Abstract
Both rectal and vaginal mucosal surfaces serve as transmission routes for pathogenic microorganisms. Vaccination through large intestinal mucosa, previously proven protective for both mucosal sites in animal studies, can be achieved successfully by direct intra-colorectal (i.c.r.) administration, which is, however, clinically impractical. Oral delivery seems preferable, but risks vaccine destruction in the upper gastrointestinal tract. Therefore, we designed a large intestine-targeted oral delivery with pH-dependent microparticles containing vaccine nanoparticles, which induced colorectal immunity in mice comparably to colorectal vaccination and protected against rectal or vaginal viral challenge. Conversely, vaccine targeted to the small intestine induced only small intestinal immunity and provided no rectal or vaginal protection, demonstrating functional compartmentalization within the gut mucosal immune system. Therefore, using this oral vaccine delivery system to target the large intestine, but not the small intestine, may represent a feasible novel strategy for immune protection of rectal and vaginal mucosa.
INTRODUCTION
Mucosal immunization has proven to be critical to induce mucosal protection1–5 and contributes to rapid and long-lasting mucosal protection in contrast to systemic immunization6. It has been shown that antigen-specific functional CD8+ cytotoxic T cells in the mucosa are critical to protect from CD4+ T cell depletion by SHIV3, while human studies indicate that a higher frequency of the antigen-specific mucosal CD8+ T cells correlates with a lower degree of herpes simplex viral infectivity as well as reduced severity of the disease7. In the mucosal tissues of HIV-infected long-term nonprogressors, there exist immunodominant CD8+ T cells and their presence is strongly correlated with HIV-1 control5,8. A variety of approaches have been proposed and employed to induce protective mucosal immunity against viral transmission through either the rectal or vaginal route1–3,9–11. However, potent but clinically practical genitorectal vaccination strategy remains unestablished for the following reasons.
Large intestinal mucosa is an optimal site to induce both rectal and vaginal immunity. Intra-colorectal (i.c.r.) vaccination directly at the large intestinal mucosa induces robust cellular and humoral immune responses in the regional lymph nodes4, more effectively than vaccination at a distant mucosa (e.g., intranasal) or by a parenteral route1–5. However, for mass human vaccination, i.c.r. administration appears to be clinically too cumbersome and unpalatable. In addition, this procedure could potentially be traumatic without adequate caution. Given that the intranasal route, although practical and relatively easy, poses the risk of inoculum invasion into the central nervous system by olfactory nerve transport12, a truly safe vaccine delivery route is needed. The oral route is the safest and most practical. However, except for a few live attenuated vaccines inducing systemic responses, simple oral delivery is ineffective at protecting either rectal or genital mucosa13. The failure is mostly attributed to the enzymatic destruction in the proximal gut and likely inadequate antigen uptake in the large intestine.
We here aimed to discover a way to selectively deliver a vaccine to the large intestinal mucosa through the oral route, which has not previously been accomplished. To mimic the “gold standard” i.c.r. immunization while circumventing the limitations of oral delivery, we encapsulated a peptide or protein vaccine into biologically compatible poly(DL-lactic-co-glycolic acid) (PLGA) nanoparticles14,15 to be used for site-specific immunization. The depot effect of PLGA nanoparticles offers an additional feature that controlled release of entrapped vaccines over extended time periods provides a longer antigen exposure to the immune system. PLGA particle size, adjustable during manufacturing, was engineered in nanometers because size-dependent mucosal uptake is most effective within nanometer ranges and impeded when the size is over 1 micron16. Selective combinations of TLR ligands can induce synergistic activation of T cells17–19. We adjuvanted the vaccine with MALP-2, poly(I:C) and CpG ODN, which have been shown to synergistically induce mucosal anti-viral protection after i.c.r. immunization20.
To bypass the harmful effects of digestive low pH and enzymatic destruction and to selectively deliver the particles to the lower gastrointestinal (GI) tract intact, the PLGA nanoparticle surface was coated with methacrylate-based polymer Eudragit FS30D21, an anionic tripolymer comprising poly(methyl acrylate, methyl methacrylate, methacrylic acid) in a 7:3:1 ratio. The ratio of free carboxyl groups to ester groups is ~1:10. It is pH sensitive and soluble in intestinal fluids at pH > 7.0, seen only in the terminal ileum, thereby preventing contents from degradation more proximally. These coated particles are ≥ 10 micrometers in diameter in order to avoid premature uptake in the small intestine primarily by Peyer’s patches, which can significantly take up particles up to 1 μm16. Our design is shown in Supplementary Fig. 1.
