Abstract
Retinal infection is the most common clinical manifestation of toxoplasmosis. The route by which circulating Toxoplasma gondii tachyzoites cross the vascular endothelium to enter the human retina is unknown. Convincing studies using murine encephalitis models have strongly implicated leukocyte taxis as one pathway used by the parasite to access target organs. To establish whether tachyzoites might also interact directly with vascular endothelium, we populated a transwell system with human ocular endothelial cells. Human retinal endothelial monolayers permitted transmigration of tachyzoites of RH and three natural isolate strains. Antibody blockade of intercellular adhesion molecule-1 significantly reduced this migration, but did not impact tachyzoite movement across an endothelial monolayer derived from the choroid, which lies adjacent to the retina within the eye. In demonstrating that tachyzoites are capable of independent migration across human vascular endothelium in vitro, this study carries implications for the development of therapeutics aimed at preventing access of tachyzoites to the retina.
Keywords: ICAM-1, vascular endothelium, migration, human retina, Toxoplasma gondii, tachyzoite
Introduction
Toxoplasma gondii is an apicomplexan parasite that infects approximately two billion persons worldwide.1 The most common clinical manifestation of toxoplasmosis is an ocular infection involving the retina (i.e., retinitis), with secondary involvement of the adjacent choroid in severe cases (i.e., retinochoroiditis). Ocular toxoplasmosis typically affects healthy adults, and causes blindness in 24% of affected eyes.2 In immunocompromised patients, particularly those who suffer from acquired immunodeficiency syndrome, infection with the parasite may result in a fatal encephalitis.1
After entering the human host, T. gondiis preads to the central nervous system via the bloodstream in tachyzoite form. Two independent groups have convincingly demonstrated that infected monocytes and/or dendritic cells carry tachyzoites across the blood-brain barrier in a murine model of toxoplasmic encephalitis.3, 4 However, as stated by Tardieux and Ménard,5 a “central question” relates to “whether tachyzoites interact with endothelial barriers”. The human retinal vasculature is characterized by a dense capillary network,6 and circulating leukocytes move slowly through retinal capillaries due to their wider diameter, stiff viscoelastic properties and tendency to adhere to the endothelium.7 If lysis of a tachyzoite-infected leukocyte occurred during trans-capillary passage, the potential would exist for a direct interaction between tachyzoite and retinal vascular endothelium. Both free and intracellular tachyzoites circulate in the blood of individuals infected with T. gondii.8
