertension2, mechanisms by which obesity increases blood pressure (BP) are incompletely understood. Since hypertension and insulin resistance commonly present together in obese patients3-5, insulin resistance has been presented as (choose “a” vs. “the”) potential link between metabolic dysfunctions induced by obesity and hypertension6.
Whether and how insulin resistance might produce an increase in blood pressure has been the subject of intense investigation. Supporting evidence stems from observations in rats on a high sugar diet in which moderate increases in insulin resistance are associated with increases in tail cuff pressure7, 8. Moreover, insulin stimulates pathways that would be considered pro-hypertensive such as sympathetic outflow9, 10 or vasoconstriction in compromised beds11. In contrast to these observations is emerging evidence that high-sugar diets do not cause hypertension when pressure is assessed by catheter-based methods12, 13 and blood pressure effects of pharmacologic modulators of insulin signaling is neutral, mixed or lacking sufficient data to draw conclusions14. The question of whether insulin resistance in the context of obesity elevates blood pressure remains unanswered.
To address the role of insulin resistance in the development of hypertension associated with obesity, we generated an obese insulin sensitive mouse model by deleting the molecular restraint of the insulin signaling pathway, protein tyrosine phosphatase 1B (PTP1B)15, in leptin receptor deficient mice (db/db). We hypothesized that improving insulin sensitivity without affecting body weight, in obese animals, would improve BP. Metabolic profiling was used to assess the degree of insulin sensitivity of the animals while radiotelemeter probes were implanted to record their blood pressure. Potential mechanisms of hypertension were assessed by examining pressure variability, aldosterone production and kidney injury.
Material and Methods
Animal model
To study the cardiovascular consequences of correcting insulin resistance in obese mice, four groups of mice were generated by crossing obese leptin receptor deficient (db/db) mice with mice presenting an increase in insulin sensitivity thanks to the deletion of protein tyrosine phosphatase 1B16, 17. As previously described15, this breeding strategy yields i) dual heterozygous littermates (HdbHPTP1B), used as lean control, ii) lean insulin sensitive mice heterozygous for db but deficient in PTP1B (HdbKPTP1B), iii) obese insulin resistant animals deficient in db and heterozygous for PTP1B (KdbHPTP1B) and iiii) obese insulin sensitive mice deficient in db and PTP1B genes (KdbKPTP1B). Mice were housed in an American Association of Laboratory Animal Care–approved animal care facility at Georgia Health Sciences University, and the Institutional Animal Care and Use Committee approved all protocols.
Metabolic Measurements
Details regarding plasma measurements of glucose, cholesterol, triglycerides, NEFA, insulin, leptin and thyroid hormones can be found in the online-only Data Supplement available at http://www.hypertensionaha.org.
In vivo blood pressure measurement
Details regarding blood pressure measurements in conscious and unconscious mice can be found in the online-only Data Supplement available at http://www.hypertensionaha.org.
Vascular adrenergic tone
Mesenteric arteries from another set of mice were isolated and mounted on a pressurized myograph to determine their contractile response adrenergic stimulation (Phenylephrine, 10 nM to 100μM), as previously described18.
Renal Morphology
Details regarding the analysis of the renal morphology can be found in the online-only Data Supplement available at http://www.hypertensionaha.org.
Statistical Analysis
All data are presented as mean±sem. Differences in means among groups for non-repeated variables were compared by 1-way ANOVA. Differences in means among groups and treatments, with repeated variables, were compared by 2- or 3-way ANOVA with repeated measures, when appropriate. Bonferroni and Fisher least significant difference tests were used as the post hoc test (SigmaStat).
Results
Indices of the metabolic and renal function
The effects of the deletion of the db and PTP1B genes on the mice phenotype were determined by measuring body weight and baseline plasma chemistry. As summarized in Table 1, leptin receptor deficiency induced a significant increase in body weight in KdbHPTP1B mice. PTP1B deletion did not affect body weight, neither in obese KdbKPTP1B mice nor in lean HdbKPTP1B mice. Obesity, induced by the deletion of the leptin receptor, was associated with a significant increase in fasting blood glucose, in the percentage of glycosylated hemoglobin (HbA1c), and in plasma insulin levels but also with increased circulating lipids levels (cholesterol, triglycerides and NEFA). These data confirm that the KdbHPTP1B mice represent a good model to study the cardiovascular consequences of obesity-induced insulin-resistance. While the deletion of PTP1B did not affect fasting blood glucose and insulin levels, in lean and obese mice, it significantly improved HbA1c, an index of the total glycemia. This is consistent with previous descriptions of this model indicating that PTP1B deletion improved peripheral insulin sensitivity but not hepatic insulin sensitivity15. Plasma thyroid hormones levels as well as plasma levels of resistin and C-Reactive Protein were not affected by obesity or by the deletion of PTP1B.
Table 1.
