Abstract
Our research to seek active compounds against human colorectal cancer from the root of Alkanna tinctoria (L.) Tausch led to the isolation of two naphthoquinones, alkannin (1) and angelylalkannin (2). The antiproliferative effects of the two compounds on human colon cancer cells HCT-116 and SW-480 were determined by MTS method. Cell cycle profile and cell apoptosis were determined using flow cytometry. Both of the two compounds showed significant inhibitory effects on the cancer cells. For alkannin (1) and angelylalkannin (2), the IC50 values were 2.38 and 4.76 µM for HCT-116 cells, while for SW-480 cells, they were 4.53 and 7.03 µM, respectively. The potential antiproliferative mechanisms were also explored. At concentrations between 1–10 µM, both compounds arrested the cell cycle at the G1 phase and induced cell apoptosis.
Keywords: Alkanna tinctoria (L.) Tausch, naphthoquinone, alkannin, angelylalkannin, human colorectal cancer cells, antiproliferation, cell cycle, apoptosis
INTRODUCTION
Colorectal cancer is one of the leading causes of cancer-related death and one of the most commonly diagnosed cancers in the West and worldwide (Engstrom et al., 2005; Siegel et al., 2012). In addition to surgical resection, many patients often need supplemental chemotherapy or radiotherapy. Commonly used chemotherapeutic agents have obvious adverse events and dose-limiting toxicity. Adding more drugs or increasing the dosages also increases the probability of adverse effects, potentially leading to patients’ refusal to continue the potentially curative chemotherapy (Schnell, 2003; Venook, 2005). As a result, improvements in treating patients with colorectal cancer are necessary.
Throughout the last two decades, botanicals with effective anticancer activities have been evaluated (Garodia et al., 2007; Li et al., 2010). The use of herbal medicines is also on the rise in cancer patients (Lee et al., 2006; Tian et al., 2010). Long-term consumption of certain botanicals might be associated with reduced cancer incidence and thus has an epidemiological basis for further investigation. Research that explores new botanical candidates with potential anticancer effects is needed for the development of safe and efficacious anticancer therapies (Hemaiswarya and Doble, 2006; Bemis et al., 2006).
Alkanna tinctoria (L.) Tausch (Boraginaceae) is widely distributed in Europe and western Asia. Its root has been used as a dye for fabrics, cosmetics, and food and as botanical drugs for ulcers, inflammation, and wounds (Papageorgiou et al., 1977). Previous phytochemical studies on the plant have resulted in the isolation of a series of naphthoquinone pigments, alkannin and its derivatives (Papageorgiou et al., 1977; Papageorgiou et al., 1980). Some of these compounds have been found to exhibit biological activities, including cytotoxic, antimicrobial, anti-Leishmanial, and anti-inflammatory activities (Papageorgiou et al., 1977; Papageorgiou et al., 1979; Papageorgiou et al., 1980; Plyta et al., 1998; Assimopoulou and Papageorgiou, 2005; Ali et al., 2011). However, the effects of alkannin and its derivatives on human colorectal cancer have not been evaluated.
In this study, chromatographic purification afforded two major naphthoquinones, alkannin (1) and angelylalkannin (2), from the root of A. tinctoria. Both of the compounds showed significant growth inhibitory effects on two human colorectal cancer cell lines, HCT-116 and SW-480. To further explore the potential mechanisms of antiproliferation, the activities of the two compounds on cell cycle and apoptosis were determined.
MATERIALS AND METHODS
Instrument procedures
Optical rotations were obtained using a DIP-360 digital polarimeter (JASCO, Easton, USA). NMR spectra were recorded on a JEOL ECX 400 NMR spectrometer (JEOL, Tokyo, Japan). ESI-TOFMS experiments utilized a JEOLAccuTOF™LC 1100 mass spectrometer (JEOL, Tokyo, Japan). Column chromatography was performed on silica gel 60 (230–400 mesh, Nacalai Tesque Inc., Kyoto, Japan) and Sephadex LH-20 gel (Uppsala, Sweden). TLC was performed on Kieselgel 60 F254 (Merck, Damstadt, Germany) plates. Spots were visualized by spraying with 10% aqueous H2SO4 solution, followed by heating.