Using a conventional transwell migration system populated with human ocular vascular endothelial cells, we investigated the possibility that, in addition to ‘taxiing’ in a leukocyte, T. gondii tachyzoites might be capable of accessing the human retina by migrating unassisted across the retinal vascular endothelium.
Results
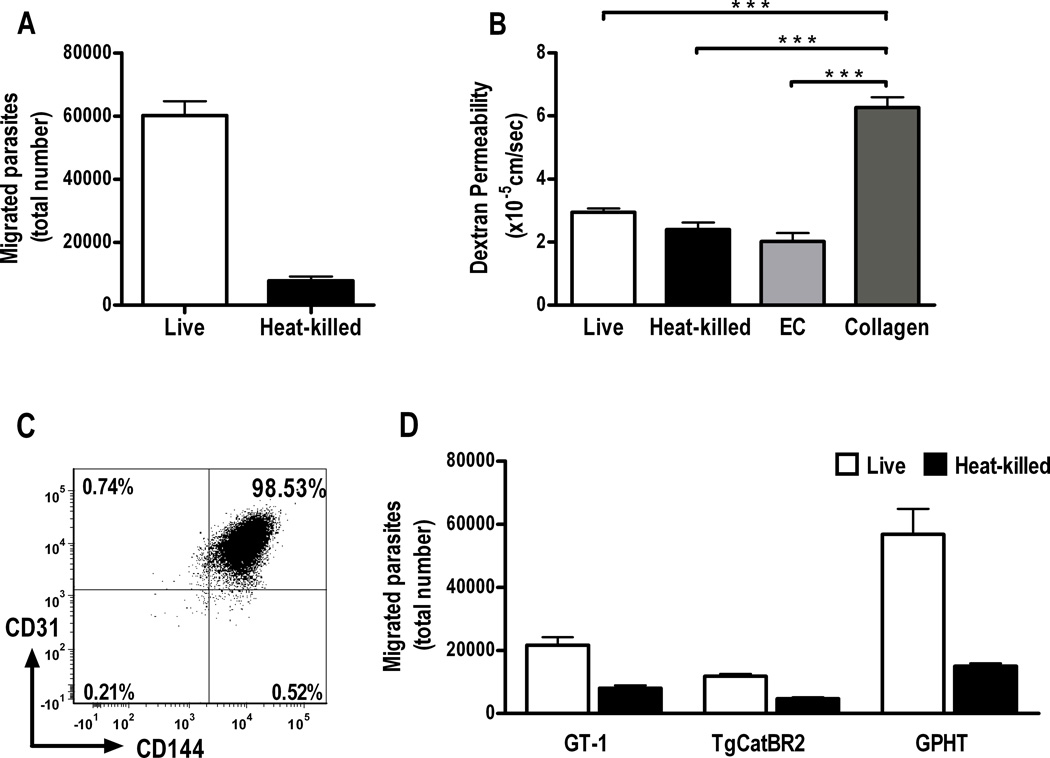
We observed migration of live T. gondii tachyzoites through a simulated human retinal vascular endothelium in a transwell system over a 4-hour period (Figure 1A). Smaller numbers of heat-killed tachyzoites, loaded into upper chambers of control transwells, were also recovered from lower chambers at this time. However, there was no significant difference (p > 0.05) in permeability to high molecular weight dextran for endothelial monolayers incubated with live, heat-killed or no tachyzoites, and permeability under these conditions remained significantly less (p < 0.001) than permeability of wells containing membranes coated with collagen alone (Figure 1B), suggesting that the endothelial monolayer remained intact for the duration of the experiment. Universal expression of CD144 (VE-cadherin) by CD31-positive retinal endothelial cells after extended confluent culture (Figure 1C), also was consistent with the formation of intercellular junctions across the monolayers. To address the possibility that transendothelial movement was peculiar to RH strain tachyzoites, we performed the same assay, but separately substituted one of three different natural parasite isolates for the clonal strain. The same result was obtained in this series of experiments (Figure 1D).
Figure 1. Toxoplasma gondii tachyzoites transmigrate a simulated human retinal vascular endothelium.
(A) Graph showing number of tachyzoites recovered from lower chambers of transwells divided by a human retinal endothelial monolayer cultured on type 1 collagen, 4 hours after upper chambers were loaded with 1 × 106 live or heat-killed RH strain T. gondii tachyzoites (n=6 wells, parasite viability = 44%). Representative of two independent experiments.
(B) Graph showing dextran permeability of transwells presented in A, as well as dextran permeability of transwells incubated in parallel, but without tachyzoites, and containing endothelial monolayers or type I collagen alone. There was no significant difference in dextran permeability of transwells containing endothelial monolayers and incubated with live tachyzoites (live), heat-killed tachyzoites (heat-killed) or without tachyzoites (EC) (p > 0.05), but a highly significant difference between dextran permeability of these wells and wells containing membranes that were coated with collagen I alone (collagen) (***p < 0.001) (n=3–6 wells). ANOVA with post-hoc Tukey tests. Dextran permeability was determined in all experiments.