General characteristics of the 4 groups of mice.
| Lean | Obese | |||
|---|---|---|---|---|
| Parameter | HdbHPTP1B | HdbKPTP1B | KdbHPTP1B | KdbKPTP1B |
| Body Weight (g) | 30 ± 0.5 | 31 ± 0.5 | 53 ± 1* | 50 ± 1* |
| Cholesterol (mg/dl) | 63 ±5 | 67 ± 4 | 144 ± 13* | 131 ± 10* |
| Triglycerides (mg/dl) | 53 ±7 | 79 ± 5 | 119 ± 19* | 67 ± 10† |
| NEFA (mg/dl) | 0.24 ± 0.02 | 0.37 ± 0.07 | 0.75 ± 0.19* | 0.59 ± 0.13# |
| Glucose (mg/dl) | 115 ± 5 | 136 ± 12 | 236 ± 18* | 199 ± 23* |
| HbA1c (%) | 4.7 ± 0.7 | 4.9 ± 0.2 | 9.7 ± 0.3* | 7.6 ± 0.2† |
| Insulin (ng/ml) | 0.45 ± 0.1 | 0.54 ± 0.1 | 5.44 ± 0.7* | 4.68 ± 0.6* |
| Leptin (ng/ml) | 2.57 ± 0.3 | 1.38 ± 0.3* | 9.8 ± 0.9* | 8.5 ± 1.1 |
| T3 (ng/dl) | 18 ± 3 | 20 ± 1.2 | 23 ± 1.4 | 25 ± 1.3 |
| T4 (μg/dl) | 11.3 ± 0.6 | 12.1 ± 1.2 | 11.1 ± 0.7 | 9.5 ± 0.6 |
| Intact PTH (pg/ml) | 16.8 ± 2.1 | 14.4 ± 2.2 | 15.1 ± 4.1 | 19.4 ± 4.8 |
| C-Reactive Protein (ng/ml) | 76 ± 5 | 80 ± 3 | 69 ± 4 | 72.4 ± 4 |
| Resistin (ng/ml) | 3.9 ± 0.2 | 3.2 ± 0.2 | 3.9 ± 0.2 | 3.9 ± 0.2 |
Data are mean± sem, n≥8 per group.
p<0.05 vs. HdbHPTP1B
p<0.05 vs. KdbHPTP1B.
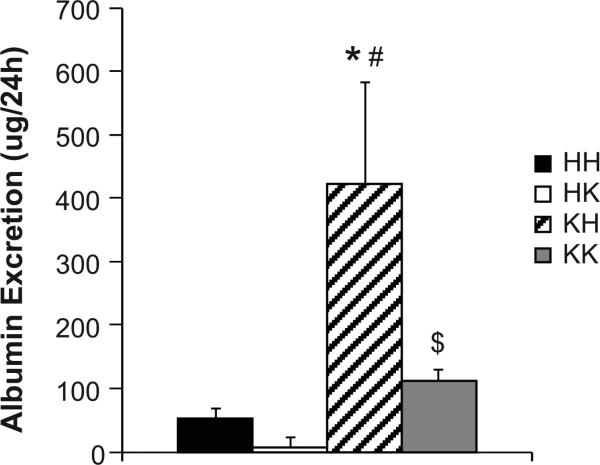
Data from measurements in metabolic cages are reported in Table 2. Obesity was associated with a significant increase in food and water intake, independent of the presence or absence of the PTP1B gene, and likely leading to the increase in urinary output. Although improving insulin sensitivity by PTP1B deletion did not affect Na+ and K+ excretion in lean and obese mice, it significantly reduced albumin excretion likely suggesting that improving insulin sensitivity in obese animals prevented kidney damage (Fig 1).
Table 2.
Metabolic parameters.
| Lean | Obese | |||
|---|---|---|---|---|
| Parameter | HdbHPTP1B | HdbKPTP1B | KdbHPTP1B | KdbKPTP1B |
| Food Intake (g/day) | 2.5 ± 0.5 | 2.0 ± 0.6 | 5.0 ± 1.9 | 4.6 ± 0.7 |
| Water Intake (ml/day) | 3.9 ± 0.3 | 3.5 ± 0.8 | 9.0 ± 2.0* | 8.0 ± 1.5* |
| Urine Volume (ml) | 1.0 ± 0.2 | 0.8 ± 0.2 | 4.7 ± 1.7* | 5.0 ± 1.2* |
| Na+ Excretion (mg/d) | 2.1 ± 0.6 | 1.6 ± 0.4 | 4.3 ± 1.0* | 4.8 ± 1.2* |
| K+ Excretion (mg/day) | 13.4 ± 3.9 | 10.4 ± 2.9 | 36.3 ± 8.8* | 38.2 ± 10.2* |
Data are mean± sem, n≥8 per group.
p<0.05 vs. HdbHPTP1B.
Figure 1. Restoration of insulin sensitivity in obese mice prevents kidney damage.
24h albumin excretion. Data are mean ± sem, *p<0.05 vs. HH, #p<0.05 vs. HK, $p<0.05 vs. KH, n=8 per group.