RESULTS
Proof of principle study on the PLGA nanoparticle vaccine
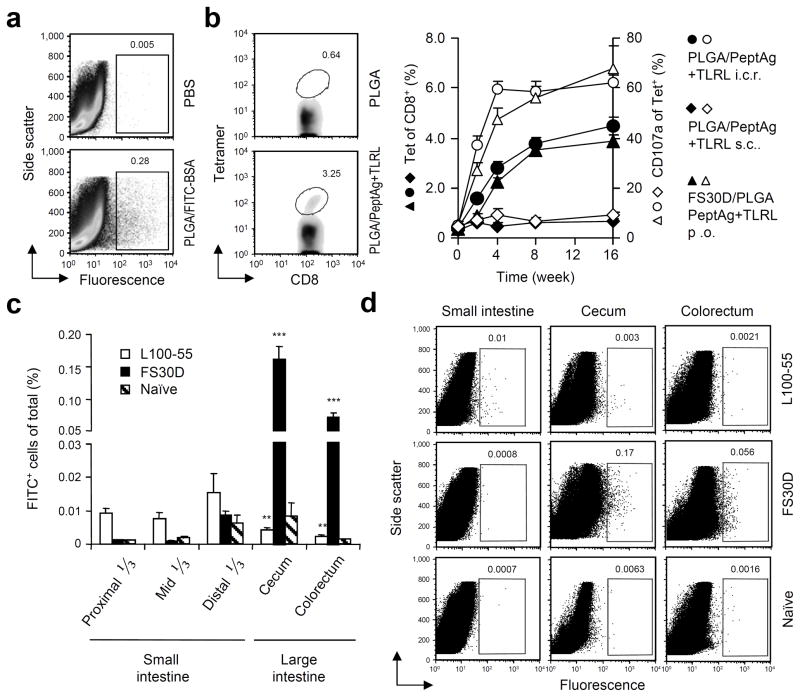
Uncoated PLGA nanoparticles were manufactured in the range from 300 500 nm (418 nm ± 88 SD) (Supplementary Fig. 2a), with 90% encapsulation efficacy (Supplementary Fig. 2b). We first conducted a proof of principle experiment in which fluorescent fluorescein-containing PLGA/FITC-BSA nanoparticles were delivered directly to the colon of mice by i.c.r. administration. At day 3, a proportion of the cells isolated from the large intestinal lamina propria were detected positive for fluorescence expression (Fig. 1a), indicating an uptake of the nanoparticles. Nanoparticle uptake was primarily found in CD11b+B220int macrophages and secondarily in CD11c+CD11b+ dendritic cells (DCs) in the lamina propria of the large intestine (Supplementary Fig. 3a). Transmission electron microscopy indicates PLGA nanoparticles in the cytoplasm (Supplementary Fig. 3b). Uptake of PLGA nanoparticles by DCs (derived from bone marrow) was also confirmed in vitro by flow cytometry (Supplementary Fig. 4a), fluorescence microscopy (Supplementary Fig. 4b) and transmission electron microscopy (Supplementary Fig. 4c).
Figure 1.
I.C.R. delivered nanoparticles enter the large intestinal mucosa and orally delivered FS30D/PLGA nanoparticle-releasing microparticles selectively targets the large intestinal mucosa for uptake. (a) Colorectal mucosal uptake of PLGA nanoparticles after i.c.r. delivery of PLGA/FITC-BSA nanoparticles. Cells were isolated from the colorectum 3 days after administration and measured for fluorescence-positive cells. (P < 0.01 between PLGA/FITC-BSA and PBS treated. Representative of three independent experiments, n = 12 15 per group). (b) Induction of antigen-specific colorectal mucosal T cells after i.c.r. delivery of PLGA nanoparticles encapsulating PCLUS3-18IIB and MALP2+poly(I:C)+CpG vaccine (PLGA/PeptAg+TLRL). Three weeks after one immunization, colorectal cells were isolated and measured for P18-I10 specific CD8+ T cells by tetramer staining. P < 0.01 between PLGA/PeptAg+TLRL and PLGA alone. Representative of two independent experiments. n = 10 per group. (c) Gut mucosal uptake of PLGA particles after oral delivery of FS30D/PLGA or L100-55/PLGA. Cells were isolated from the small and large intestine at day 2 for measurement of fluorescence-positive cells. **P < 0.02 on white bar indicates the difference from small intestine. ***P < 0.001 on black bar indicates the difference from the small intestine. n = 9 12 per group. (d) Representative examples of flow cytometry from experiments shown in c.