(C) Analysis of CD144 expression on human retinal endothelial cells cultured at confluence for 3 days, i.e., at least one day less than endothelial cells plated in transwells. Cells were gated on the basis of forward and side scatter to exclude debris and dead cells, and plotted for CD31 and CD144 expression. Numbers indicate percentage of gated cells expressing CD31 and/or CD144. Representative of two independent experiments. (D) Graph showing results of the same transmigration assay, conducted with three natural isolates (i.e., GT-1, TgCatBR2 and GPHT) in place of the RH laboratory strain (n=6 wells, parasite viability = GT-1, 58%; TgCatBR2, 27%; GPHT, 23%). Data were obtained in three independent experiments. In all graphs, bars represent mean and error bars represent standard error of mean.
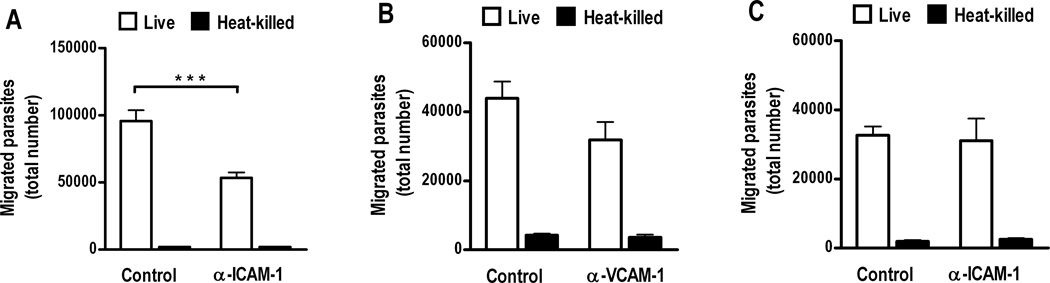
To investigate a possible role for the cell adhesion molecule, intercellular adhesion molecule (ICAM)-1, in migration of T. gondii tachyzoites across human retinal vascular endothelium, we performed endothelial transmigration assays after pre-incubating endothelial monolayers with anti-human ICAM-1 antibody or control immunoglobulin G1 (IgG1). ICAM-1 blockade significantly reduced tachyzoite movement across the endothelium by approximately 50% (Figure 2A, p < 0.001). In contrast, specific antibody blockade of the related cell adhesion molecule, vascular cell adhesion molecule (VCAM)-1, did not significantly impact tachyzoite migration (Figure 2B, p > 0.05). To determine whether ICAM-1-mediated endothelial transmigration within the eye was specific to retina, we performed the same experiment, but seeded the transwell membrane with endothelial cells that were isolated from choroid, in place of the retina. Choroid is the ocular tissue that encircles the retina. Consistent with specificity of the retinal endothelial interaction, ICAM-1 antibody blockade did not impact tachyzoite migration across simulated human choroidal vascular endothelium (Figure 2C, p > 0.05). When studies were repeated, we observed variation in absolute numbers of migrated parasites, but relationships between experimental conditions remained consistent.
Figure 2. ICAM-1 blockade significantly inhibits Toxoplasma gondii tachyzoite migration across simulated human retinal, but not choroidal, vascular endothelium.
Graphs showing number of tachyzoites recovered from lower chambers of transwells divided by (A and B) human retinal endothelial cell monolayers or (C) human choroidal endothelial cell monolayerscultured on type 1 collagen, 4 hours after upper chambers were loaded with 1 × 106 live or heat-killed RH strain T. gondii tachyzoites (n=6 wells, parasite viability = A, 55%; B, 52%;C, 95%). Monolayers were pre-incubated for 2 hours with (A and C) mouseanti-human ICAM-1 IgG1 (α-ICAM-1) or (B) mouse anti-human VCAM-1 IgG1 (α-VCAM-1), or control mouse IgG1 (control). There was a significant reduction in tachyzoite migration across the retinal endothelial monolayer in the presence of anti-ICAM-1 antibody (***p < 0.001), but not anti-VCAM-1 antibody (p > 0.05) (Student’s t-test). Anti-ICAM-1 antibody did not reduce tachyzoite migration across the choroidal endothelial monolayer (p > 0.05) (Student’s t-test). Each graph presents results that are representative of two independent experiments. In all graphs, bars represent mean and error bars represent standard error of mean.
