Abstract
Trauma results in a persistent depression in adaptive immunity which contributes to patient morbidity and mortality. This state of immune paralysis following trauma is characterized by a change in cell-mediated immunity, specifically a depression in T-cell function and a shift towards Th2 T-cell phenotype. Upregulation of iNOS is well-recognized after injury and contributes to the inflammatory response and organ damage early after trauma. However, it is unknown whether iNOS plays a role in adaptive immune dysfunction after trauma. This study utilized a murine model of severe peripheral tissue injury to show that iNOS is rapidly upregulated in macrophages and a (Gr-1hi-CD11bhi) MDSC subpopulation in the spleen. Through the use of iNOS knockout mice, a specific iNOS inhibitor and an NO scavenger this study demostrates that iNOS-derived NO is required for the depression in T lymphocyte proliferation, interferon-gamma and interleukin-2 production within the spleen at 48hr after trauma. These findings support the hypothesis that iNOS regulates immune suppression following trauma, and suggest that targeting the sustained production of NO by iNOS may attenuate post-traumatic immune depression.
Keywords: Injury, T lymphocyte, Immunosuppression, MDSC, iNOS
Introduction
Severe trauma induces an immuno-inflammatory response that contributes to both early and delayed clinical complications in trauma patients. The initial response is dominated by excessive activation of innate immune pathways manifested by a pronounced systemic inflammatory response and organ injury. If the host survives this initial period, a persistent depression in adaptive immunity remains that renders the trauma patient susceptible to nosocomial infections [1]. This state of immune paralysis is characterized by a shift towards a Th2 T-cell phenotype and a depression in T-cell responses.
Initiator and effector mechanisms involved in driving both the innate inflammatory response, and the reduced adaptive immune response, are still under investigation. Recent studies suggest that innate immune receptors that detect tissue injury, such as TLR 4 [2, 3] and TLR 9 [4], are involved in the initial pro-inflammatory response observed in models of experimental trauma. Key effector pathways and mediators, such as cytokine/chemokines and complement [5], are also known to be involved in this initial pro-inflammatory response. In addition, an important effector pathway upregulated early after hemorrhagic shock is inducible nitric oxide synthase (iNOS) [6,7].
Studies in both animal models and humans have shown that hemorrhagic shock and severe systemic injury lead to an early upregulation of iNOS in the liver, lung, spleen and vascular system [6, 7, 8]. Studies in hemorrhagic shock models using knockout mice and selective iNOS inhibitors have shown that iNOS contributes to the propagation of the inflammatory response and to organ damage that manifests within the first few hours [6, 7, 8, 9]. However, relatively little is known about whether iNOS regulates the delayed immune depression observed following trauma. It has been well established that iNOS-mediated nitric oxide (NO) has a regulatory role on T-cell responses in several disease settings [10] and it has been shown to influence differentiation, proliferation, function and survival in multiple T-cell populations. Isayama et al [11] recently reported that iNOS knockout (−/−) mice had fewer regulatory CD4+ T-cells in the spleen and thymus following hemorrhagic shock than wild type (WT) mice, however, the precise role of iNOS in the functional changes in T-cell populations following traumatic injury remain unknown.
In this study, we postulated that iNOS would play a key role in driving the depression in lymphocyte responses following injury. We used a model of severe peripheral tissue injury previously established by our laboratory known as pseudofracture [12, 13] to show that iNOS is upregulated in specific cell populations in the spleen, and that iNOS and NO are required for the depression in lymphocyte proliferation and pro-inflammatory cytokine production. Our data demonstrate a previously unidentified role of iNOS in the regulation of T-cell dysfunction following peripheral tissue trauma. These findings support the notion that targeting the sustained production of NO by iNOS could attenuate the immune depression following trauma.
Materials & Methods
Animal Care
This research protocol complied with the regulations regarding the care and use of experimental animals published by the National Institutes of Health, and was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Age-matched wild type (WT) C57BL/6 mice were obtained from Charles River Laboratories International (Wilmington, MA). iNOS−/− mice were bred within our facility and were originally created by the gene-targeted disruption method and backcrossed onto the C57Bl/6 background through 10 generations [14]. All mice were male, aged 8-12 weeks and weighed 20-30g. Animals were maintained in the University of Pittsburgh Animal Research Center with a 12hr light/dark cycle and free access to standard laboratory food and water. All mice were acclimated to their surroundings for at least 1 week before being used for experiments.
Experimental Groups
The mice were randomly allocated to trauma or control groups. Trauma groups included pseudofracture (PF), laparotomy (Lap), combined PF+laparotomy. Control mice were used to obatin physiologic baseline results. These mice were euthanized under inhalational anesthesia with no experimental manipulation performed. All experimental mice were anesthestized with pentobarbital sodium (70mg/kg, IP; Ovation Pharmaceuticals, Deerfield, IL) and inhalational isoflurane (Abbott Laboratories, Chicago, IL) was additionally administered as needed to maintain sufficient depth of anesthesia. Buprenorphine (0.1mg/kg SC; Bedford Laboratories, Bedford, OH) was administered after recovery from anesthesia as an analgesic. Pseudofracture was performed as previously described [2, 12, 13, 15]. In brief, the soft tissue injury in the form of a muscle crush injury to the thigh musculature bilaterally, was followed by an injection of a crushed bone solution. The bone solution was prepared from the femurs and tibias of age- and weight-matched syngeneic donor mice. Harvested bones were crushed in a sterile fashion with a pestle and mortar and then resuspended in 2ml of phosphate buffered saline (PBS) for injection. The bone solution was used within 1h of preparation and kept at 4°C until use. Sterility was checked by culture of the solution overnight on agar plates. A large hemostat was used to perform the crush injury on both lower extremities, and was clamped to the same pressure around the posterior thigh musculature for 30s. Using a 20 gauge needle, 0.15ml of the bone solution was injected into the crushed thigh musculature on each leg The animals were allowed full freedom after the procedure. Mortality of the procedure was 0%.