We subsequently confirmed that antigen-specific T cells responses could be induced in the colon after a single i.c.r. delivery (Fig. 1b), but not after s.c. administration (Supplementary Fig. 5), of PLGA nanoparticles encapsulating PCLUS3-18IIIB (CD4+ T cell helper epitopes fused with HIV Env CD8+ CTL epitope) and TLR ligands (MALP2+poly(I:C)+CpG)20. Therefore, PLGA nanoparticles can serve as an effective vaccine delivery system when they are deposited in the large intestinal lumen.
Oral delivery of nanoparticle-releasing microparticle vaccines
The Eudragit served to make 10 50 μm particles and released contents substantially as early as 1 h at pH 7.4, in contrast to at pH 2.5 at which the particles were stable (Supplementary Fig. 6). After oral delivery of Eudragit FS30D containing PLGA/FITC-BSA nanoparticles, nanoparticle uptake was found almost exclusively in the large intestine (Fig. 1c,d). The cecum is the first part of the large intestine encountered, but in humans, where the cecum is relatively small, the balance between cecum and colon may be different. We further contrasted FS30D with L100-55 microparticles containing the same nanoparticles for lower pH release (pH > 5.5) in the small intestine and found that oral administration of Eudragit L100-55-coated nanoparticles resulted in primary uptake rather in the small intestine (Fig. 1c,d). Likewise, uncoated PLGA nanoparticles, to the extent any successfully traversed the stomach, were primarily delivered to the small intestine (Supplementary Fig. 7). Thus, the distribution of nanoparticles was altered by coating them with FS30D, such that a majority now reached and were taken up by cells in the large intestine (Fig. 1c,d and Supplementary Fig. 7). These results validate the approach of coating PLGA nanoparticles with FS30D to protect them during transit through the upper GI tract for increased delivery to the large intestine.
We next used a luciferase reporter system to confirm the large intestine targeted delivery with FS30D. Luciferase expression was measured in intestinal sections after oral administration of FS30D/PLGA containing a luciferase DNA plasmid (Supplementary Fig. 8).
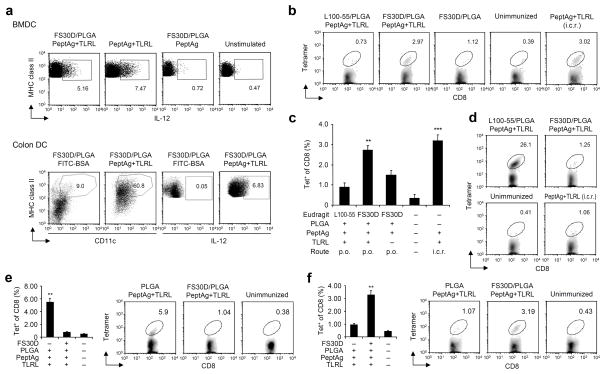
After confirming that formulated vaccine components within the micro/nanoparticles retained TLR agonist activity in in vitro settings or after oral administration (Fig. 2a), we examined intestinal immune responses in mice after two immunizations with the two different Eudragit coatings of the PLGA nanoparticle vaccine given orally with a two-week interval. Three weeks after the second immunization, tetramer positive CD8+ T cells were detected in the large intestine, indicating successful induction of colorectal immunity with the oral FS30D-coated vaccine (Fig. 2b,c). In contrast, the L100-55-coated vaccine induced a minimal level of colonic antigen-specific CD8+ T cells (Fig. 2b,c). In fact, the L100-55-coated vaccine induced a T cell response primarily in the small intestine, where the FS30D-coated vaccine was only marginally effective (Fig. 2d). Without Eudragit coating, PLGA nanoparticle vaccines did not elicit significant immune responses in the large intestine but did induce responses in the small intestine (Fig. 2e,f). The results affirm that the enteric coating with FS30D is essential for oral delivery of PLGA nanoparticles to the large intestine.
Figure 2.