Discussion
Elegant studies conducted in murine toxoplasmosis have shown clearly that T. gondii tachyzoites infect phagocytic leukocytes, which traffic parasites from the vascular tree to the brain.3, 4 However, tachyzoites use a form of independent motility known as ‘gliding’ that is parasite actin-dependent.9 By gliding, they cross polarized epithelial and trophoblastic monolayers,10 and move through tissues such as intestine, in which they navigate from the epithelium to submucosal vascular endothelium.11 These observations led us to hypothesize that T. gondii tachyzoites were also capable of crossing from the circulation to a target organ by direct interaction with the vascular endothelium. Recognizing that the most common clinical manifestation of toxoplasmosis was retinitis, we used a transwell system populated with human retinal endothelial cells to test our hypothesis. Our experiments demonstrated that tachyzoites were indeed capable of moving through a simulated human retinal endothelium.
We first evaluated migration of RH strain tachyzoites across a human retinal endothelial monolayer. Although RH is the most widely studied parasite strain, it has remained in culture for multiple decades, and, in addition to exhibiting heightened virulence in comparison to natural isolates of the same haplogroup, it has lost the ability to complete a full life cycle.12 Therefore, it was important to replicate our initial experiments using recently acquired “natural isolates”, which had more direct relevance to human ocular toxoplasmosis. We tested three such strains. These strains included GT-1, from haplogroup 1, and TgCatBR2 and GPHT, from haplogroup 6.13 Haplogroup 1 is believed to be responsible for many cases of toxoplasmic retinochoroiditis in immunocompetent individuals in the United States and Europe. Haplogroup 6 is the only group known to have a worldwide distribution, including in South America, where disease is relatively common and frequently aggressive. Each of the three natural isolates transmigrated human retinal vascular endothelium.
Barragan et al.10 reported that T. gondii tachyzoite migration through epithelial and trophoblastic monolayers depended on interaction with the cell surface immunoglobulin superfamily member, ICAM-1, and further, that human ICAM-1 immunoprecipitated parasite adhesin, MIC2. Our previous studies have demonstrated high constitutive expression of ICAM-1 transcript by human retinal endothelial cells, plus a significant increase in expression when these cells are infected with tachyzoites.14 Thus, we hypothesized that ICAM-1 mediated migration of T. gondii tachyzoites across human retinal endothelium. In support of this hypothesis, ICAM-1 antibody blockade significantly reduced tachyzoite movement in the transwell. Similar blockade of another member of the immunoglobulin superfamily, VCAM-1, did not significantly decrease tachyzoite movement. The choroid lies immediately adjacent to the retina within the eye, but is not the primary target of T. gondii in ocular toxoplasmosis. Choroidal endothelial cells express low levels of ICAM-1, and expression is not significantly increased following infection with T. gondii tachyzoites.14 Consistent with these facts and our hypothesis, ICAM-1 blockade did not impact tachyzoite migration across simulated human choroidal vascular endothelium. The observations made using simulated retinal versus choroidal endothelium may explain, in part, why T. gondii preferentially infects the retina within the eye. If MIC2 expression varies with parasite haplogroup, there may be inter-strain variation in the ability of thetachyzoite to interact with endothelial ICAM-1 and enter the retina, but our data do not address this question.