Plant material and purification of compounds
The roots of A. tinctoria were donated from Prof. Tibor Wenger, Semmelweis University, Budapest, Hungary and authenticated by Prof. Yukihiro Shoyama. A voucher specimen (201112) was deposited at the Herbarium of Faculty of Pharmaceutical Science, Nagasaki International University. The air-dried sample (900 g) was pulverized and then extracted with 95% EtOH under sonication (2 L × 3), and the combined extracts were concentrated in vacuo to dryness. The obtained EtOH residue (35 g) was suspended in H2O (0.5 L) and partitioned with CHCl3 (0.5 L × 3) to obtain CHCl3-soluble fraction (pigment fraction; 14 g). The pigment fraction was fractionated on a silica gel column with a gradient of hexane-EtOAc (20:1→0:1) to obtain five fractions, Fr.1.1–1.5. Fr.1.1 (2.1 g) was repeatedly chromatographed on a silica gel column with hexane-EtOAc (20:1), followed by a sephadex LH-20 column with MeOH as an eluent to obtain angelylalkannin [2, 950 mg, red syrup, = −28° (c 0.20, CHCl3), ESI-MS m/z 371 [M+H]+]. Fr.1.4 (500 mg) was subjected to a silica gel column with hexane-EtOAc (5:1), followed by a sephadex LH-20 column with MeOH as an eluent to obtain alkannin [1, 25 mg, red amorphous powder, = −34° (c 0.30, CHCl3), ESI-MS m/z 289 [M+H]+].
Bioassay materials and reagents
All cell culture plasticware were obtained from Falcon Labware (Franklin Lakes, NJ) and Techno Plastic Products (Trasadingen, Switzerland). Trypsin, McCoy’s 5A and Leibovitz's L-15 media, fetal bovine serum and insulin-like growth factor, and phosphate buffered saline were obtained from Mediatech, Inc. (Herndon, VA). Dulbecco’s Modified Eagle’s medium was obtained from ATCC (Manassas, VA). Penicillin G/streptomycin and irinotecan were obtained from Sigma-Aldrich (St. Louis, MO). An MTS assay kit, CellTiter 96 Aqueous One Solution Cell Proliferation Assay, was obtained from Promega (Madison, WI). PI/RNase staining buffer and annexin V-FITC apoptosis detection kit were obtained from BD Biosciences (Rockville, MD).
Cell culture
Human colorectal cancer cells (HCT-116 and SW-480) and rat intestinal epithelial cells (IEC-6, as normal cells) were obtained from American Type Culture Collection (ATCC). Cells were routinely grown in McCoy's 5A medium (for HCT-116) or Leibovitz's L-15 medium (for SW-480) or Dulbecco’s Modified Eagle’s medium (for IEC-6) supplemented with 10% fetal bovine serum and penicillin/streptomycin (50 units/ml). Cells were maintained in a tissue culture flask and kept in a humidified incubator (5% CO2 in air at 37°C). The medium was changed every 2–3 days. When the cells reached 70%–80% confluence, they were trypsinized, harvested, and seeded into a new tissue culture flask.
Cell proliferation analysis
The effects of alkannin (1) and angelylalkannin (2) on the proliferation of colorectal cancer cells were determined using an MTS assay. Cancer cells were plated into a 96-well plate at a density of 1×104 cells/well. After seeding for 24 h, the cells were treated with alkannin (1) or angelylalkannin (2) at various concentrations. Data from this study were then compared to our previous reports using 5-FU in the same experimental condition to serve as a positive control. All experiments were performed in triplicate. At the end of the sample exposure period, the medium of each well was discarded and 100 µL of fresh medium and 20 µL of CellTiter 96 aqueous solution were added. The plate was returned to the incubator where it remained for 1–4 h in a humidified atmosphere at 37°C. Then, 60 µL of medium from each well was transferred to an ELISA 96-well plate, and the absorbance of the formazan product was measured at 490 nm. The blank control was recorded by measuring the absorbance at 490 nm with wells containing medium mixed with Cell Titer 96 aqueous solution but no cells. Results were expressed as a percentage of control (vehicle set at 100%).
Cell cycle analysis
The cell cycle profile was assayed by flow cytometry after staining with PI/RNase, and the assay data from alkannin (1) and angelylalkannin (2) were compared. HCT-116 cells were seeded in 24-well tissue culture plates. On the second day, the medium was changed, and cells were treated with alkannin (1) or angelylalkannin (2) at different concentrations. Cells were incubated for 48 h before harvesting. The cells were fixed gently with 80% ethanol before being placed in a freezer for 2 h. They were then treated with 0.25% Triton X-100 for 5 min in an ice bath. The cells were resuspended in 30 µL of phosphate buffered saline (PBS) containing 40 µg/mL propidium iodide and 0.1 mg/mL RNase. Cells were incubated in a dark room for 20 min at room temperature before cell cycle analysis with a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) and FlowJo software (Ashland, OR). For each measurement, at least 10,000 cells were counted.