Orally delivered FS30D-coated PLGA nanoparticle vaccine induces antigen-specific T cells in the large intestine, while L100-55-coated vaccine induces the T cells in the small intestine. (a) Activation of DC after 20-h incubation with supernatants from FS30D-coated PLGA containing PCLUS3-18IIIB+TLRL (FS30D/PLGA/PeptAg+TLRL), antigen (FS30D/PLGA/PeptAg) or vaccine only (PeptAg+TLRL) dissolved in PBS at pH 7.4 for 16 h (upper panels). Intracellular IL-12 was measured by flow cytometry (n = 6 per group). The micro/nanoparticles were also given orally and DC activation in vivo was assessed ex vivo (lower panels), n = 5 per group. (b–d) Induction of T cell responses after oral delivery of FS30D/PLGA/PeptAg+TLRL or L100-55/PLGA/PeptAg+TLRL. Oral administration was conducted twice with a two-week interval. Tetramer positive cells in the colorectum (b and c) or upper part of the small intestine (d) were measured three weeks after. The i.c.r. group was immunized with vaccine only without nanoparticles but formulated in DOTAP. **P < 0.01, ***P < 0.001 indicate the significant difference between the group with asterisks and each of the groups without asterisks. There are no differences between the two groups with asterisks. Representative of experiments (c) is summarized in b. In d, P < 0.001 for L100-55 vs other groups. n = 8 12 per group. (e and f) T cell responses induced after oral delivery of uncoated or FS30D-coated PLGA/PeptAg+TLRL. Tetramer positive cells in the upper small intestine (e) or the colorectum (f) were measured. **P < 0.01 indicates the significant difference between groups (n = 7 per group).
Induction of T-cell immunity against viral infection
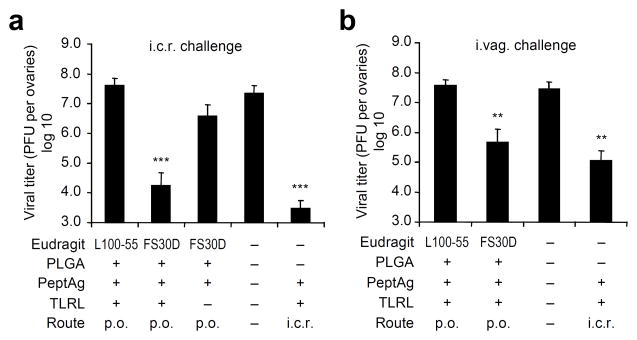
To evaluate the protective efficacy of this vaccine, after prime and boost oral immunization, we challenged mice rectally with a replication-competent vaccinia virus vPE-16, which expresses the HIV Env epitope P18-I10 used in the peptide vaccine. Mice immunized with the L100-55-coated vaccine were not protected from the virus challenge. However, the FS30D-coated vaccine reduced viral load almost equally as well as the peptide vaccine given i.c.r. (Fig. 3a). Therefore, oral delivery of FS30D-coated PLGA vaccine targeting the large intestine is effective for local colorectal vaccination against viral infection.
Figure 3.
Orally delivered FS30D-coated PLGA nanoparticle peptide vaccine confers T-cell mediated resistance to virus infection in the rectal or vaginal tract. FS30D/PLGA/PeptAg+TLRL or L100-55/PLGA/PeptAg+TLRL was given orally to mice twice with a two-week interval, followed by i.c.r. (a) or i.vag. (b) challenge with 2×107 or 1×107 PFU of vPE16, respectively, three weeks after the last immunization. Ovaries (where this virus primarily replicates) were removed at day 6 for viral titer assessment. **P < 0.01, ***P < 0.001 indicate the significant difference in viral titer between the group with asterisks and each of the groups without asterisks (n = 12 15 per group).
It has been previously shown that i.c.r. vaccination with adenovirus-based vaccines induced effective protection against virus challenge (vaccinia and HSV-2) not only in the rectal but also in vaginal mucosa4. To determine whether vaginal mucosal protection can be induced by the orally administered FS30D-coated PLGA vaccine, we immunized mice orally with the vaccine (FS30D/PLGA/PCLUS3-18IIIB+TLR ligands) twice with a two-week interval, followed by intravaginal (i.vag.) challenge with vPE-16. Compared to i.c.r. immunization, oral delivery of FS30D-coated vaccine induced almost equal clearance of virus after vaginal challenge (Fig. 3b), whereas, again, the L100-55 formulation that delivers the vaccine to the small intestine was not effective. Thus, delivery to the large intestine was more effective than to the small intestine in protecting against both genital and rectal challenge. This efficacy was largely T-cell mediated, as the virus does not incorporate gp160 into virions and is not sensitive to anti-gp160 neutralization.