T. gondii tachyzoites may take a transcellular or paracellular route across the retinal vascular endothelium, as is well described for leukocytes crossing an endothelium from circulation to target organ.15 One interesting observation that applied to both clonal and natural isolates, was transendothelial passage of some heat-killed tachyzoites that were studied in parallel with the live parasites. A limited breach of the endothelial monolayer, undetectable by tracking diffusion of high molecular weight dextran, cannot be excluded. However, another potential explanation for the observation is endothelial transcytosis of tachyzoites. Certain bacterial and fungal species are known to use this mechanism to cross the blood-brain barrier.16, 17
In conclusion, our findings indicate that, in addition to taxiing in leukocytes, T. gondii may enter the human retina by crossing the local vascular endothelium unassisted. This migration relies in part on retinal endothelial ICAM-1. Current treatments for ocular toxoplasmosis fail to eradicate the parasite from the eye, leaving the patient at risk for recurrent disease.1 Our results have implications for the development of more effective therapeutics, aimed at preventing access of tachyzoites to the retina following exposure of the human host to the parasite.
Methods
Culture of Toxoplasma gondii tachyzoites
Per the classification of Khan et al,13 clonal (RH: haplogroup 1), natural common (GT-1: haplogroup 1) and natural exotic (TgCatBR2 and GPHT: haplogroup 6) T. gondii isolates were used in these studies. Natural isolates were generously provided by L. David Sibley, PhD (Washington University, St Louis, MO). Parasites were maintained in tachyzoite form by serial passage in confluent monolayers of human neonatal dermal fibroblasts (Cascade Biologics, Portland, OR) in Dulbecco’s modified Eagle’s medium (Catalogue number: 12100; Invitrogen-Gibco, Grand Island, NY), supplemented with 44 mM sodium bicarbonate, and 1% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, UT), at 37°C and 5% CO2. Parasite viability was evaluated by plaque assay for every experiment; viability of 20% or greater was required, consistent with the previous description of the viability of a freshly egressed, high-passaged natural isolate from haplogroup 1.12
Isolation and immortalization of human ocular endothelial cells
Retinal and choroidal endothelial cells were obtained from non-diseased eyes of human cadaveric donors (Lions VisionGift, Portland, OR), adhering closely to previously published methods.14 In brief, retina or choroid were dissected from posterior eye cups and digested with graded solutions of ≤ 0.5 mg/ml Dispase (Invitrogen-Gibco) and ≤ 3 mg/ml type II collagenase (Sigma-Aldrich, St. Louis, MO) in MCDB-131 medium (Sigma-Aldrich) with 2% FBS. Endothelial cells were isolated from the digested tissue using magnetic Dynabeads (Dynal-Invitrogen, Oslo, Norway) coated with mouse monoclonal anti-human CD31 antibody (BD Pharmingen, San Diego, CA), per the manufacturer’s instructions. Cells were cultured at 37 °C and 5% CO2 in MCDB-131 medium (Sigma-Aldrich) with 2 – 10% FBS and EGM-2 SingleQuots supplement, omitting gentamicin, hydrocortisone and FBS (Clonetics-Lonza, Walkerville, MD).
To produce sufficient numbers for study, endothelial cells were immortalized by transduction with the murine recombinant amphotropic retrovirus, LXSN16E6E7, which encodes for human papilloma virus E6 and E7 oncogenes, as well as a G418-resistence gene.18 LXSN16E6E7 virus was kindly gifted by Denise A. Galloway, PhD (Fred Hutchinson Cancer Institute). Endothelial cells were cultured for 24 hours with LXSN16E6E7 virus in supernatant harvested from PA317 packaging cell cultures. Percentage of immortalized cells was maximized by transduction in the presence of 5 mg/ml hexadimethrine bromide (Sigma-Aldrich) (retinal endothelial cells), or by post-transduction exposure to 200 µg/ml G418 antibiotic (Mediatech-Cellgro, Manassas, VA) (choroidal endothelial cells). Following immortalization, cultures were purified by selection with anti-CD31 antibody-coated magnetic Dynabeads. Immortalized cells retained the endothelial phenotype as evidenced by: cobblestone morphology; expression of CD31 and von Willebrand factor; and capillary-like tube formation on provisional extracellular matrix (data not shown).