Apoptotic analysis
HCT-116 cells were seeded in 24-well tissue culture plates. After 24 h, the medium was changed and alkannin (1) or angelylalkannin (2) were added. After treatment for 48 h, cells floating in the medium were collected. The adherent cells were detached with 0.05% trypsin. Then culture medium containing 10% FBS (and floating cells) was added to inactivate trypsin. When gentle pipetting was completed, the cells were centrifuged for 5 min at 1500g. The supernatant was removed and cells were stained with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) according to the manufacturer's instructions. Untreated cells were used as control for double staining. Immediately after staining, the cells were analyzed by a FACScan flow cytometer. For each measurement, at least 20,000 cells were counted.
Statistical analysis
Data are presented as mean ± standard error (S.E.). A one-way ANOVA was employed to determine statistical significance of results. In some cases, Student’s t-test was used for comparing two groups. The level of statistical significance was set at P< 0.05.
RESULTS AND DISCUSSION
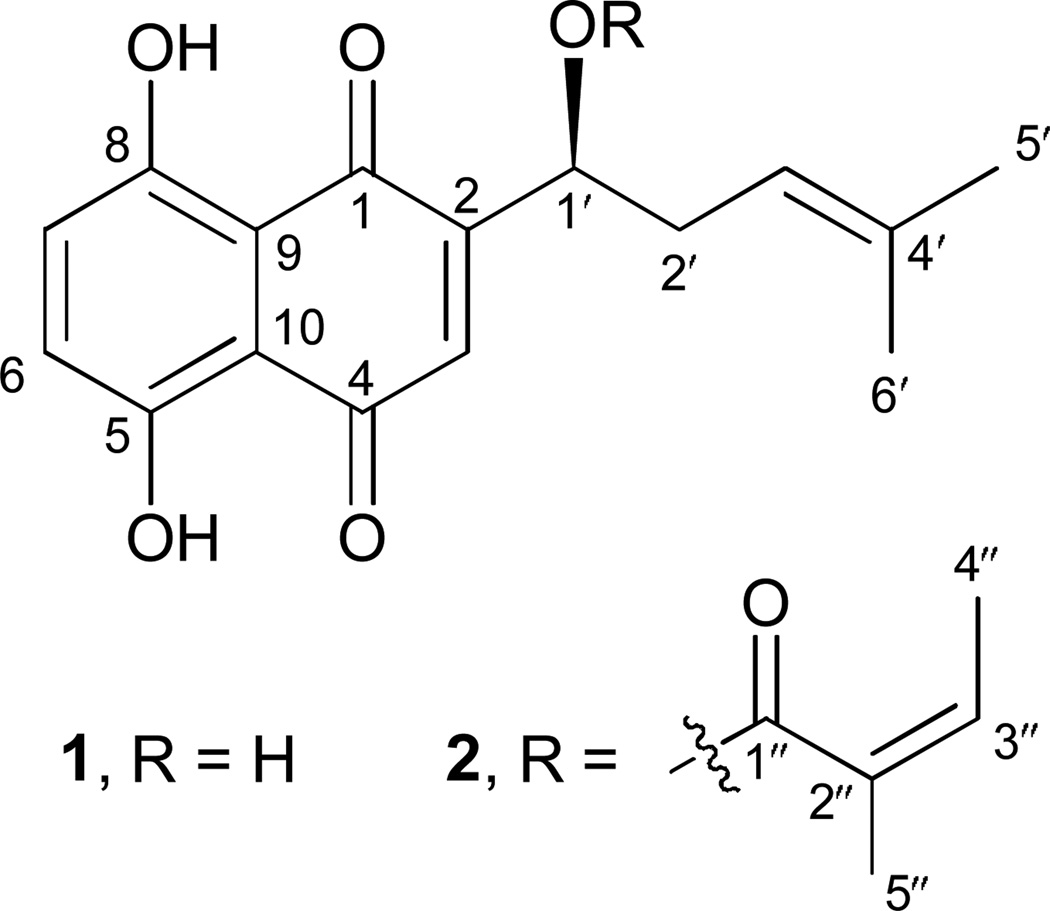
The ethanolic extract of the root of A. tinctoria was partitioned between CHCl3 and water to give the fraction containing pigments, followed by column chromatography on silica gel and Sephadex LH-20 to yield alkannin (1) and angelylalkannin (2) (Fig. 1). Their structures were confirmed by NMR and MS spectra and compared with those previously reported (Papageorgiou et al., 1977; Papageorgiou et al., 1980).
Figure 1.
The structures of alkannin (1) and angelylalkannin (2).