Induction of antibody immunity against viral infection
The humoral response also plays an important role in protection in the gut and genital mucosal immunity. Therefore, we examined whether encapsulating whole viral proteins in the FS30D-coated PLGA vaccine can induce antibody-mediated protective immunity at both mucosal sites after challenging mice with pathogenic vaccinia strain WR. Vaccinia A33 and L1 are immunogens of the extracellular enveloped virion and intracellular mature virion, respectively. Antibody responses induced by the combination of both types of viral antigens encoded by plasmid DNA22 or as recombinant proteins23 can protect animals from lethal challenge of WR. Of note, CTLs specific for the vaccinia protein A33 or L1 have not been reported in BALB/c mice24. TLR ligands have been shown to activate B cells directly and contribute to antigen-specific antibody responses25,26, including in the gut27. Here we found that the triple TLR ligands previously shown to synergistically activate naïve T cells20 could also synergistically activate B cells (Supplementary Fig. 9a) as determined by CD6928. I.c.r.. immunization with the combination of recombinant A33 and L1 mixed with the triple TLR ligands in DOTAP induced antibody responses in the blood (Supplementary Fig. 9b).
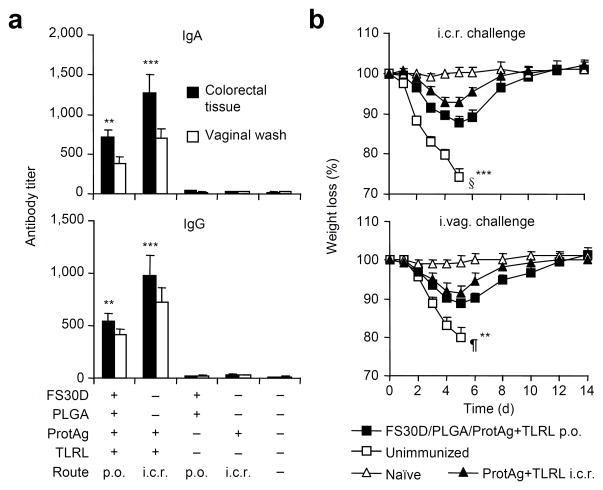
The ability of PLGA nanoparticles containing the above vaccine components to induce antibody responses was assessed by immunization through either the i.c.r. or s.c. routes (Supplementary Fig. 10a), and the results further showed that CD4+ T cells were also activated by such a vaccine regimen (Supplementary Fig. 10b). We therefore constructed FS30D-coated PLGA/A33+L1+TLRL vaccine microparticles and administered them orally. Vaccinia-specific IgA and IgG antibody responses were significantly induced in both the large intestine (tested in tissue homogenates) and vaginal tracts (tested in vaginal washes) (Fig. 4a). Further, orally immunized mice resisted the WR virus challenge by either the rectal or vaginal route (Fig. 4b) as determined by weight loss and by 0 vs 75% or 50% mortality, respectively. Therefore, the FS30D-coated PLGA vaccine system can also be used to induce antibody-mediated mucosal protection at both mucosal transmission sites.
Figure 4.
Orally delivered FS30D-coated PLGA nanoparticle protein vaccine confers antibody-mediated resistance to virus infection in the rectal or vaginal tract. FS30D-coated PLGA containing antigen proteins A33 and L1 and TLR ligands (ProtAg+TLRL) was administered orally with a two-week interval. (a) Serum and local IgA (top) and IgG (bottom) antibodies against both A33 and L1 (together) measured at three weeks after the last immunization. Both FS30D/PLGA/ProtAg+TLRL p.o. and ProtAg+TLRL i.c.r. groups have significantly higher antibody titers than the other groups (P < 0.02). **P < 0.01 and ***P < 0.001 indicate difference (for both sites) from each of the bars without asterisks. Results from two independent experiments (n = 10 per group). (b) Disease course of the mice after challenge with WR by the i.c.r. (4×107 PFU) or i.vag. (1×107 PFU) route three weeks after the last immunization. **P < 0.02, ***P < 0.001 indicate the differences between the FS30D/PLGA/ProtAg+TLRL and unimmunized groups in weight loss (n = 10 14 per group). §, 75% mortality; ¶, 50% mortality.