Effects of obesity and PTP1B deletion on blood pressure and heart rate
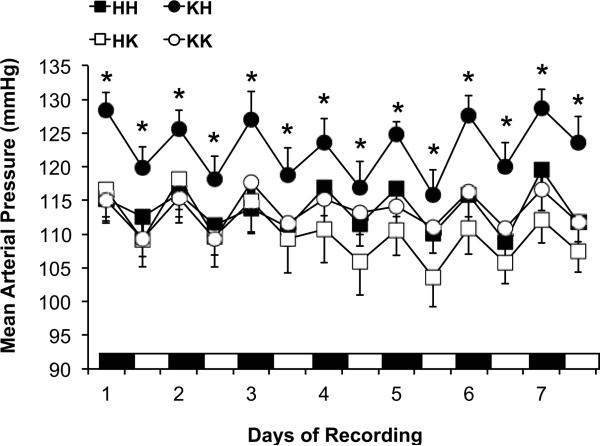
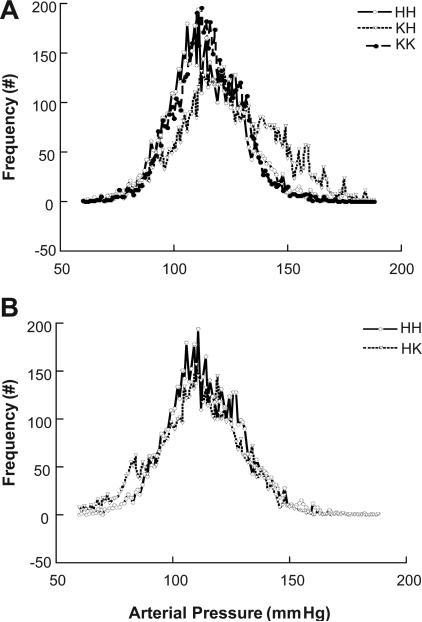
The effects of obesity on BP were assessed with a 7-day continuous BP recording via radiotelemetry. As reported in Figure 2, obesity (KdbHPTP1B) induced a significant increased in mean arterial pressure as well as in systolic and diastolic BP (Table 3) with no effect on circadian variation. Although the deletion of PTP1B in lean mice (HdbKPTP1B) did not affect BP, improving peripheral insulin sensitivity by the deletion of PTP1B in obese mice significantly improved BP in KdbKPTP1B mice. Indeed, despite obesity, KdbKPTP1B mice present a MAP as well as a systolic and diastolic BP that are similar to the BP of the lean mice (HdbHPTP1B, HdbKPTP1B). Heart rate was neither affected by obesity nor by the deletion of PTP1B (Table 3). The distribution of the mean arterial pressure values was analyzed over the 7-day BP recording and plot on Figure 3. As represented in Fig. 3A, and summarized in Table 3, lean control mice (HdbHPTP1B) present a Gaussian distribution of their MAP distribution centered around a value of 114±2 mmHg. Obesity (KdbHPTP1B) not only induced a shift of the MAP distribution toward higher MAP values but also increased the standard deviation of the MAP and the frequency of the MAP values above 140 mmHg (Table 3). This suggests that the vasculature and the kidney of the KdbHPTP1B mice are more often submitted to higher pressure values, likely above the limits of autoregulation. Restoration of insulin sensitivity in obese mice with the deletion of PTP1B completely restored the distribution of the MAP. The deletion of PTP1B in lean mice did not affect the distribution of the MAP (Fig 3B) confirming that the effects observed are not the direct consequence of the deletion of PTP1B.
Figure 2. Restoration of insulin sensitivity in obese mice prevents hypertension.
Nocturnal and diurnal mean arterial pressure of the heart rate over the 7 days of recording. Data are mean± sem, *p<0.05 vs. HH, n=12-14 per group.
Table 3.
Baseline Cardiovascular Parameters, Blood Pressure Response to Ganglionic Blockade and Statistical descriptors of individual frequency distribution curves from HdbHPTP1B, HdbKPTP1B, KdbHPTP1B and KdbKPTP1B mice.
| Lean | Obese | |||
|---|---|---|---|---|
| Parameter | HdbHPTP1B | HdbKPTP1B | KdbHPTP1B | KdbKPTP1B |
| MAP (mmHg) | 114 ± 2 | 108 ± 4 | 127 ± 2* | 115 ± 2† |
| DBP | 105 ± 3 | 100 ± 4 | 114 ± 2* | 103 ± 3† |
| SBP | 124 ± 3 | 119 ± 4 | 133 ± 3* | 125 ± 3 |
| HR | 522 ± 15 | 551 ± 16 | 488 ± 9 | 492 ± 18 |
| Response to Ganglionic Blockade | ||||
| Δ MAP (%) | -26 ± 3 | -31 ± 2 | -39 ± 4* | -35 ± 4* |
| Statistical Descriptors of Frequency Distribution Curves (Figure 2) | ||||
| SKEW | -0.06 ± 0.15 | -0.79 ± 0.44 | -0.22 ± 0.35 | 0.17 ± 0.05 |
| STD of MAP | 14.6 ± 0.8 | 13.1 ± 3.5 | 21.4 ± 2.2* | 14.1 ± 1.7† |
| Frequency over 140 mmHg (%) | 7 ± 3 | 4 ± 2 | 28 ± 5* | 4 ± 2† |
p<0.05 vs. HdbHPTP1B
p<0.05 vs. KdbHPTP1B.
Figure 3. Preservation of insulin sensitivity in obese mice improves blood pressure variability.
Frequency distribution of mean arterial blood pressure in (A) HH, KH and KK mice, (B) HH and HK mice. Data represent summary curves averaged from a sub-set of mice (n=6) from those averaged in Figure 1.