Laparotomy was performed to insert intraperitoneal Alzet mini-osmotic pumps (Durect Corp., Cupertino, CA) that administered a continuous infusion of NOX-100 (NO-scavenger; 30mg/kg/hr) or vehicle control (sterile water) over a 48h period. NOX-100, an NO scavenger was provided by Ching-San Lai, PhD, Medinox (San Diego, CA). A 1.5cm midline laparotomy incision was performed, pumps were inserted into the peritoneal cavity and the abdomen was closed in two layers with sutures. The mice that underwent the combined PF + Lap procedure received the PF directly after abdominal closure. Some experimental mice were given 5 mg/kg 1400W [N-(3-(Aminomethyl)benzyl)acetamidine], an iNOS inhibitor (Sigma-Aldrich Co., St.Louis, MO), or saline control intraperitonally within 30 min of trauma.
Reagents
Saponin was from Sigma-Aldrich Co. (St.Louis, MO); mouse IL-6, IL-10, IFN-g, IL-2 enzyme-linked immunosorbent assay (ELISA) kits from R&D systems Inc. (Minneapolis, MN); mouse myeloperoxidase (MPO) ELISA kits from Cell sciences (Canton, MA); RPMI 1640 and L-Glutamine from Lonza BioWhittaker (Walkersville, MD); heat-inactivated fetal bovine serum (FBS), non-essential amino acids (NEAA) and sodium pyruvate from Hyclone Lab., Fisher Scientific, (Logan, UT); penicillin-streptomycin (pen/strep) and 2-mercaptoethanol (2-ME) from Gibco, Life Technologies Corp., (Grand Island, NY); concanavalin A (con A) from GE healthcare Corp. (Piscataway, NJ) and antiCD3e mAb (clone 145-2C11), anti-CD16/CD32, BD cytofix/cytoperm from BD Biosciences (San Jose, CA, USA). Fluorescently labeled antibodies for flow cytometry were purchased from either eBiosciences (San Diego, CA) or BD Biosciences (San Jose, CA).
Sample collection and preparation
Mice were euthanized at 1hr, 3hr, 6hr, 12hr, 24hr, 48hr and 72 hr after trauma, or without experimental manipulation as controls. At the conclusion of each experiment, animals were sacrificed with an overdose of isoflurane, a thoracotomy was performed and blood was harvested by a cardiac puncture. Immediately after cardiac puncture, liver, lungs and spleen were aseptically harvested, snap frozen in liquid nitrogen and stored at −80°C until further analysis. Parallel experiments also provided spleens that were harvested either directly for preparation of single cell suspensions, or prepared for immunofluorescence staining.
Plasma was collected by centrifugation of heparinized blood samples at 3500 rpm for 10min at 4°C. Samples were then aliquoted and stored at 4°C for measurement of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels with HESKA Dri-chem 4000 (Heska Loveland CO; slides from FUJIFILM Corp., Japan) analysis. Alternative plasma aliquots were stored at −80°C for later analysis of IL-6, IL-10 levels by ELISA, which were performed according to the manufacturer’s instructions. As a marker for neutrophil (PMN) infiltration, MPO activity was quantified in lung tissue lysates. Lung tissue was thawed, homogenized in lysis buffer and an MPO ELISA was performed following manufacturer’s protocol. NF-κB DNA-binding activity was measured by electrophoretic mobility shift assay (EMSA) using nuclear extracts prepared from liver tissue as previously described [12, 15].
Relative mRNA quantification
Total mRNA was extracted from snap-frozen spleen. iNOS mRNA expression was quantified by standardized SYBR Green two-step real-time reverse transcription-PCR (RT-PCR). Relative fold increase of the iNOS gene was quantified over the murine house-keeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The sequences for the primers used were: iNOS: (F) GTGACGGCAAACATGACTTCAG and (R) GCCATCGGGCATCTGGTA; GAPDH: (F) TTCACCACCATGGAGAAGGC and (R) GGCATGGACTGTGGTCATGA.
Immunofluorescence and Confocal Microscopy
For immunofluorescence staining, animals were perfused with PBS followed by 2% paraformaldehyde (2%PFA). After further fixation in 2%PFA for 2h, spleen samples were rehydrated in 30% sucrose for 24h, and sucrose was changed 3 times. The samples were then cryopreserved in liquid nitrogen-cooled 2-methylbutane and stored at −80°C until ready for cryosectioning (6μm). Sections were incubated with 2% bovine serum albumin (BSA) in PBS for 1h, followed by 5 washes with PBS+0.5% BSA (PBB). iNOS and F4/80 content was measured with the following markers: FITC-iNOS (1:200, BD Transduction) and F4/80 (1:100, BD Transduction) for 1h at 37°C in PBB, followed by incubation in PBB with Alexa647 Fluor phalloidin (1:250, Invitrogen) and Cy3 (1:1,000, Jackson ImmunoResearch Laboratories, West Grove, PA). Samples were washed three times with PBB, followed by a PBS wash prior to 30s incubation with Hoechst nuclear stain. Samples were washed with PBS prior to being coverslipped using Gelvatol (23g polyvinyl alcohol 2000, 50 ml glycerol, 0.1% sodium azide to 100 ml PBS). Positively stained cells in 5 random fields were imaged on a Fluoview 1000 confocal scanning microscope (Olympus, Melville, NY). Imaging conditions were maintained at identical settings with original gating performed within each antibody-labeling experiment with the negative control (no primary antibody).
Splenocyte proliferation and flow cytometry
Spleens were harvested and single cell suspensions were prepared by digestion with collagenase D (1mg/ml; Roche Diagnostics Corp., Indianapolis, IN) followed by mechanical break-up through a 70mM mesh strainer. The spleens were then washed with PBS, centrifuged at 1200rpm for 10 min. Erythrocytes were lysed with RBC lysing buffer (Sigma-Aldrich, St.Louis, MO) and the cells were washed again in PBS. The cell pellet was resuspended in RPMI 1640 supplemented with 10%FBS, l-glutamine, pen/strep, NEAA, sodium pyruvate, 2-ME to a final concentration of 1 × 106 cells/ml. Cell viability of 90% was confirmed with trypan blue stain.