Human ocular endothelial transmigration assay
Polyethylene terephthalate transwell (PET) membranes (3 micron pore-size, 0.3 cm2 diameter: BD Labware, Franklin Lakes, NJ) were inserted into 24-well plates, coated with 50 µg/ml bovine type I collagen (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 hours at 37 °C, and subsequently seeded with 30,000 endothelial cells suspended in MCDB-131 medium with 10% FBS and EGM-2 SingleQuots. Transwells were incubated at 37 °C and 5% CO2 for 4 to 5 days. One million freshly egressed live or heat-killed (i.e., exposed to 55 °C for 60 minutes)10 tachyzoites, suspended in phenol red-free endothelial basal medium (Clonetics-Lonza) with 2.5% FBS and EGM-2 SingleQuots at 1:4 dilution, were loaded in upper chambers, and lower chambers were loaded with the same medium (n=4–6 wells). After a 4-hour incubation at 37 °C and 5% CO2, parasites in lower chambers were counted by hematocytometer. In some experiments, upper chambers were pre-incubated for 2 hours with 10 µg/ml mouse anti-human ICAM-1 antibody (isotype IgG1; clone P2A4; Millipore, Temecula, CA) or 30 µg/ml mouse anti-human VCAM-1 antibody (isotype IgG1; clone BBIG-V1; R&D Systems, Minneapolis, MN), or control mouse IgG1 (clone 11711; R&D Systems) at the same concentration. Endothelial monolayer integrity was determined by permeability to 1 mg/ml Texas Red-conjugated 70,000 MW dextran (Molecular Probes-Invitrogen, Eugene, OR), per the method of Harhaj et al.19
For statistical analyses, the Student’s t-test was used to compare 2 groups and the ANOVA, with post-hoc Tukey testing, was used for multiple group comparisons. For all experiments, criteria for endothelial monolayer integrity were: (1) no significant difference (p ≥ 0.05) in dextran permeability of transwells containing endothelial monolayers and incubated with live versus heat-killed tachyzoites; (2) no significant increase (p ≥ 0.05) in permeability of transwells containing endothelial monolayers and incubated with live or heat-killed tachyzoites versus no tachyzoites; and (3) highly significant increase (p < 0.001) in permeability of transwells containing membranes coated with type I collagen alone versus wells containing endothelial monolayers and incubated with live, or heat-killed or no tachyzoites (n=3–6 wells).
Flow cytometric analysis of confluent human retinal endothelial cells
Human retinal endothelial cells were plated for confluence in a 6-cm diameter dish. After 3 days, the cells were lifted with 0.05% trypsin, and surface-stained for 30 minutes with V450-conjugated mouse anti-human CD31 antibody (isotype IgG1; clone WM59) and PE-conjugated anti-CD144 antibody (isotype IgG1; clone 55–7H1), orsimilarly labeled control mouse IgG1 (clone MOPC-21) (all obtained from BD Pharmingen, San Diego, CA) in phosphate buffered saline with 10% sodium azide and 10% FBS on ice. Subsequently, cells were washed with and suspended in the same buffer, and subjected to fluorescence activated cell sorting. Data were acquired on the BD LSRII flow cytometer (Becton-Dickinson, San Jose, CA), and analyzed using FCS Express V3 (De Novo Software, Los Angeles, CA).
Acknowledgements
This work was supported by grants from the National Institutes of Health (R21 EY019550) and Research to Prevent Blindness (unrestricted grant to Casey Eye Institute). The authors thank L. David Sibley, PhD (Washington University, St Louis, MO), who provided the natural isolates used in these studies. They also thank Dr. Sibley and Asis Khan, PhD (Washington University), for their expert advice regarding the execution of these studies. The LXSN16E6E7 viral construct was the generous gift of Denise A. Galloway, PhD (Fred Hutchinson Cancer Institute, Seattle, WA).