Sympathetic and vascular adrenergic tone
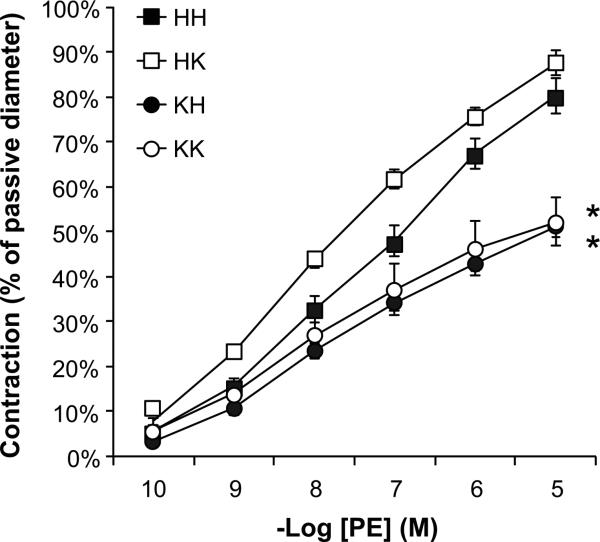
Sympathetic contribution to BP control was determined by measuring BP response to ganglionic blockade. As reported in Table 3, obesity increased BP response to mecamylamine, supporting a sumpatho-activation. Increasing insulin sensitivity with PTP1B deletion did not affect sympathetic control of BP, neither in obese nor in lean animals. Indices of sympathetic tone were also obtained by measuring vascular adrenergic tone. As represented in Figure 4, obesity reduced mesenteric adrenergic constriction to phenylephrine consistent with the aforementioned sympatho-activation. Correction of insulin resistance in obese mice did not restore vascular adrenergic tone suggesting that obesity-induced sympatho-activation is not driven by insulin resistance. PTP1B deletion in lean mice did not affect vascular adrenergic reactivity ruling out a direct effect of PTP1B on mesenteric adrenergic tone.
Figure 4. Obesity reduces vascular adrenergic tone.
Mesenteric artery response to cumulative doses of Phenylephrine. Data are mean± sem, *p<0.05 vs. HH, n=9-10 per group.
Plasma aldosterone levels
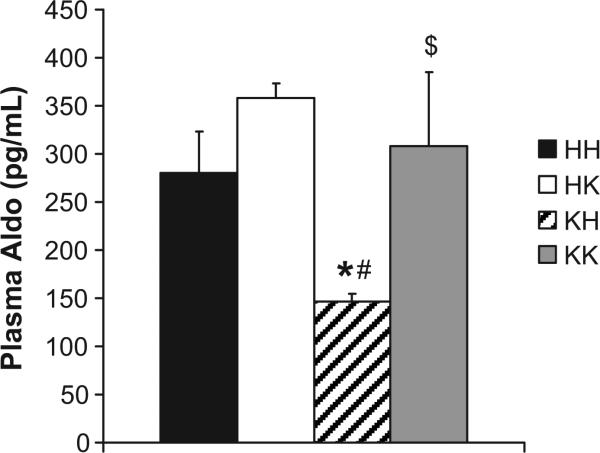
Since the renin-angiotensin-aldosterone system has been reported to be activated in obesity19, 20 we measured plasma aldosterone levels in our 4 groups of mice. As reported in Figure 5, obese insulin resistant mice (KdbHPTP1B) showed a reduction in plasma aldosterone levels compared to HdbHPTP1B mice, consistent with increased pressure and salt intake. Deletion of PTP1B did not affect plasma aldosterone levels in lean HdbKPTP1B mice but restored its levels in obese KdbKPTP1B mice, suggesting that the decrease observed in obese mice likely reflects increased blood pressure rather than salt intake in hyperphagic mice.
Figure 5. Insulin resistance reduces RAAS activation.
Plasma Aldosterone levels. Data are mean ± sem, *p<0.05 vs. HH, #p<0.05 vs. HK, $p<0.05 vs. KH, n=9-10 per group.
Renal Morphology
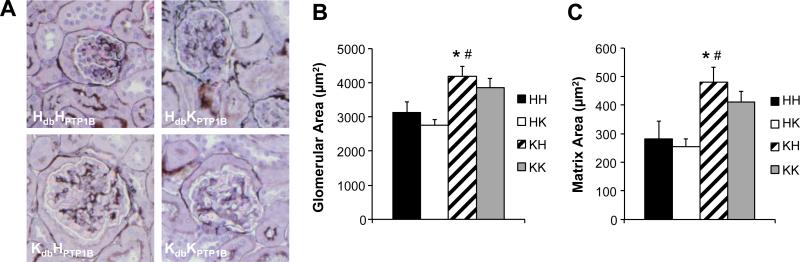
Histo-morphological analysis of the kidneys revealed that obesity (KdbHPTP1B) significantly affects renal structure by increasing glomerular and matrix areas. PTP1B deletion neither affected renal structure in lean mice (HdbKPTP1B) nor restored it in obese insulin sensitive mice (KdbKPTP1B), Figure 6.
Figure 6. Obesity impairs renal morphology.
(A) Glomerular morphology in individual experimental groups, methenamine silver stain. (B, C) Quantification of the glomerular and matrix cross sectional areas. Data are mean ± sem, *p<0.05 vs. HH, #p<0.05 vs. HK, n=5 per group.
Discussion
The goal of the present study was to determine whether restoring insulin sensitivity in obese mice would prevent the development of hypertension associated with obesity. We observed that 1) obesity is associated with reduced insulin sensitivity and impaired control of blood pressure 2) deletion of the molecular restraint of insulin signaling pathway (PTP1B) improves peripheral insulin sensitivity in obese mice and prevents increases in BP and BP variability, 3) mechanisms of this improvement are likely independent of thyroid status, the RAAS, and renal injury. Relevant to these observations are the concepts of insulin resistance and its role in the development of hypertension.