Spleen cell cultures were carried out in 96-well round-bottom tissue-culture plates (BD Falcon, BD Biosciences (San Jose, CA) at 1 × 105 viable cells/well. Parallel cultures of splenocytes were stimulated with con A (2.5 μg/ml) or antiCD3e mAb (1μg/ml) and were cultured for 72h at 37°C. Splenocyte culture supernatant was collected at the 48h time point and frozen for cytokine analysis. Tritiated thymidine (1μCi/well; Perkin Elmer Inc., Waltham, MA) was added for the last 18h of culture. Proliferation was assessed through thymidine incorporation using a Topcount scintillation counter (Perkin Elmer Inc., Waltham, MA) and measured as counts per minute (cpm).
Splenocytes in single cell suspension were washed with PBS, incubated with anti-CD16/CD32 (Fc block) for 10min at 4°C. Cells were then stained for 20min at 4°C with the following fluorescently labeled mAbs: anti-CD3, anti-CD8, anti-CD4, anti-CD25, anti-CD19, anti-CD90.2, anti-CD11b, anti-CD11c, anti-Gr-1, anti-B220, anti-F4/80. Cells to be analyzed for cell surface markers alone were then washed with PBS and suspended in 1% paraformaldehyde and kept at 4°C in the dark for later analysis. Cells to be stained for intracellular iNOS expression were washed with PBS after surface marker staining and then incubated with BD cytofix/cytoperm for 20min at 4°C. These cells were then washed with 0.1% saponin, stained with anti-iNOS (clone 6/iNOS/NOS Type II) at room temperature for 1h, washed with 0.1% saponin buffer and immediately analyzed. A Becton Dickinson LSR II flow cytometer was used for analysis of samples; FlowJo v.7.6.5 software (Tree Star Inc., Ashland, OR) was used for data analysis.
Statistical analysis
Statistical significance (p<0.05) was assessed by Student’s t-test and ANOVA analysis using SigmaPlot 11.0 software (Systat Software Inc., San Jose, California, USA). The results presented in the study are expressed as the mean ± standard error or mean (SEM).
Results
Pseudofracture results in early upregulation of systemic inflammation and later depression of splenocyte responses
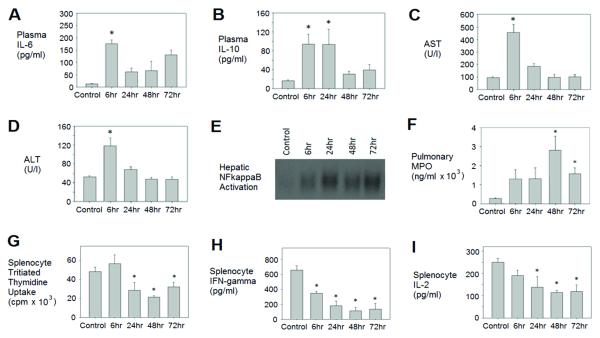
The pseudofracture model has been shown to simulate severe peripheral tissue trauma that recapitulates the systemic and end organ responses observed following bilateral femur fracture, and at the same time allows for late term immunological studies [13]. Previous studies showed comparable inflammatory responses and organ dysfunction at early time points in mice subjected to either pseudofracture or bilateral femur fracture [12]. In the present study we confirmed this early systemic inflammation, as well as characterized a later suppressed immune response over time after pseudofracture. As shown in figure 1, cytokine markers of the systemic inflammatory response including IL-6 and IL-10 were elevated early (6-24h) (Fig. 1A & 1B) after injury as expected. A second elevation in circulating IL-6 was also noted at 72h. Circulating levels of AST and ALT, used as markers of tissue and organ damage, were also elevated early (6h) but then returned to baseline by 24h (Fig. 1C & 1D). Interestingly, hepatic NFκB activation remained elevated throughout the 72h time period measured (Fig. 1E). Pulmonary neutrophil sequestration, shown by MPO, peaked at 48h (Fig. 1F), which lagged behind the maximal increases in cytokine levels.
Figure 1. Pseudofracture produces a biphasic immune response following trauma.
C57BL/6 mice were euthanized at 6, 24, 48 and 72h following pseudofracture or as unmanipulated controls. Plasma IL-6 (A) and IL-10 (B); plasma aspartate aminotranferase (AST) (C) and alanine aminotranferase (ALT) (D); hepatic NFκB activation (E); pulmonary (whole lung) myeloperoxidase (MPO) levels (F); splenocyte function in culture after concanavalin A (2.5ug/mL) stimulation including proliferation measured in counts per minute (cpm) of tritiated thymidine uptake (G), IFN-gamma release (H) and IL-2 release (I). Data shown as mean±SEM; * = p < 0.05 vs. Control; n=6-10mice/group at each time point; representative of 3 independent experiments.
We used in vitro proliferative and cytokine responses of splenocytes isolated from control and injured animals to assess a parameter of immune function known to be depressed after injury. Cells were stimulated to proliferate with either antiCD3ε antibody or concanavalin A (ConA). Splenocyte responses to Con A remained intact at 6h post-injury, but were then significantly depressed at the 24, 48 and 72h time points compared with controls (Fig. 1G). Splenocyte production of Th1-type proinflammatory cytokines interferon-gamma and IL-2 were also significantly decreased in injured mice in comparison with controls, with a nadir at 48h (Fig 1H & 1I). Parallel studies using antiCD3ε for mitogenic stimulation of splenocytes in culture showed the same pattern of diminished proliferation and proinflammatory cytokine responses of splenocytes (data not shown). The greatest depression in splenocyte responses was shown to occur at 48h after pseudofracture (Fig. 1G, 1H & 1I) and hence this time point was selected for all further splenocyte work within this study. These results therefore established that our model not only recapitulates early inflammatory responses to trauma, but also recapitulates the subsequent immune suppression observed in trauma patients.
We then determined whether there was any change in percentages of immune cell populations within spleen after trauma that would explain the observed reduction in T-cell response. We used flow cytometry to immunophenotype cells in the spleen at time points out to 48h after pseudofracture (Table 1). No significant change was observed in the percentage of the different T-lymphocyte populations (CD3+CD4+, CD3+CD8+ and CD4+CD25+ lymphocytes) within the spleen following pseudofracture. Percentages of B-lymphoctyes, dendritic cells (DC) and F4/80+ macrophages were also not different at any time point after pseudofracture. There were statistical differences observed in the percentage of a small subset of myeloid-derived suppressor cells (MDSC) that were Gr-1hi-CD11bhi. The percentage of these cells increased early after pseudofracture (1h and 6h) with maximal numbers at 6h, which then declined by 12h (Table 1). These data suggest that the depressed splenocyte proliferation and cytokine release observed after trauma is not attributable to major variations in lymphocyte composition within the splenocyte cultures, and that MDSC subsets upregulated early may influence subsequent lymphocyte suppression.