References
- 1.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Bosch-Driessen LE, Berendschot TT, Ongkosuwito JV, Rothova A. Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology. 2002;109:869–878. doi: 10.1016/s0161-6420(02)00990-9. [DOI] [PubMed] [Google Scholar]
- 3.Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gatel D, Tardieux I. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107:309–316. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert H, Hitziger N, Dellacasa I, Svensson M, Barragan A. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell Microbiol. 2006;8:1611–1623. doi: 10.1111/j.1462-5822.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- 5.Tardieux I, Menard R. Migration of Apicomplexa across biological barriers: the Toxoplasma and Plasmodium rides. Traffic (Copenhagen, Denmark) 2008;9:627–635. doi: 10.1111/j.1600-0854.2008.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuwabara T, Cogan DG. Studies of retinal vascular patterns. I. Normal architecture. Arch Ophthalmol. 1960;64:904–911. doi: 10.1001/archopht.1960.01840010906012. [DOI] [PubMed] [Google Scholar]
- 7.Nishiwaki H, Ogura Y, Kimura H, Kiryu J, Miyamoto K, Matsuda N. Visualization and quantitative analysis of leukocyte dynamics in retinal microcirculation of rats. Invest Ophthalmol Vis Sci. 1996;37:1341–1347. [PubMed] [Google Scholar]
- 8.Silveira C, Vallochi AL, Rodrigues da Silva U, Muccioli C, Holland GN, Nussenblatt RB, et al. Toxoplasma gondii in the peripheral blood of patients with acute and chronic toxoplasmosis. Br J Ophthalmol. 2011;95:396–400. doi: 10.1136/bjo.2008.148205. [DOI] [PubMed] [Google Scholar]
- 9.Sibley LD. Intracellular parasite invasion strategies. Science. 2004;304:248–253. doi: 10.1126/science.1094717. [DOI] [PubMed] [Google Scholar]
- 10.Barragan A, Brossier F, Sibley LD. Transepithelial migration of Toxoplasma gondii involves an interaction of intercellular adhesion molecule 1 (ICAM-1) with the parasite adhesin MIC2. Cell Microbiol. 2005;7:561–568. doi: 10.1111/j.1462-5822.2005.00486.x. [DOI] [PubMed] [Google Scholar]
- 11.Barragan A, Sibley LD. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J Exp Med. 2002;195:1625–1633. doi: 10.1084/jem.20020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan A, Behnke MS, Dunay IR, White MW, Sibley LD. Phenotypic and gene expression changes among clonal type I strains of Toxoplasma gondii. Eukaryot Cell. 2009;8:1828–1836. doi: 10.1128/EC.00150-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan A, Fux B, Su C, Dubey JP, Darde ML, Ajioka JW, et al. Recent transcontinental sweep of Toxoplasma gondii driven by a single monomorphic chromosome. Proc Natl Acad Sci U S A. 2007;104:14872–14877. doi: 10.1073/pnas.0702356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JR, Choi D, Chipps TJ, Pan Y, Zamora DO, Davies MH, et al. Unique gene expression profiles of donor-matched human retinal and choroidal vascular endothelial cells. Invest Ophthalmol Vis Sci. 2007;48:2676–2684. doi: 10.1167/iovs.06-0598. [DOI] [PubMed] [Google Scholar]
- 15.Engelhardt B, Wolburg H. Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004;34:2955–2963. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- 16.Stins MF, Badger J, Sik Kim K. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb Pathog. 2001;30:19–28. doi: 10.1006/mpat.2000.0406. [DOI] [PubMed] [Google Scholar]
- 17.Kronstad JW, Attarian R, Cadieux B, Choi J, D'Souza CA, Griffiths EJ, et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat Rev Microbiol. 2011;9:193–203. doi: 10.1038/nrmicro2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halbert CL, Demers GW, Galloway DA. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991;65:473–478. doi: 10.1128/jvi.65.1.473-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci. 2006;47:5106–5115. doi: 10.1167/iovs.06-0322. [DOI] [PubMed] [Google Scholar]