Abstract
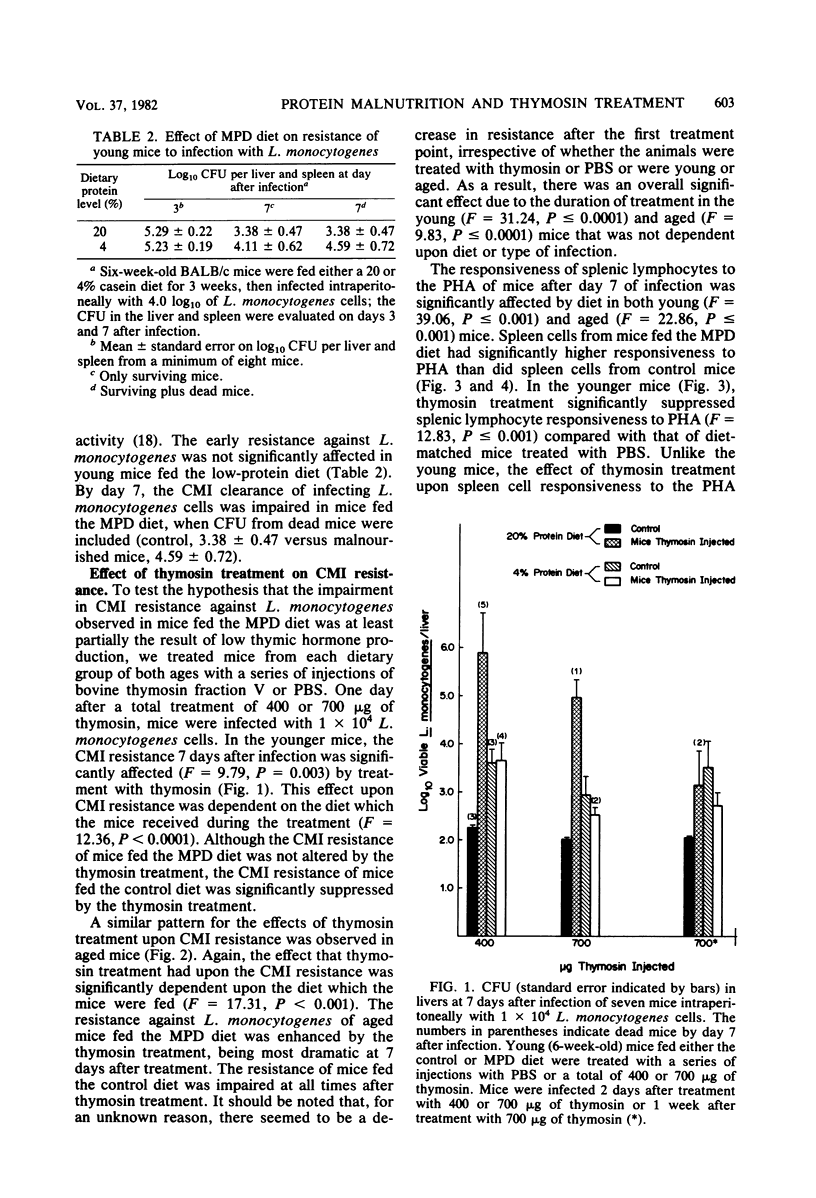
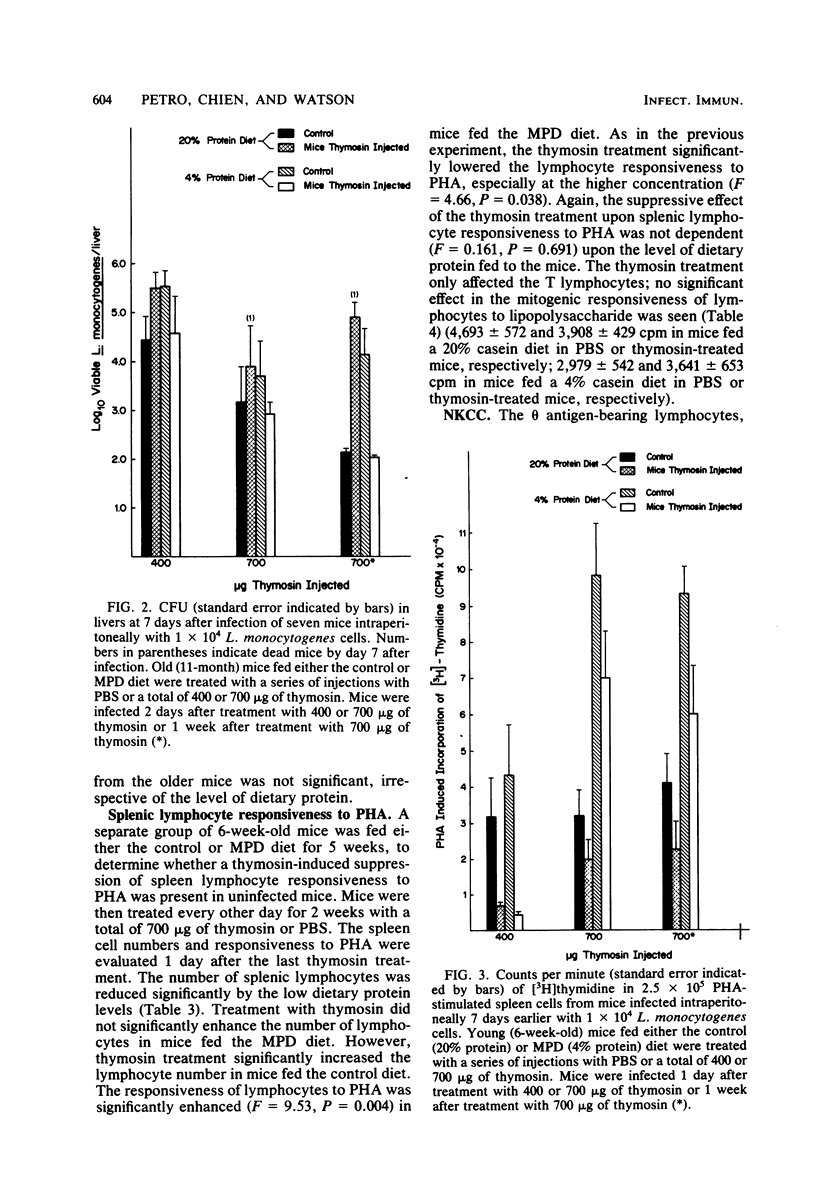
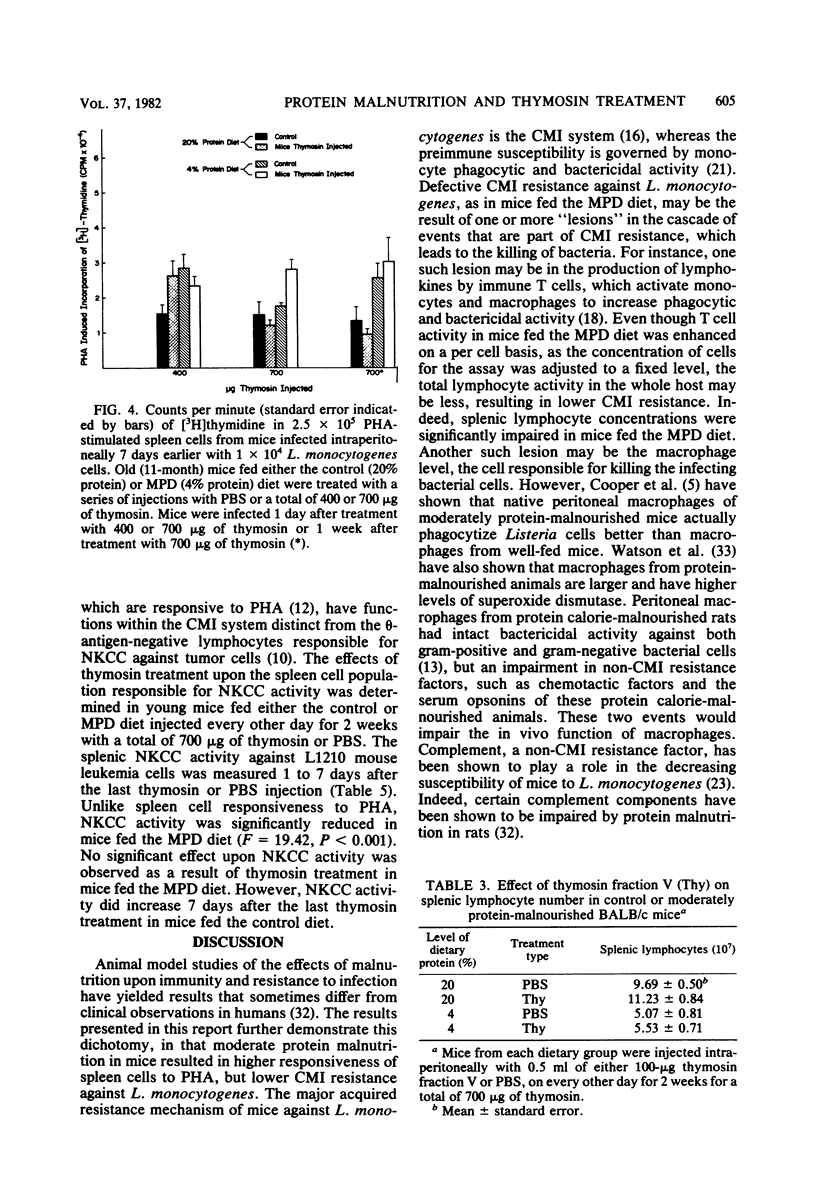
Cell-mediated immune reactivity, measured by lymphocyte responsiveness to phytohemagglutinin, was higher in both young or aged mice fed a 4% casein diet compared with age-matched controls. Treatment in vivo with bovine thymosin fraction V decreased the responsiveness to phytohemagglutinin of lymphocytes from mice fed either the control or moderately protein-deficient diets when compared with mice treated in vivo with saline. Resistance against Listeria monocytogenes, known to be a cell-mediated immune function, was impaired in young and aged mice which were fed the low-protein diet. Treatment with thymosin was able to significantly improve the cell-mediated immune resistance to L. monocytogenes of moderately protein-malnourished mice. Thymosin treatment impaired the resistance to L. monocytogenes of young or aged mice fed the control diet. The splenic natural killer cell cytotoxicity of protein-malnourished mice was impaired compared with that of mice fed the control diet. Treatment with thymosin did not restore the natural killer cell cytotoxic activity in protein-malnourished mice, but did enhance that activity in control mice.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beatty D. W., Dowdle E. B. The effects of kwashiorkor serum on lymphocyte transformation in vitro. Clin Exp Immunol. 1978 Apr;32(1):134–143. [PMC free article] [PubMed] [Google Scholar]
- Bongiorni-Malavé I., Pocino M. Abnormal regulatory control of the antibody response to heterologous erythrocytes in protein-calorie malnourished mice. Clin Immunol Immunopathol. 1980 May;16(1):19–29. doi: 10.1016/0090-1229(80)90162-2. [DOI] [PubMed] [Google Scholar]
- Bounous G., Kongshavn P. A. The effect of dietary amino acids on immune reactivity. Immunology. 1978 Aug;35(2):257–266. [PMC free article] [PubMed] [Google Scholar]