DISCUSSION
As direct rectal or vaginal vaccine delivery may be impractical for mass human vaccination, despite the efficacy of the i.c.r route, oral delivery is a desirable alternative route potentially allowing safe, simple, and rapid delivery. We hypothesized that if the vaccine is able to survive the passage to, and is not absorbed before reaching the large intestine, the vaccination should mimic the efficacy of i.c.r. vaccination. Accordingly, we designed a two-part FS30D/PLGA nanoparticle-releasing microparticle system and showed that this novel system can bypass the small intestine and deliver orally administered vaccines highly specifically into the large intestinal mucosa and induce almost equal protective immunity not only in the rectal but also vaginal mucosa.
Uncoated PLGA particles given orally either failed to traverse the stomach or reached only the small intestine, and did not elicit significant colonic responses, but only small intestinal immune responses. These results underline the need for combinatorial use of Eudragit FS30D and PLGA to ensure specific targeting of the large intestine by orally delivered vaccine. Our study also indicates that TLR ligand activity was maintained after encapsulation within PLGA, and thus, PLGA is an ideal delivery vehicle for vaccines containing TLR agonists.
Further, we designed a contrasting vaccine coated nanoparticle (L100-55/PLGA) that delivered antigen to and induced T cell responses in the small intestine selectively,, whereas conversely FS30D-coated vaccines induced responses in the large but not small intestine, providing a comparison of these sites for the first time with the same vaccine. Thus, we have made a fundamental discovery of cellular immune compartmentalization within the gut mucosal immune system, which is reminiscent of the sub-compartmentalized gut humoral immunity29, highlighting the need for site-specific delivery of vaccines to the large intestinal mucosa for protection against infections transmitted by the rectal or vaginal route, and we demonstrated a novel way to accomplish this via the more practical oral route. Our findings thus strengthen the conception that the common mucosal immune system is somehow sub-compartmentalized.
There are many potential applications of our delivery technology beyond the vaccination against viral infection. This novel strategy is applicable for many forms of vaccines such as DNA, recombinant proteins, peptides, and a wide variety of adjuvants, and may be adapted in the development of vaccination strategies combating certain sexually transmitted infections caused by not only viruses but possibly also other types of pathogens. It also suggests a new approach to the development of a preventive or therapeutic vaccination against mucosal malignancies such as colorectal as well as cervical cancer. With this technology, in-depth study of the mucosal immunological subcompartmentalization between the large and small intestines can be easily conducted. For example, besides vaccination, one could use this approach to localize the site of induction of oral tolerance. The technology may be incorporated with other technologies to devise therapies in which selective targeting within the gut mucosa is needed.
Our targeted micro/nanoparticle system lends itself to practical large-scale clinical applications because of its 1) great stability in a dry-powder formulation; 2) easy shipment and storage without refrigeration, and long shelf life; and 3) economical large batch GMP processing. These features would be highly desired for effective industrial manufacturing and clinical management. PLGA is contained in several FDA-approved products. Although fine tuning of the formulation may be necessary to account for longer transit times in the gut of non-human primates and humans, as well as slightly higher pH values (around 7.3) in the distal small intestine of humans, this study demonstrates a promising proof of concept that this type of formulation may be practical and effective for oral vaccination to induce lower GI immunity without the need for intrarectal delivery. Capsules either coated with or comprised of Eudragit have been investigated in humans for site-targeted release in the distal GI tract30–32. Clinical approaches would be extended to consider packaging PLGA nanoparticles with these colon-specific capsules, which may represent a preferable carrier for targeted colon vaccination in humans.
In conclusion, we have demonstrated functional immune compartmentalization of the gut mucosa and developed a nanoparticle-releasing microparticle oral delivery system and demonstrated that it is an easy, non-invasive vaccination strategy effective against viral infection occurring through the rectal or vaginal mucosa. Such a vaccination strategy would represent a conceptually novel approach to the development of vaccines against mucosal infections and potentially against mucosal cancers and to advanced study of immunobiological mechanisms involving mucosal compartmentalization.
Supplementary Material
Supplementary Figure 1 Design for oral delivery of vaccines for immunization of the large intestinal mucosa.
Supplementary Figure 2 PLGA nanoparticle size distribution and release rate.
Supplementary Figure 3 Colorectal mucosal uptake of PLGA nanoparticles administered i.c.r..
Supplementary Figure 4 Uptake of PLGA nanoparticles by mouse bone marrow derived DCs, and colorectal mucosal cells.