Model
The metabolic dysfunction associated with obesity is characterized by a loss of sensitivity to insulin in metabolically active tissues. The pervasiveness of this observation along with significant levels of hypertension in these obese populations has led to the intriguing hypothesis that insulin resistance is indeed the root cause of hypertension in obesity. Hyperinsulinemia is highly correlated with hypertension in human patients and has been shown to raise blood pressure in insulin resistant rats21. However, controversies have emerged, including the lack of effects in obese insulin resistant dogs22-24 and rats13, 25. Previously, we generated an insulin sensitive mouse model despite obesity by deleting the molecular restraint of the insulin signaling pathway (PTP1B). Deletion of PTP1B did not affect body weight but significantly improved peripheral insulin sensitivity as reflected by the reduced HbA1c, improved glucose clearance15 and reduced levels of circulating lipids. However PTP1B deletion in db/db mice does not correct fasting blood glucose suggesting that these mice retain a defect in their hepatic gluconeogenesis that likely explains the persistent hyperglycemia observed in the KdbKPTP1B mice. The deletion of PTP1B did not affect the metabolic profile or the BP of the lean mice. A previous study from our group demonstrated that PTP1B deletion only modifies leptin-mediated control of BP in mice on a Balb/C background26. Therefore, the mouse model generated, on a C57Bl/6 background, provide us an important tool to assess the role of insulin and insulin resistance in hypertension related to obesity without the confounding effects of changes in body weight or effects of PTP1B on leptin signaling.
Insulin sensitivity and blood pressure regulation
In the present study we observed that our obese insulin resistant mice (KdbHPTP1B) not only develop hypertension but also present an increase in BP variability. As a consequence, the distribution of their BP values is shifted towards higher BP values (Figure 3) and also covers a broader range of values as reflected by an increase in the standard deviation of the MAP (Table 3). The net result of these changes is that KdbHPTP1B mice present a fourfold increase in the frequency of their MAP values over 140 mmHg. This likely suggests a fourfold increase in the probability to reach the upper autoregulatory limit of the kidney, a phenomenon likely leading to kidney damage27. By measuring albumin excretion, we observed that the obese insulin resistant mice present a significant increase in protein excretion compared to lean controls indicating a functional consequence (the development of kidney damage) to the increase in BP observed in the KdbHPTP1B mice. We further demonstrated that restoring insulin sensitivity in the obese KdbKPTP1B mice prevented the development of hypertension associated with obesity and completely restored BP variability. This improvement occurred despite no improvement in the structural effects of obesity and metabolic dysfunction on the kidney indicating that the improvement in albumin excretion likely reflects the improvement of blood pressure. Finally, the analysis of the cardiovascular phenotype of the HdbKPTP1B mice did not reveal any effects of PTP1B deletion on the BP and BP variability of the lean mice, ruling out a direct effect of PTP1B deletion on BP regulation. Taken together, these data support the hypothesis that in the context of obesity, the insulin resistance state is a major determinant of altered regulation of blood pressure.
Potential mechanisms
The central hypothesis of the current study is that insulin resistance is a contributing mechanism to impaired regulation of blood pressure in obesity. This hypothesis is supported by the primary data of the study but the mechanisms are only indirectly revealed.
Aldosterone
Obesity has long been associated with activation of the reninangiotensin-aldosterone system19, 20. In the current study, we found that aldosterone, a major end-product of the RAAS, is not increased but rather decreased. This finding is consistent with previous studies of obesity showing reduced renin and aldosterone in non-diabetic rodent models of obesity 28 and also consistent with humans in early stages of obesity in which salt-insensitive increases in blood pressure are evident29. Moreover, aldosterone is restored to normal levels when insulin resistance is corrected by deletion of PTP1B suggesting that the decrease in aldosterone was simply a reflection of the increase in BP as sodium intake was similar between groups. While aldosterone does not account for all the pressor actions of angiotensin30, there were also no differences in sodium excretion between obese groups of mice whether insulin resistance was corrected or not. Thus, while an effect of angiotensin independent of aldosterone cannot be entirely ruled out as a mechanism, it would require a pathway with no impact on sodium balance. The simplest conclusion, therefore, is that the increase in blood pressure and its normalization with deletion of PTP1B does not involve the RAAS to a major extent.
Renal Injury
Another common hemodynamic insult in obesity and metabolic disease is damage to the kidney, reflected by glomerular injury, matrix expansion and the presence of protein in the urine31. In the current study, we observed that combination of high blood pressure and hyperglycemia resulted in all three of these insults but that only microalbuminuria was resolved by correction of insulin resistance. From these observations, we conclude that insulin resistance as manifested in elevated lipids and HbA1c is not the root cause of anatomic injury to the kidney. As fasting hyperglycemia persists in these obese mice with deletion of PTP1B, this finding is consistent with clinical observations that aggressive management of blood glucose limits diabetic nephropathy32. The correction of albuminuria may reflect the correction of blood pressure rather than a direct effect on glomerular function. Thus, while it is clear that the kidney is damaged in the obese, metabolically compromised state, this injury may be the resultant rather than the cause of derangements in blood pressure control.