- Chandra R. K. Cell-mediated immunity in nutritional imbalance. Fed Proc. 1980 Nov;39(13):3088–3092. [PubMed] [Google Scholar]
- Chandra R. K. Serum thymic hormone activity in protein-energy malnutrition. Clin Exp Immunol. 1979 Nov;38(2):228–230. [PMC free article] [PubMed] [Google Scholar]
- Cooper W. C., Good R. A., Mariani T. Effects of protein insufficiency on immune responsiveness. Am J Clin Nutr. 1974 Jun;27(6):647–664. doi: 10.1093/ajcn/27.6.647. [DOI] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. Effect of dietary proteins and amino acids on the susceptibility of mice to bacterial infections. J Exp Med. 1958 Jul 1;108(1):69–81. doi: 10.1084/jem.108.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Hof H. Cell-mediated resistance to infection with Listeria monocytogenes in nude mice. Infect Immun. 1977 Feb;15(2):382–385. doi: 10.1128/iai.15.2.382-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracey M., Suharjono, Sunoto, Stone D. E. Microbial contamination of the gut: another feature of malnutrition. Am J Clin Nutr. 1973 Nov;26(11):1170–1174. doi: 10.1093/ajcn/26.11.1170. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. II. discriminating stimulation of lymphocyte subpopulations by phytomitogens and heterologous antilymphocyte sera. Clin Exp Immunol. 1972 Mar;10(3):525–536. [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Douglas S. D., Hammer G., Braden K. Antibacterial functions of macrophages in experimental protein-calorie malnutrition. II. Cellular and humoral factors for chemotaxis, phagocytosis, and intracellular bactericidal activity. J Infect Dis. 1978 Aug;138(2):134–142. doi: 10.1093/infdis/138.2.134. [DOI] [PubMed] [Google Scholar]