Supplementary Figure 5 Induction of antigen-specific colorectal mucosal T cells after i.c.r. but not s.c. delivery of PLGA nanoparticles encapsulating PCLUS3-18IIB and MALP2+poly(I:C)+CpG vaccine.
Supplementary Figure 6 Eudragit FS30D microparticle size distribution and dissolution.
Supplementary Figure 7 Differences between uncoated and FS30D-coated PLGA in GI mucosal uptake after oral delivery.
Supplementary Figure 8 Site specific delivery with FS30D given by the oral route.
Supplementary Figure 9 Synergistic activation of B cells by a combination of TLR ligands and induction of antibody responses.
Supplementary Figure 10 Induction of the antibody and CD4 T-cell response by PLGA vaccines.
Acknowledgments
The authors thank B. Moss and P. Earl (US National Institutes of Health) for generous provision of vPE16, G. Cohen (University of Pennsylvania) for vaccinia antibodies, NIH Tetramer Facility for tetramers P18-I10, and BEI resources for vaccinia recombinant proteins. We also thank J. Hooper, D. Johnson, M. Dobrovolskaia, B. Zolnik, J. Gao, and X. Liu for professional comments and help, and J. FitzGerald for electron microscopy. We appreciate Z. Xia, D. Pendleton, D. Li and L. Smith for their technical and secretarial assistance. This research was supported by the Intramural Research Program of the US National Institutes of Health, National Cancer Institute, Center for Cancer Research, and the Intramural AIDS Targeted Antiviral Program (IATAP), a grant from the National Natural Science Foundation of China (31170872), and a CRADA with Nanotherapeutics Inc.
Footnotes
AUTHOR CONTRIBUTIONS
Q.Z., R.J.M., and J.A.B. designed the experiments, interpreted the data and wrote the manuscript. Q.Z. executed many of the experiments, Z.W. conducted some of experiments, and J.T., R.C.W., J.K. and B.E. produced micro/nanoparticles containing vaccines and were involved in micro- and nanoparticle characterization and in vitro release experiments. T.C. participated in experiment design and the initial experiments. G.Z. performed electron microscopy. D.M.K. provided CpG ODN and contributed to analysis and discussion. I.M.B., S.G., and Y.S. participated in planning and discussion. J.A.B. oversaw the overall execution of the projects.
COMPETING FINANCIAL INTERESTS
J.T. is the CEO and J.K. and B.E. are employees of Nanotherapeutics Inc., a for-profit company with patent rights to the NanoDRYR technology (US Patent Application 20050175707) used herein.
References
- 1.Belyakov IM, et al. The importance of local mucosal HIV-specific CD8(+) cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. The Journal of clinical investigation. 1998;102:2072–2081. doi: 10.1172/JCI5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belyakov IM, et al. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nature medicine. 2001;7:1320–1326. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- 3.Belyakov IM, Isakov D, Zhu Q, Dzutsev A, Berzofsky JA. A novel functional CTL avidity/activity compartmentalization to the site of mucosal immunization contributes to protection of macaques against simian/human immunodeficiency viral depletion of mucosal CD4+ T cells. J Immunol. 2007;178:7211–7221. doi: 10.4049/jimmunol.178.11.7211. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Q, et al. Immunization with adenovirus at the large intestinal mucosa as an effective vaccination strategy against sexually transmitted viral infection. Mucosal Immunol. 2008;1:78–88. doi: 10.1038/mi.2007.3. [DOI] [PubMed] [Google Scholar]
- 5.Critchfield JW, et al. Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status. PLoS ONE. 2008;3:e3577. doi: 10.1371/journal.pone.0003577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price GE, et al. Single-dose mucosal immunization with a candidate universal influenza vaccine provides rapid protection from virulent H5N1, H3N2 and H1N1 viruses. PLoS One. 2010;5:e13162. doi: 10.1371/journal.pone.0013162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiffer JT, et al. Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18973–18978. doi: 10.1073/pnas.1006614107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferre AL, et al. Immunodominant HIV-specific CD8+ T-cell responses are common to blood and gastrointestinal mucosa, and Gag-specific responses dominate in rectal mucosa of HIV controllers. J Virol. 2010;84:10354–10365. doi: 10.1128/JVI.00803-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehner T, et al. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nature medicine. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 10.Miller CJ, McGhee JR. Progress towards a vaccine to prevent sexual transmission of HIV. Nature medicine. 1996;2:751–752. doi: 10.1038/nm0796-751. [DOI] [PubMed] [Google Scholar]