Sympathetic tone
A third major component of the cardiovascular dysfunction associated with obesity is the overactivation of the sympathetic nervous system. Consistent with previous studies performed with obese patients or animal models of obesity, we find that obesity enhances sympathetic contribution to BP. Indeed, an increased BP response to ganglionic blockade was observed in KdbHPTP1B and KdbKPTP1B mice (Table 3). Obesity-induced sympatho-activation was further supported by a “vascular adrenergic escape” reported in mesenteric artery (Figure 4). As we and others previously documented18, 26, 33, 34, sympathoactivation is associated with reduced vascular adrenergic tone. In the present study, we made the singular observation that correction of peripheral insulin resistance does not prevent sympathoactivation. These data minimalize the role of sympathoactivation in the development of hypertension associated with obesity and suggest that insulin resistance is not the factor triggering sympathoactivation in mice. Nevertheless, these data do not rule out a role for insulin per se in sympathoactivation. Indeed, whereas PTP1B deletion corrected peripheral insulin resistance, it did not restore insulin levels in obese mice. This is consistent with studies reporting that hyperinsulinemia increases sympathetic tone but not BP in human9, 10, 35 and dogs22 but in contradiction with studies demonstrating that hypersinulinemia raises BP though a sympatho-mediated mechanism in rats21, 36. These data present mice as a better model than rats to study the role of insulin and insulin resistance in the development of obesity-induced hypertension.
Nitric oxide
Another pathway implicated in the cardiovascular effects of obesity is a reduction in the actions and/or production of nitric oxide. In consideration of cardiovascular control, the two primary sites of NO action are in the vasculature, where it regulates peripheral resistance37, 38 and in the kidney, where it is regulates salt balance39. An extensive characterization of our mouse model15 discovered that NO-mediated dilation is sharply curtailed in KdbHPTP1B mice, an effect almost completed corrected by deletion of PTP1B. Vascular NO has been documented to be an important contributor of BP variability in animals40 and humans41, 42 and BP variability, most likely due to progressive renal injury, contributes to fixed hypertension43.
The concept that loss of vascular NO is the cause of obesity-related increases in BP is further supported by studies in other disease states and conditions. Brands and coworkers44 have documented that type 1 diabetes fails to produce hypertension unless NO production is blocked, despite the significant renal injury present in this model. Moreover, very recent work from do Carmo et al., demonstrated that peripheral NO was essential to prevent hypertension with activation of the melanocortin system45
In the present study, we demonstrate that insulin resistance plays a key role in the loss of blood pressure control, both mean and variability, related to obesity. In this experimental model, obesity in young animals produces these defects without involvement of aldosterone or other endocrine deficits. A survey of potential mechanisms suggests that BP variability associated with the loss of vascular NO is a likely culprit, especially when super-imposed on a compromised kidney. Correction of NO-mediated dilation restores BP control. We conclude that at least during early stages of obesity and metabolic dysfunction, correction of insulin resistance would be an important step in the prevention of long-term hypertension. The extent to which insulin resistance is involved in more established hypertension associated with more pervasive renal damage remains to be determined.
Perspective
The growing epidemic of obesity and type II diabetes increases the need for pharmacological therapies to prevent body weight gain and its adverse effects on the metabolic and cardiovascular functions. Due to its key role in the control of insulin and leptin signaling, the Protein Tyrosine Phosphatase 1B is suggested to be a key therapeutic target for the treatment of obesity and type II diabetes. The potential of this therapeutic target has been greatly enhanced by the demonstration that PTP1B KO mice are protected against obesity and type II diabetes. However, whether PTP1B inhibition could be beneficial for the cardiovascular function remained to be fully elucidated. In the present study we tested the hypothesis that improving insulin sensitivity, by the deletion of PTP1B, will prevent the development of hypertension associated with obesity. By deleting PTP1B in leptin receptor deficient mice, we demonstrated that improving peripheral insulin sensitivity is sufficient to prevent obesity-induced hypertension in mice. While further studies are still needed to determine the precise mechanisms involved, this study clearly supports the role of PTP1B as a potential therapeutic target against obesity related cardiovascular dysfunction and presents insulin resistance as a key component of the development of hypertension associated with obesity.
Supplementary Material
Novelty and Significance.
What Is New? - The new information in the current study is that:
Correction of insulin signaling in muscle and fat of obese mice via deletion of PTP1B normalizes elevated blood pressure
Obese mice have reduced aldosterone levels that are driven by the increased pressure, not their elevated salt intake
Obese mice have renal injury and sympathoactivation but this cannot be explained by improved in peripheral insulin sensitivity
What Is Relevant? – The risk factors that determine increased blood pressure in obese individuals remain poorly understood. These data indicate that some component of the insulin resistant state is driving increases in blood pressure in a manner that does not related to increased aldosterone, SNS activity or physical injury to the kidney. Exact mechanisms are likely complex and remain to be fully elucidated.
Summary – Obesity increases arterial pressure in a manner that is driven in part by the metabolic defects caused by obesity. Correcting these defects, perhaps with drugs that target PTP1B, may improve hypertension in obese patients.
Acknowledgements
The authors acknowledge the helpful editorial assistance of J. Blake Norman in the preparation of the written document.
Sources of funding
This work was supported by the National Institute of Health, (R01, D.W.S) and the American Heart Association (11SDG5060006, E.B.C).