- Khorshidi M., Mohagheghpour N. Effect of protein deficiency on suppressor cells. Infect Immun. 1979 Jun;24(3):770–773. doi: 10.1128/iai.24.3.770-773.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low T. L., Thurman G. B., Chincarini C., McClure J. E., Marshall G. D., Hu S. K., Goldstein A. L. Current status of thymosin research: evidence for the existence of a family of thymic factors that control T-cell maturation. Ann N Y Acad Sci. 1979;332:33–48. doi: 10.1111/j.1749-6632.1979.tb47095.x. [DOI] [PubMed] [Google Scholar]
- Malavé I., Layrisse M. Immune response in malnutrition. Differential effect of dietary protein restriction on the IgM and IgG response to alloantigens. Cell Immunol. 1976 Feb;21(2):337–343. doi: 10.1016/0008-8749(76)90061-7. [DOI] [PubMed] [Google Scholar]
- Mandel T. E., Cheers C. Resistance and susceptibility of mice to bacterial infection: histopathology of listeriosis in resistant and susceptible strains. Infect Immun. 1980 Dec;30(3):851–861. doi: 10.1128/iai.30.3.851-861.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall G. D., Jr, Thurman G. B., Rossio J. L., Goldstein A. L. In vivo generation of suppressor T cells by thymosin in congenitally athymic nude mice. J Immunol. 1981 Feb;126(2):741–744. [PubMed] [Google Scholar]
- Petit J. C. Resistance to listeriosis in mice that are deficient in the fifth component of complement. Infect Immun. 1980 Jan;27(1):61–67. doi: 10.1128/iai.27.1.61-67.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petro T. M., Bhattacharjee J. K. Effect of dietary essential amino acid limitations upon the susceptibility to Salmonella typhimurium and the effect upon humoral and cellular immune responses in mice. Infect Immun. 1981 Apr;32(1):251–259. doi: 10.1128/iai.32.1.251-259.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petro T. M., Watson R. R. Resistance to L1210 mouse leukemia cells in moderately protein-malnourished BALB/c mice treated in vivo with thymosin fraction V. Cancer Res. 1982 Jun;42(6):2139–2145. [PubMed] [Google Scholar]