Table 1. Percentages of spleen cell populations up to 48h after pseudofracture.
| Cell type (surface markers) |
Control | Time after pseudofracture | ||||
|---|---|---|---|---|---|---|
| 1h | 6h | 12h | 24h | 48h | ||
|
T cells (CD3+,CD4+) |
12.7±0.9 | 10.7±1.7 | 9.6±1.4 | 10.2±1.1 | 11.1±1.9 | 11.0±0.8 |
|
T cells (CD3+,CD8+) |
8.7±0.6 | 11.1±1.3 | 8.3±0.3 | 9.7±1.7 | 7.3±0.4 | 7.6±0.6 |
|
T cells (%CD4+,CD25+ of CD4+) |
8.2±0.5 | 9.3±1.7 | 9.6±1.1 | 10.2±1.5 | 10.5±2.3 | 11.1±1.5 |
|
B cells (B220+) |
37.2±1.8 | 40.0±7.4 | 38.0±4.2 | 51.1±3.0 | 50.2±5.8 | 49.4±4.4 |
|
DCs (CD11c+) |
1.2±0.1 | 1.1±0.1 | 1.2±0.2 | 1.3±0.1 | N.D. | N.D. |
|
Macrophages (F4/80+) |
2.1±0.2 | 2.4±0.3 | 2.1±0.2 | 2.2±0.1 | N.D. | N.D. |
|
MDSC (CD11b+,Gr-1+) |
2.4±0.3 | 2.9±0.4 | 2.2±0.1 | 3.2±0.3 | N.D. | N.D. |
|
MDSC subset (Gr-1hi-CD11bhi) |
0.2±0.0 | 1.2±0.2* | 1.8±0.6* | 0.6±0.1 | N.D. | N.D. |
CD4+CD25+ cells are presented as a percent of total CD4+ cells.DC = dendritic cells; MDSC = myeloid-derived suppressor cells. Data shows mean±SEM; n=4-6 mice/group at each time point; data representative of several independent experiments. Shaded rows identify T lymphocytes.
= p<0.05 vs. respective control
N.D.= not determined.
Splenic iNOS expression is upregulated after pseudofracture
Since iNOS is known to play an important role in early responses to trauma, and has been implicated in T-cell dysfunction in other diseases, we next investigated iNOS expression in the spleen following pseudofracture. To do this we used a combination of quantitative RT-PCR and cellular immunofluoresence. Levels of iNOS mRNA were elevated over baseline as early as 1 h after pseudofracture, but then declined at 3h and 6h (Fig. 2A). Levels of iNOS mRNA then increased again at 12h and peaked at 24h after pseudofracture with expression around 6 times greater than control. Similarly, immunofluoresence staining for iNOS in spleen revealed low levels of iNOS proteinexpression at baseline, which increased appreciably by 12h following pseudofracture (Fig. 2B). Spleen sections were also co-stained with macrophage marker F4/80 (Fig. 2B – red staining), and this revealed that some iNOS positive cells were macrophages, although many macrophages did not upregulate iNOS at this time point. These data confirm that iNOS is upregulated following pseudofracture as would be expected after trauma.
Figure 2. Upregulation of iNOS expression in spleen after pseudofracture.
Relative iNOS mRNA expresssion in whole spleen up to 48h after pseudofracture in comparison with controls (A). Data shown as mean±SEM; * = p < 0.05 vs. Control; n=2-4mice/group at each time point; data representative of 2 independent experiments. Immunofluoresence detection in spleen of iNOS: green, actin: blue, F4/80: red, in unmanipulated control mice (left) and 12h after pseudofracture (right). Magnification x20, (x60-inset) (B). FACS analysis of intracellular iNOS in Gr-1hi-CD11bhi MDSCs following trauma (C – histogram). Representative flow cytogram of Gr-1+CD11b+ spleen cells at 12h after trauma after gating through a CD19+CD90.2+ exclusion gate (C-inset). Gr-1hi-CD11bhi MDSC subset circled. Data is representative of more than 3 independent experiments.
Splenic Gr-1hi-CD11bhi MDSC upregulate iNOS expression after trauma
We next wanted to further identify the cell subsets that upregulated iNOS expression after injury. Several splenic cell types have been previously shown to express iNOS, including MDSC, DC, macrophages and B-cells [16], and we used flow cytometry to identify intracellular iNOS expression within each of these cell types in the spleen at various times after trauma. As shown in the histogram inlay (Fig. 2C), Gr-1+CD11b+ positive MDSC cells within the spleen formed 5 distinct subsets and each subpopulation was separately evaluated for iNOS expression at time points after trauma. Intracellular iNOS was only found to be significantly upregulated in the Gr-1hi-CD11bhi MDSC subset at 12h following trauma and not in any other evaluated cell type (Fig. 2C). This is the same population of MDSC that was identified earlier to increase in percentage in the spleen early after trauma (Table 1). Together these findings suggest a potential role for MDSC-derived iNOS in the immune dysregulation observed after trauma.
Increased iNOS expression contributes to immune dysfunction following injury
To determine whether iNOS contributed to the injury-induced changes in splenocyte responses, we assessed splenocyte proliferation and cytokine responses in splenocytes isolated from WT and iNOS−/− mice at 48h following pseudofracture. Splenocyte proliferation in iNOS−/− mice was similar to WT at baseline (Fig. 3A). However, in contrast to the suppression of splenocyte proliferation observed in WT mice, iNOS−/− splenocytes had significantly increased proliferation at 48h after injury (Fig. 3A). Similarly, the post-traumatic suppression of splenocyte IFN-gamma (Fig. 3A) and IL-2 (data not shown) production observed in WT mice was not observed in splenocytes from injured iNOS-deficient mice. In fact there was a significant increase in IFN-gamma from iNOS−/− splenocytes stimulated with ConA compared with their controls (Fig. 3A). These findings suggest that iNOS−/− mice were less susceptible to post-traumatic immune changes than wild type counter parts.