- 11.Boyer JD, et al. Protection against simian/human immunodeficiency virus (SHIV) 89.6P in macaques after coimmunization with SHIV antigen and IL-15 plasmid. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18648–18653. doi: 10.1073/pnas.0709198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Ginkel FW, Jackson RJ, Yuki Y, McGhee JR. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J Immunol. 2000;165:4778–4782. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- 13.McConnell EL, Basit AW, Murdan S. Colonic antigen administration induces significantly higher humoral levels of colonic and vaginal IgA, and serum IgG compared to oral administration. Vaccine. 2008;26:639–646. doi: 10.1016/j.vaccine.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 14.O'Hagan DT, Singh M, Ulmer JB. Microparticle-based technologies for vaccines. Methods (San Diego, Calif. 2006;40:10–19. doi: 10.1016/j.ymeth.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Mundargi RC, Babu VR, Rangaswamy V, Patel P, Aminabhavi TM. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharmaceutical research. 1996;13:1838–1845. doi: 10.1023/a:1016085108889. [DOI] [PubMed] [Google Scholar]
- 17.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nature reviews. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 18.Manicassamy S, Pulendran B. Modulation of adaptive immunity with Toll-like receptors. Seminars in immunology. 2009;21:185–193. doi: 10.1016/j.smim.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Q, et al. Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: implications for vaccines. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16260–16265. doi: 10.1073/pnas.0805325105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Q, et al. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. The Journal of clinical investigation. 2010;120:607–616. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bott C, et al. In vivo evaluation of a novel pH- and time-based multiunit colonic drug delivery system. Aliment Pharmacol Ther. 2004;20:347–353. doi: 10.1111/j.1365-2036.2004.02033.x. [DOI] [PubMed] [Google Scholar]
- 22.Hooper JW, Custer DM, Schmaljohn CS, Schmaljohn AL. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology. 2000;266:329–339. doi: 10.1006/viro.1999.0096. [DOI] [PubMed] [Google Scholar]
- 23.Fogg C, et al. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J Virol. 2004;78:10230–10237. doi: 10.1128/JVI.78.19.10230-10237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooper JW, Custer DM, Thompson E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology. 2003;306:181–195. doi: 10.1016/S0042-6822(02)00038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 26.Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS One. 2007;2:e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shang L, et al. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529–538. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, Ross SR. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2281–2286. doi: 10.1073/pnas.042355399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cronkhite RI, Michael JG. Sub-compartmentalization of the gastrointestinal (GI) immune system determined with microbeads that differ in release properties. Vaccine. 2004;22:2106–2115. doi: 10.1016/j.vaccine.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Cole ET, et al. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int J Pharm. 2002;231:83–95. doi: 10.1016/s0378-5173(01)00871-7. [DOI] [PubMed] [Google Scholar]
- 31.Schellekens RC, et al. Pulsatile drug delivery to ileocolonic segments by structured incorporation of disintegrants in pH-responsive polymer coatings. J Control Release. 2008;132:91–98. doi: 10.1016/j.jconrel.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Han M, et al. In vitro and in vivo evaluation of a novel capsule for colon-specific drug delivery. Journal of pharmaceutical sciences. 2009;98:2626–2635. doi: 10.1002/jps.21627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Design for oral delivery of vaccines for immunization of the large intestinal mucosa.
Supplementary Figure 2 PLGA nanoparticle size distribution and release rate.
Supplementary Figure 3 Colorectal mucosal uptake of PLGA nanoparticles administered i.c.r..
Supplementary Figure 4 Uptake of PLGA nanoparticles by mouse bone marrow derived DCs, and colorectal mucosal cells.
Supplementary Figure 5 Induction of antigen-specific colorectal mucosal T cells after i.c.r. but not s.c. delivery of PLGA nanoparticles encapsulating PCLUS3-18IIB and MALP2+poly(I:C)+CpG vaccine.
Supplementary Figure 6 Eudragit FS30D microparticle size distribution and dissolution.
Supplementary Figure 7 Differences between uncoated and FS30D-coated PLGA in GI mucosal uptake after oral delivery.
Supplementary Figure 8 Site specific delivery with FS30D given by the oral route.
Supplementary Figure 9 Synergistic activation of B cells by a combination of TLR ligands and induction of antibody responses.
Supplementary Figure 10 Induction of the antibody and CD4 T-cell response by PLGA vaccines.