- Purkayastha S., Kapoor B. M., Deo M. G. Influence of protein deficiency on homograft rejection and histocompatibility antigens in rats. Indian J Med Res. 1975 Aug;63(8):1150–1154. [PubMed] [Google Scholar]
- Schopfer K., Douglas S. D. In vitro studies of lymphocytes from children with kwashiorkor. Clin Immunol Immunopathol. 1976 Jan;5(1):21–30. doi: 10.1016/0090-1229(76)90146-x. [DOI] [PubMed] [Google Scholar]
- Scrimshaw N. S., Taylor C. E., Gordon J. E. Interactions of nutrition and infection. Monogr Ser World Health Organ. 1968;57:3–329. [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wara D. W., Goldstein A. L., Doyle N. E., Ammann A. J. Thymosin activity in patients with cellular immunodeficiency. N Engl J Med. 1975 Jan 9;292(2):70–74. doi: 10.1056/NEJM197501092920204. [DOI] [PubMed] [Google Scholar]
- Watson R. R., Haffer K. Modifications of cell-mediated immune responses by moderate dietary protein stress in immunologically immature and mature BALB/c mice. Mech Ageing Dev. 1980 Mar;12(3):269–278. doi: 10.1016/0047-6374(80)90050-0. [DOI] [PubMed] [Google Scholar]
- Watson R. R., McMurray D. N. The effects of malnutrition on secretory and cellular immune processes. CRC Crit Rev Food Sci Nutr. 1979 Dec;12(2):113–159. doi: 10.1080/10408397909527275. [DOI] [PubMed] [Google Scholar]
- Watson R. R., Rister M., Baehner R. L. Superoxide dismutase activity in polymorphonuclear leukocytes and alveolar macrophages of protein malnourished rats and guinea pigs. J Nutr. 1976 Dec;106(12):1801–1808. doi: 10.1093/jn/106.12.1801. [DOI] [PubMed] [Google Scholar]
- Weindruch R. H., Suffin S. C. Quantitative histologic effects on mouse thymus of controlled dietary restriction. J Gerontol. 1980 Jul;35(4):525–531. doi: 10.1093/geronj/35.4.525. [DOI] [PubMed] [Google Scholar]
- Wolf R. E. Thymosin-induced suppression of proliferative response of human lymphocytes to mitogens. J Clin Invest. 1979 Apr;63(4):677–683. doi: 10.1172/JCI109350. [DOI] [PMC free article] [PubMed] [Google Scholar]