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the united states 1999 to 2000: A rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 2.Kannel W, Brand M, Skinner J, Dawber T, McNamara P. The relation of adiposity to blood pressure and development of hypertension: The framingham study. Ann Intern Med. 1967;67:48–59. doi: 10.7326/0003-4819-67-1-48. [DOI] [PubMed] [Google Scholar]
- 3.Modan M, Halkin H, Almog S, Lusky A, Eshkol A, Shefi M, Shitrit A, Fuchs Z. Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J Clin Invest. 1985;75:809–817. doi: 10.1172/JCI111776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas CP, Estigarribia JA, Darga LL, Reaven GM. Insulin and blood pressure in obesity. Hypertension. 1985;7:702–706. doi: 10.1161/01.hyp.7.5.702. [DOI] [PubMed] [Google Scholar]
- 5.Manicardi V, Camellini L, Bellodi G, Coscelli C, Ferrannini E. Evidence for an association of high blood pressure and hyperinsulinemia in obese man. J Clin Endocrinol Metab. 1986;62:1302–1304. doi: 10.1210/jcem-62-6-1302. [DOI] [PubMed] [Google Scholar]
- 6.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities -- the role of insulin resistance and the sympathoadrenal system. New England Journal of Medicine. 1996;334:374–382. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 7.Bunnag P, Hori MT, Ormsby B, Berger ME, Golub MS, Tuck ML. Impaired in vivo adrenergic responses in diet-induced hypertensive rats. Hypertens Res. 1997;20:17–21. doi: 10.1291/hypres.20.17. [DOI] [PubMed] [Google Scholar]
- 8.Nishimoto Y, Tomida T, Matsui H, Ito T, Okumura K. Decrease in renal medullary endothelial nitric oxide synthase of fructose-fed, salt-sensitive hypertensive rats. Hypertension. 2002;40:190–194. doi: 10.1161/01.hyp.0000024267.71656.0d. [DOI] [PubMed] [Google Scholar]
- 9.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson EA, Balon TW, Hoffman RP, Sinkey CA, Mark AL. Insulin increases sympathetic activity but not blood pressure in borderline hypertensive humans. Hypertension. 1992;19:621–627. doi: 10.1161/01.hyp.19.6.621. [DOI] [PubMed] [Google Scholar]
- 11.Eringa EC, Stehouwer CDA, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese zucker (fa/fa) rats. American Journal of Physiology - Endocrinology And Metabolism. 2007;293:E1134–E1139. doi: 10.1152/ajpendo.00516.2006. [DOI] [PubMed] [Google Scholar]
- 12.Brands MW, Garrity CA, Holman MG, Keen HL, Alonso-Galicia M, Hall JE. High-fructose diet does not raise 24-hour mean arterial pressure in rats. American journal of hypertension. 1994;7:104–109. doi: 10.1093/ajh/7.1.104. [DOI] [PubMed] [Google Scholar]
- 13.D'Angelo G, Elmarakby AA, Pollock DM, Stepp DW. Fructose feeding increases insulin resistance but not blood pressure in sprague-dawley rats. Hypertension. 2005;46:806–811. doi: 10.1161/01.HYP.0000182697.39687.34. [DOI] [PubMed] [Google Scholar]
- 14.Ovalle F. Cardiovascular implications of antihyperglycemic therapies for type 2 diabetes. Clinical Therapeutics. 2011;33:393–407. doi: 10.1016/j.clinthera.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Ali MI, Ketsawatsomkron P, Belin de Chantemele EJ, Mintz JD, Muta K, Salet C, Black SM, Tremblay ML, Fulton DJ, Marrero MB, Stepp DW. Deletion of protein tyrosine phosphatase 1b improves peripheral insulin resistance and vascular function in obese, leptin-resistant mice via reduced oxidant tone. Circ Res. 2009;105:1013–1022. doi: 10.1161/CIRCRESAHA.109.206318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1b. Dev Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 17.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1b gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 18.Romanko OP, Stepp DW. Reduced constrictor reactivity balances impaired vasodilation in the mesenteric circulation of the obese zucker rat. Am J Physiol Heart Circ Physiol. 2005;289:H2097–2102. doi: 10.1152/ajpheart.00213.2005. [DOI] [PubMed] [Google Scholar]
- 19.de Paula RB, da Silva AA, Hall JE. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension. 2004;43:41–47. doi: 10.1161/01.HYP.0000105624.68174.00. [DOI] [PubMed] [Google Scholar]
- 20.Saiki A, Ohira M, Endo K, Koide N, Oyama T, Murano T, Watanabe H, Miyashita Y, Shirai K. Circulating angiotensin ii is associated with body fat accumulation and insulin resistance in obese subjects with type 2 diabetes mellitus. Metabolism. 2009;58:708–713. doi: 10.1016/j.metabol.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Brands MW, Hildebrandt DA, Mizelle HL, Hall JE. Sustained hyperinsulinemia increases arterial pressure in conscious rats. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1991;260:R764–R768. doi: 10.1152/ajpregu.1991.260.4.R764. [DOI] [PubMed] [Google Scholar]
- 22.Hall JE, Brands MW, Zappe DH, Dixon WN, Mizelle HL, Reinhart GA, Hildebrandt DA. Hemodynamic and renal responses to chronic hyperinsulinemia in obese, insulin-resistant dogs. Hypertension. 1995;25:994–1002. doi: 10.1161/01.hyp.25.5.994. [DOI] [PubMed] [Google Scholar]