Figure 3. iNOS/NO contributes to immune cell dysfunction following pseudofracture.
(A): Splenocyte responses to either ConA (2.5ug/mL - left) or Anti-CD3ε (1ug/mL - right) from WT and iNOS−/− mice at 48h after pseudofracture in comparison with controls. Splenocyte proliferation and IFN-gamma release are shown as percent change from baseline control. Data shown as mean ± SEM. * = p < 0.05 vs. respective Control; n=6-10 mice/gp. (B): Splenocyte responses to either Con A (2.5ug/mL - left) or Anti-CD3ε (1ug/mL - right) from WT mice with or without 1400W (5mg/kg IP) at 48h after pseudofracture in comparison with controls. Splenocyte proliferation and IFN-gamma release are shown as percent change from baseline control. Data shown as mean ± SEM. * = p < 0.05 vs. respective Control; n=5-10mice/group; representative of 2 independent experiments. (C): Splenocyte responses to Con A (2.5ug/mL) from WT mice at 48h after traumas in comparison with controls. PF=pseudofracture, Lap[Veh]=Laparotomy with vehicle pump insertion, Lap[NOX100]= Laparotomy with NOX100 pump insertion, PF+Lap[Veh]= pseudofracture & laparotomy with vehicle pump insertion, PF+Lap[NOX100]= pseudofracture & laparotomy with NOX100 pump insertion. Splenocyte proliferation (left) is measured by absolute counts per minute (c.p.m.) of tritated thymidine uptake; splenocyte IFN-gamma release (right). Data shown as mean ± SEM. * = p<0.05 vs. Controls; # = p<0.05 vs. Lap [Veh]; ^ = p=0.05 vs. PF+Lap [Veh]; n=6-7mice/group.
We next wanted to confirm the importance of iNOS in post-traumatic immune suppression. To do this we blocked iNOS enzymatic activity in WT mice using the highly selective iNOS inhibitor, 1400W. The half-life of 1400W is reported to be less than 3 hours by Rota et al. [17]. C57BL/6 mice were given 5mg/kg 1400W, or saline control, intraperitoneally within 30min of pseudofracture. WT mice without 1400W showed the expected depression in splenocyte proliferation at 48h after trauma (Fig. 3B). However, in mice treated with a single dose of 1400W, trauma induced depression of splenocyte proliferation was not observed (Fig. 3B). Splenocyte ability to produce IFN-gamma was similarly unaffected by trauma in mice treated with iNOS-inhibitor (Fig. 3B). Taken together our findings suggest that iNOS plays an important role in driving post-traumatic T-cell suppression.
Extracellular NO-scavenger attenuates post-traumatic immune dysfunction
To determine if NO release accounted for injury-induced changes in splenocyte function, we used a potent NO scavenger, NOX-100, given to mice via intraperitoneal Alzet osmotic pumps as a continuous infusion over a 48h period. We then compared splenocyte proliferation responses in unmanipulated control mice, with experimental groups of mice undergoing pseudofracture alone, laparotomy with pump insertion alone, or the combination of pseudofracture with laparotomy and pump insertion. We observed the expected decrease in splenocyte proliferation and cytokine production at 48h after pseudofracture, and this immunosuppression was also observed after the surgical trauma of the laparotomy for pump insertion, with or without pseudofracture (Fig. 3C). The continuous in vivo administration of a NO scavenger, but not vehicle control, over the 48h time period following trauma prevented this immune dysfunction (Fig. 3C). Similar results were obtained by stimulating cells with CD3ε to induce proliferation and cytokine responses (data not shown). These findings imply that NOX-100 was protective against lymphocyte changes following trauma. Our data therefore suggest that iNOS-induced NO drives the depressed responses of splenocytes after trauma.
Early pro-inflammatory responses and organ injury are not iNOS-dependent after pseudofracture
iNOS has been shown to contribute to the early inflammatory response and organ damage in models of hemorrhagic shock [6] We additionally chose to evaluate the involvement of iNOS in the early proinflammatory phase after pseudofracture. Interestingly, there was no difference in the increase in liver enzymes (AST and ALT) at 6h after trauma between iNOS −/− and WT mice (Fig. 4A & 4B). Similar findings were observed in trauma mice that received iNOS-specific inhibitor,1400W. There was no difference in the increase in plasma IL-6 levels or in ALT in mice with or without iNOS-inhibition after pseudofracture (Fig. 4C & 4D). Thus, the early inflammatory response induced by tissue trauma is not dependent on iNOS, in contrast to the findings after hemorrhagic shock.
Figure 4. Early pro-inflammatory responses and organ injury are not iNOS-dependent after pseudofracture.
Plasma alanine aminotranferase (ALT) (A) and aspartate aminotranferase (AST) (B) at 6h after pseudofracture in comparison to unmanipulated controls in both iNOS−/− and WT mice. Levels of circulating IL-6 (C) and ALT (D) at 6h following pseudofracture in WT mice with and without 1400W (5mg/kg IP). Data shown as mean ± SEM. * = p < 0.05 vs. respective Control.
Discussion
Traumatic tissue injury initiates a set of immuno-inflammatory responses that lead to complications which contribute to trauma patient morbidity and mortality [18]. Early complications can result from excessive activation of innate immune pathways and are characterized by a pronounced systemic inflammatory response and organ injury. If the trauma patient survives this initial period, they remain at risk for delayed complications which result from a persistent depression in adaptive immunity, and which render the trauma patient susceptible to nosocomial infections [1]. Immune paralysis following trauma is characterized by a change in cell-mediated immunity, specifically a shift towards Th2 T-cell phenotype and a depression in T-cell function [19]. This lymphocyte dysfunction after injury has been shown to correlate with the increased susceptibility to infections and death from sepsis [20]. Our study shows that iNOS-derived NO, potentially from a subgroup of spelnocyte MDSC, contributes to T-cell dysfunction induced by peripheral tissue trauma. However, in clear contrast to findings in hemorrhagic shock, iNOS is not required for the early pro-inflammatory changes and liver damage in this model of trauma, suggesting a disconnect between the mechanisms driving early and late post-traumatic immune responses.