- 23.Pamies-Andreu E, Fiksen-Olsen M, Rizza RA, Romero JC. High-fructose feeding elicits insulin resistance without hypertension in normal mongrel dogs. American journal of hypertension. 1995;8:732–738. doi: 10.1016/0895-7061(95)00118-9. [DOI] [PubMed] [Google Scholar]
- 24.Rocchini AP, Yang JQ, Gokee A. Hypertension and insulin resistance are not directly related in obese dogs. Hypertension. 2004;43:1011–1016. doi: 10.1161/01.HYP.0000123073.48855.e9. [DOI] [PubMed] [Google Scholar]
- 25.Bezerra RMN, Ueno M, Silva MS, Tavares DQ, Carvalho CRO, Saad MJA, Gontijo JAR. A high-fructose diet induces insulin resistance but not blood pressure changes in normotensive rats. Brazilian Journal of Medical and Biological Research. 2001;34:1155–1160. doi: 10.1590/s0100-879x2001000900008. [DOI] [PubMed] [Google Scholar]
- 26.Belin de Chantemele EJ, Muta K, Mintz J, Tremblay ML, Marrero MB, Fulton DJ, Stepp DW. Protein tyrosine phosphatase 1b, a major regulator of leptin-mediated control of cardiovascular function. Circulation. 2009;120:753–763. doi: 10.1161/CIRCULATIONAHA.109.853077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navar LG. Renal autoregulation: Perspectives from whole kidney and single nephron studies. American Journal of Physiology - Renal Physiology. 1978;234:F357–F370. doi: 10.1152/ajprenal.1978.234.5.F357. [DOI] [PubMed] [Google Scholar]
- 28.Osmond JM, Mintz JD, Stepp DW. Preventing increased blood pressure in the obese zucker rat improves severity of stroke. American Journal of Physiology - Heart and Circulatory Physiology. 2010;299:H55–H61. doi: 10.1152/ajpheart.01111.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall JE, Brands MW, Hildebrandt DA, Mizelle HL. Obesity-associated hypertension. Hyperinsulinemia and renal mechanisms. Hypertension. 1992;19:I45–55. doi: 10.1161/01.hyp.19.1_suppl.i45. [DOI] [PubMed] [Google Scholar]
- 30.Lea WB, Kwak ES, Luther JM, Fowler SM, Wang Z, Ma J, Fogo AB, Brown NJ. Aldosterone antagonism or synthase inhibition reduces end-organ damage induced by treatment with angiotensin and high salt. Kidney Int. 2009;75:936–944. doi: 10.1038/ki.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. Journal of the American Society of Nephrology. 2001;12:1211–1217. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 32.The ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New England Journal of Medicine. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 33.Belin de Chantemele EJ, Mintz JD, Rainey WE, Stepp DW. Impact of leptin-mediated sympatho-activation on cardiovascular function in obese mice. Hypertension. 2011;58:271–279. doi: 10.1161/HYPERTENSIONAHA.110.168427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogikyan RV, Supiano MA. Arterial alpha-adrenergic responsiveness is decreased and sns activity is increased in older humans. American Journal of Physiology - Endocrinology And Metabolism. 1994;266:E717–E724. doi: 10.1152/ajpendo.1994.266.5.E717. [DOI] [PubMed] [Google Scholar]
- 35.Tsutsu N, Nunoi K, Kodama T, Nomiyama R, Iwase M, Fujishima M. Lack of association between blood pressure and insulin in patients with insulinoma. J Hypertens. 1990;8:479–482. doi: 10.1097/00004872-199005000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Meehan W, Buchanan T, Hsueh W. Chronic insulin administration elevates blood pressure in rats. Hypertension. 1994;23:1012–1017. doi: 10.1161/01.hyp.23.6.1012. [DOI] [PubMed] [Google Scholar]
- 37.Stamler JS, Loh E, Roddy MA, Currie KE, Creager MA. Nitric oxide regulates basal systemic and pulmonary vascular resistance in healthy humans. Circulation. 1994;89:2035–2040. doi: 10.1161/01.cir.89.5.2035. [DOI] [PubMed] [Google Scholar]
- 38.Rees DD, Palmer RM, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proceedings of the National Academy of Sciences. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and na excretion via nitric oxide. Hypertension. 2008;51:1605–1610. doi: 10.1161/HYPERTENSIONAHA.107.108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chowdhary S, Vaile JC, Fletcher J, Ross HF, Coote JH, Townend JN. Nitric oxide and cardiac autonomic control in humans. Hypertension. 2000;36:264–269. doi: 10.1161/01.hyp.36.2.264. [DOI] [PubMed] [Google Scholar]
- 42.Claxton CR, Brands MW. Nitric oxide opposes glucose-induced hypertension by suppressing sympathetic activity. Hypertension. 2003;41:274–278. doi: 10.1161/01.hyp.0000049620.70909.2e. [DOI] [PubMed] [Google Scholar]
- 43.Schillaci G, Pucci G, Parati G. Blood pressure variability. Hypertension. 2011;58:133–135. doi: 10.1161/HYPERTENSIONAHA.111.175752. [DOI] [PubMed] [Google Scholar]
- 44.Brands MW, Bell TD, Gibson B. Nitric oxide may prevent hypertension early in diabetes by counteracting renal actions of superoxide. Hypertension. 2004;43:57–63. doi: 10.1161/01.HYP.0000104524.25807.EE. [DOI] [PubMed] [Google Scholar]
- 45.do Carmo JM, Bassi M, da Silva AA, Hall JE. Systemic but not central nervous system nitric oxide synthase inhibition exacerbates the hypertensive effects of chronic melanocortin-3/4 receptor activation. Hypertension. 2011;57:428–434. doi: 10.1161/HYPERTENSIONAHA.110.163931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.