Increased iNOS expression is a known major effector pathway induced early after injury [6,7] Of the three mammalian NO synthases, neuronal NOS and endothelial NOS are constitutively expressed while iNOS is upregulated in inflammatory conditions [16, 21]. Recent studies have additionally suggested a regulatory role for both nNOS and eNOS in inflammatory processes, however the predominant role of iNOS within the immune system remains significant [16, 21]. Our study demonstrates a previously unidentified role for iNOS in T-cell dysfunction induced by peripheral tissue trauma. Similar regulatory effects of iNOS-mediated NO on T-lymphocyte function have been well-described in various disease settings including malignancy [22], autoimmunity [23], graft vs host disease [24] and thermal injury [25]; these effects include regulation of differentiation, proliferation, function and T-cell survival. Multiple mechanisms of action have been implicated in the regulation of these functions, including inhibition of several signaling processes required for T-lymphocyte proliferation including Jak3 and STAT5 signaling, caspase activation in T-cells [26], and ribonucleotide reductase activity, as well as activation of soluble guanylate cyclase [27].
Similarly, the consumption of arginine by iNOS-expressing cells has been shown to impair lymphocyte proliferation through a number of mechanisms including down-regulation of CD3ζ chain [28], oxidant production, and suppression of polyamine synthesis. Arginine availability has long been identified as essential for normal T cell proliferation and function [28, 29]. Decreased T-lymphocyte responses and decreased plasma L-arginine levels after trauma are well described, although the mechanisms that account for the iNOS-mediated T-cell dysfunction in our model remain unclear. A study by Angele et al, showed that arginine administration during resuscitation in a trauma-hemorrhagic shock model restored depressed splenic functions found at 6h post-trauma hemorrhage [30], and iNOS did not influence these findings. Our findings, in contrast, show an iNOS-derived NO-dependent suppression of T-cell function at later time points after pseudofracture, but we also found that iNOS was not involved in the early inflammatory response after trauma. We were able to prevent post-traumatic splenocyte dysfunction using an NO scavenger, which indicates that excess NO and not arginine depletion alone contributes to suppressed splenocyte responses in our model. Other mechanisms have also been identified through which NO can regulate accessory cell functions shown to impair T cell responses. For example, MDSC have been shown to reduce T-cell proliferation when activated by NO-mediated increases in cGMP [22]. Similarly, NO-mediated DC apoptosis results in a feedback-inhibitory effect on T-cell proliferation or fails to support the secretion of IFN-gamma by T-cells. Additionally, NO can mediate the immunosuppressive activity of mesenchymal stem cells on T cell responses.
Studies in both animal models of hemorrhagic shock and injured humans have shown that iNOS is upregulated early after injury. This well-described iNOS induction following trauma has been shown in the liver [8, 31], lung [6], spleen [11], gut [32], thymus and vascular system [7]. Here we confirm that iNOS mRNA and protein are upregulated early in the spleen. Immunofluoresence showed at least some, but not all, of the iNOS positive cells were macrophages. Several splenic cell types are known to express iNOS: MDSC [33], DC, macrophages [27], B-cells [16]. We found that iNOS was induced in MDSC, specifically in a Gr-1hi-CD11bhi MDSC subset following trauma. This subpopulation also accumulated within the spleen as early as 1h after trauma with a peak of iNOS expression at 12h. Makarenkova et al. have previously found through histological assessment that MDSC (Gr-1+CD11b+ cells) accumulated in the spleen within 6h after trauma and further identified these cells as a cause of T cell dysfunction after laparotomy [33]. In contrast, we did not find changes in the general MDSC (Gr-1+CD11b+) population over time in the pseudofracture model.
Experimental studies in hemorrhagic shock models have shown that iNOS contributes to propagation of the inflammatory response and organ damage within the first few hours after injury [6, 7, 8, 9]. Our study differs from previous work in that we utilized a model of isolated tissue trauma. Whereas, the pseudofracture model leads to an early inflammatory response similar to that described in hemorrhagic shock models, we did not observe a role for iNOS in either measures of systemic inflammation or end-organ damage. Of note, the magnitude of liver damage is relatively mild in our model. Whereas inflammation in both hemorrhagic shock and peripheral tissue trauma is TLR4 dependent [3], the role of iNOS in this early phase of the response is clearly different.
The second phase of the immunoinflammatory response following pseudofracture was highlighted by a maximal decrease in splenocyte, specifically T lymphocyte, functions at 48hr after injury. Several forms of traumatic injury including femur fracture, femur fracture with hemorrhagic shock [34], laparotomy [33], laparatomy with hemorrhagic shock [30] and thermal injury [27] have been shown to be associated with immune dysfunction affecting mainly cell-mediated immunity. It has been well established that T cell function is markedly altered following traumatic injury [20]; the degree of lymphocyte suppression has been suggested to correlate with the complexity of surgery or severity of trauma [35]. Additionally, the degree of lymphocyte suppression has been shown to correlate with the subsequent development of infectious complications and mortality [20]. The proliferative and cytokine release capacities of cultured splenocytes in response to the T cell mitogens Concanavalin A and antiCD3e allows for an assessment of T lymphocyte functions.
The state of immune paralysis following trauma is also characterized by a shift towards Th2 T cell phenotype, with a depression in Th1 immunity [36]. The pattern of suppressed proliferative and Th1 cytokine release responses after pseudofracture were found to be similar to that seen in other models of injury [25, 34]. We found little to no suppression at the 6hr time point with maximal depression at 48hr following injury. Our flow cytometry studies indicate that the lymphocyte composition of the spleen was not altered by the injury over the initial 48hr. This substantiates that the depressed splenocyte proliferation and cytokine release observed after trauma [figure 1(G), 1(H) & 1(I)] were not attributable to variation in respective cell composition within the cultures. Others have reported alterations in lymphocyte numbers and CD4+:CD8+ ratio after injury however conflicting findings have been reported. These discrepancies may be due to variations in both the type of injury and lymphoid compartment assessed [35].
An additional effect of NO on immunoregulation of T-lymphocytes includes regulation of T-cell Th1/Th2 phenotypes. A study by Wei et al. demonstrated stronger Th1 responses in iNOS−/− mice [37], and this may be reflected in our findings showing increased splenocyte cytokine release in iNOS−/− cells after pseudofracture. Our results also showed that after trauma iNOS−/− mice had increased splenocyte responses in comparison with those of 1400W treated mice. We postulate that the chronic loss of the iNOS gene within the knockout mouse strain would have additional immune changes that may be the cause for this observation. Nonetheless, different levels of NO production in the context of different insults have also been shown to affect the Th1/Th2 immune polarity [21]. A biphasic T-cell response to NO has been described in which low concentrations enhance induction of Th1 cells, while higher concentrations suppress the Th1-type response [38]. In addition, a differential impact of iNOS-derived NO on T cell subpopulations has been described where mouse CD8+ T-cells were more susceptible to the anti-proliferative effect of NO than CD4+ T-cells [39]. The multidirectional effects of nitric oxide on immune cells and in particular, T-lymphocytes are well-recognized [10] and could explain the differences in measured parameters of the immune response amongst the various trauma models.
In summary, we show in this study a significant role for iNOS-generated NO-dependent suppression of lymphoproliferative responses, with a suppression of the Th1-type cytokine response after tissue trauma. This is a previously undescribed role for iNOS in the setting of traumatic injury, and suggests iNOS-induced NO plays an important role in the pathophysiology of trauma. Our findings may represent NO as a therapeutic target for immunomodulation to limit injury-induced immune suppression.
Acknowledgments
The authors would like to thank Debra Williams, Richard Shapiro and Lauryn Kohut for their generous technical assistance.
Source of Funding
This study was performed under the funding provided through National Institute of Health Grant 5R37GM044100 (TRB).
Footnotes
Conflicts of Interest
Dr.Timothy R. Billiar is a member on the scientific advisory board of Medinox, the company that provided NOX-100 used in experiments presented in this manuscript. For the remaining authors, no conflicts of interest were declared.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaudry IH, Ayala A. Mechanism of increased susceptibility to infection following hemorrhage. Am J Surg. 1993;165(2A Suppl):59S–67S. doi: 10.1016/s0002-9610(05)81208-5. [DOI] [PubMed] [Google Scholar]
- 2.Kobbe P, Kaczorowski DJ, Vodovotz Y, Tzioupis CH, Mollen KP, Billiar TR, Pape HC. Local exposure of bone components to injured soft tissue induces toll-like receptor 4-dependent systemic inflammation with acute lung injury. Shock. 2008;30(6):686–91. doi: 10.1097/SHK.0b013e31816f257e. [DOI] [PubMed] [Google Scholar]
- 3.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: Toll-like receptor 4 sentinel for the detection of tissue damage. Shock. 2006;26(5):430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai C, Gill R, Eum HA, Cao Z, Loughran PA, Darwiche S, Edmonds RD, Menzel CL, Billiar TR. Complement factor 3 deficiency attenuates hemorrhagic shock-related hepatic injury and systemic inflammatory response syndrome. Am J Physiol Regul Integr Comp Physiol. 2010;299(5):R1175–82. doi: 10.1152/ajpregu.00282.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, Peitzman AH, Billiar TR, Tweardy DJ. Essential Role of Induced Nitric Oxide in the Initiation of the Inflammatory Response after Hemorrhagic Shock. J Exp Med. 1998;187(6):917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiemermann C, Szabo C, Mitchell JA, Vane JR. Vascular hyporeactivity to vasoconstrictor agents and hemodynamic decompensation in hemorrhagic shock is mediated by nitric oxide. Proc Natl Acad Sci U S A. 1993;90:267–271. doi: 10.1073/pnas.90.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly E, Shah NS, Morgan NN, Watkins SC, Peitzman AB, Billiar TR. Physiologic and molecular characterization of the role of nitric oxide in hemorrhagic shock: evidence that type II nitric oxide synthase does not regulate vascular decompensation. Shock. 1997;7(3):157–63. doi: 10.1097/00024382-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Hierholzer C, Kalff JC, Billiar TR, Bauer AJ, Tweardy DJ, Harbrecht BG. Induced nitric oxide promotes intestinal inflammation following hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2004;286:G225–G233. doi: 10.1152/ajpgi.00447.2002. [DOI] [PubMed] [Google Scholar]
- 10.Bogdan C. Regulation of lymphocytes by nitric oxide. Methods Mol Biol. 2011;677:375–93. doi: 10.1007/978-1-60761-869-0_24. [DOI] [PubMed] [Google Scholar]
- 11.Isayama K, Murao Y, Saito F, Hirakawa A, Nakatani T. Effects of hypertonic saline on CD4+CD25+Foxp3+ regulatory T cells after hemorrhagic shock in relation to iNOS and cytokines. J Surg Res. 2010;172(1):137–45. doi: 10.1016/j.jss.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 12.Menzel CL, Pfeifer R, Darwiche SS, Kobbe P, Gill R, Shapiro RA, Loughran P, Vodovotz Y, Scott MJ, Zenati MS, Billiar TR, Pape HC. Models of Lower Extremity Damage in Mice: Time Course of Organ Damage and Immune Response. J Surg Res. 2011;166(2):149–56. doi: 10.1016/j.jss.2010.11.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darwiche SS, Kobbe P, Pfeifer R, Kohut L, Pape H, Billiar T. Pseudofracture: An Acute Peripheral Tissue Trauma Model. JoVE. 50:2011. doi: 10.3791/2074. URL: http://www.jove.com/details.php?id=2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song YK, Billiar TR, Lee YJ. Role of Galectin-3 in Breast Cancer Metastasis: Involvement of Nitric Oxide. Am J Pathol. 2002;160(3):1069–75. doi: 10.1016/S0002-9440(10)64927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeifer R, Kobbe P, Darwiche SS, Billiar TR, Pape HC. Role of Hemorrhage in the Induction of Systemic Inflammation and Remote Organ Damage: Analysis of Combined Pseudo-Fracture and Hemorrhagic Shock. J Ortho Res. 2011;29:270–274. doi: 10.1002/jor.21214. [DOI] [PubMed] [Google Scholar]
- 16.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–16. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 17.Rota C, Bergamini S, Daneri F, Tomasi A, Virgili F, Iannone A. N-Acetylcysteine Negatively Modulates Nitric Oxide Production in Endotoxin-Treated Rats Through Inhibition of NF-kB Activation. Antioxid Redox Signal. 2002;4(1):221–6. doi: 10.1089/152308602753625988. [DOI] [PubMed] [Google Scholar]
- 18.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, López MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG. the Inflammation and Host Response to Injury Large-Scale Collaborative Research Program: A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–90. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flohé SB, Flohé S, Schade FU. Invited review: deterioration of the immune system after trauma: signals and cellular mechanisms. Innate Immun. 2008;14(6):333–44. doi: 10.1177/1753425908100016. [DOI] [PubMed] [Google Scholar]
- 20.Faist E, Kupper TS, Baker CC, Chaudry IH, Dwyer J, Baue AE. Depression of cellular immunity after major injury. Its association with posttraumatic complications and its reversal with immunomodulation. Arch Surg. 1986;121(9):1000–5. doi: 10.1001/archsurg.1986.01400090026004. [DOI] [PubMed] [Google Scholar]
- 21.Wink DA, Hines HB, Cheng RYS, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89(6):873–91. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203(12):2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niedbala W, Alves-Filho JC, Fukada SY, Vieira SM, Mitani A, Sonego F, Mirchandani A, Nascimento DC, Cunha FQ, Liew FY. Regulation of type 17 helper T-cell function by nitric oxide during inflammation. Proc Natl Acad Sci U S A. 2011;108(22):9220–5. doi: 10.1073/pnas.1100667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman RA, Langrehr JM, Wren SM, Dull KE, Ildstad ST, McCarthy SA, Simmons RL. Characterization of the immunosuppressive effects of nitric oxide in graft vs host disease. J Immunol. 1993;151(3):1508–18. [PubMed] [Google Scholar]
- 25.Daniel T, Alexander M, Hubbard WJ, Chaudry IH, Choudhry MA, Schwacha MG. Nitric oxide contributes to the development of a post-injury Th2 T-cell phenotype and immune dysfunction. J Cell Physiol. 2006;208(2):418–27. doi: 10.1002/jcp.20677. [DOI] [PubMed] [Google Scholar]
- 26.Mahidhara RS, Hoffman RA, Huang S, Wolf-Johnston A, Vodovotz Y, Simmons RL, Billiar TR. Nitric oxide-mediated inhibition of caspase-dependent T lymphocyte proliferation. J Leukoc Biol. 2003;74:403–11. doi: 10.1189/jlb.0602293. [DOI] [PubMed] [Google Scholar]
- 27.Valenti L, Mathieu J, Chancerelle Y, Levacher M, Chanaud B, De Sousa M, Strzalko S, Dinh-Xuan AT, Giroud JP, Florentin I. Nitric oxide inhibits spleen cell proliferative response after burn injury by inducing cytostasis, apoptosis, and necrosis of activated T lymphocytes: role of the guanylate cyclase. Cell Immunol. 2003;21(1):50–63. doi: 10.1016/s0008-8749(03)00064-9. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, Ochoa JB, Ochoa AC. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol. 2003;171(3):1232–9. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 29.Bronte V, Zanovello P. Regulation of immune responses by l-arginine metabolism. Nat Rev Immunol. 2005;5(8):641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 30.Angele MK, Smail N, Knoferl MW, Ayala A, Cioffi WG, Chaudry IH. L-Arginine restores splenocyte functions after trauma and hemorrhage potentially by improving splenic blood flow. Am J Physiol Cell Physiol. 1999;276:C145–C151. doi: 10.1152/ajpcell.1999.276.1.C145. [DOI] [PubMed] [Google Scholar]
- 31.Collins JL, Vodovotz Y, Hierholzer C, Villavicencio RT, Liu S, Alber S, Gallo D, Stolz DB, Watkins SC, Godfrey A, Gooding W, Kelly E, Peitzman AB, Billiar TR. Characterization of the expression of inducible nitric oxide synthase in rat andhuman liver during hemorrhagic shock. Shock. 2003;19(2):117–122. doi: 10.1097/00024382-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Smail N, Catania RA, Wang P, Cioffi WG, Bland KI, Chaudry IH, Gut and Liver The Organs Responsible for Increased Nitric Oxide Production After Trauma-Hemorrhage and Resuscitation. Arch Surg. 1998;133:399–405. doi: 10.1001/archsurg.133.4.399. [DOI] [PubMed] [Google Scholar]
- 33.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ Myeloid Suppressor Cells Cause T Cell Dysfunction after Traumatic Stress. J Immunol. 2006;176:2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 34.Mack VE, McCarter MD, Naama HA, Calvano SE, Daly JM. Dominance of T-helper 2-type cytokines after severe injury. Arch Surg. 1996;131(12):1303–8. doi: 10.1001/archsurg.1996.01430240057007. [DOI] [PubMed] [Google Scholar]
- 35.Schäffer M, Barbul A. Lymphocyte function in wound healing and following injury. Br J Surg. 1998;85(4):444–60. doi: 10.1046/j.1365-2168.1998.00734.x. [DOI] [PubMed] [Google Scholar]
- 36.O’Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222(4):482–90. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375(6530):408–11. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 38.Niedbala W, Wei XQ, Piedrafita D, Xu D, Liew FY. Effects of nitric oxide on the induction and differentiation of Th1 cells. Eur J Immunol. 1999;29(8):2498–505. doi: 10.1002/(SICI)1521-4141(199908)29:08<2498::AID-IMMU2498>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 39.Hoffman RA, Mahidhara RS, Wolf-Johnston AS, Lu L, Thomson AW, Simmons RL. Differential modulation of CD4 and CD8 T-cell proliferation by induction of nitric oxide synthesis in antigen presenting cells. Transplantation. 2002;74(6):836–45. doi: 10.1097/00007890-200209270-00018. [DOI] [PubMed] [Google Scholar]