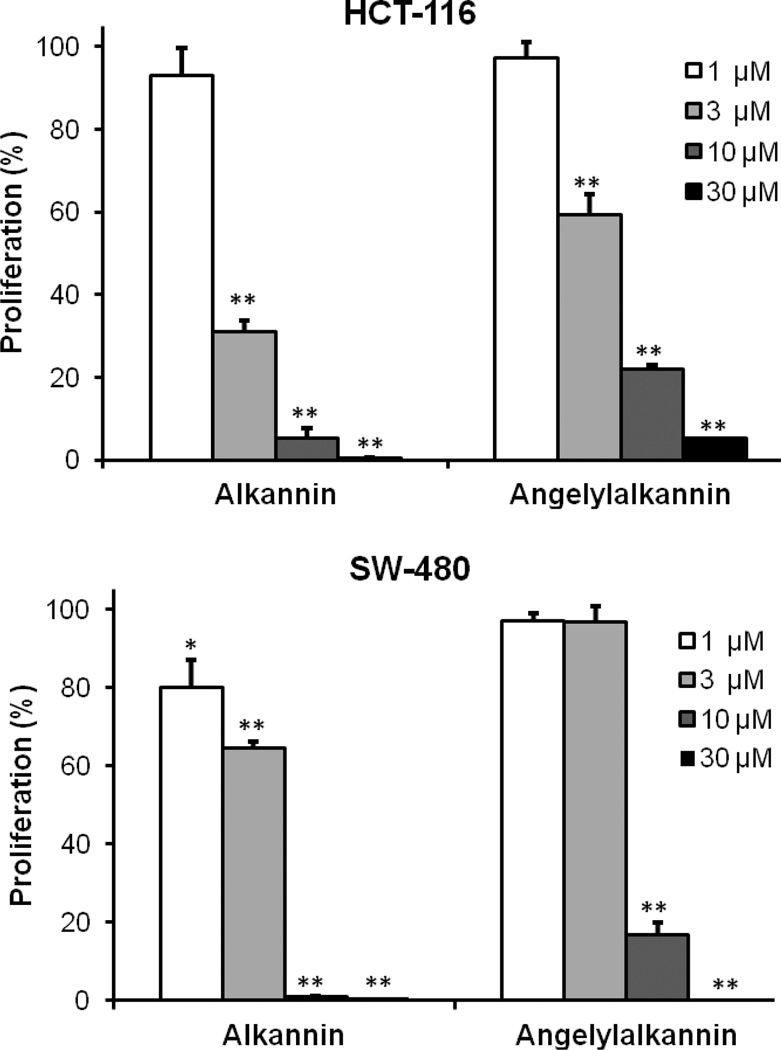
After treatment with alkannin (1) or angelylalkannin (2) for 48 h, the proliferation of HCT-116 and SW-480 cells was suppressed dose dependently. Concentrations of each compound at 1, 3, and 10 µM were tested (Fig. 2). For the HCT-116 cells, after treatment with 1, 3, and 10 µM of alkannin (1) for 48 h, cell proliferation was inhibited by 6.9%, 68.9% and 94.8%, respectively; while 1, 3, and 10 µM of angelylalkannin (2) inhibited cell growth by 2.7%, 40.7% and 78.1%, respectively. On SW-480 cells, the antiproliferative effect of 1, 3, and 10 µM of alkannin (1) was 20.1%, 35.5% and 99.2%, respectively; while results with angelylalkannin (2) were 2.9%, 3.3% and 83.3%, respectively. With 30 µM of either compound, cell growth was inhibited absolutely in both cell lines. The IC50 of alkannin (1) and angelylalkannin (2) for HCT-116 cells were 2.38 µM and 4.76 µM; on SW-480 cells they were 4.53 µM and 7.03 µM, respectively.
Figure 2.
Antiproliferative effects of alkannin (1) and angelylalkannin (2) on human colorectal cancer cells. HCT-116 and SW-480 cells were treated with the compounds at various concentrations for 48 h. Cell proliferation was determined using MTS method. Data are presented as mean ± standard error. *, P<0.05; **, P<0.01 vs. control.
We previously evaluated the effects of a commonly used chemotherapeutic agent, fluorouracil (5-FU), on HCT-116 and SW-480 cell lines (Wang et al., 2007; Li et al., 2009) in the same experimental conditions as this study. Thus, our 5-FU data can be considered a positive control for the present study. We observed that at a 10 µM concentration, 5-FU showed approximately 35% antiproliferative activity. In this study, we observed that compared to 5-FU, alkannin (1) and angelylalkannin (2) possessed significantly stronger antiproliferative activity at the same concentration.
In this study, we also used rat intestinal epithelial cells (IEC-6) to evaluate the effect of alkannin (1) and angelylalkannin (2) on these normal intestinal cells. We observed that at 10 µM of alkannin (1) and angelylalkannin (2), the proliferation was 75.5% and 88.3%, respectively. However, the proliferations of alkannin (1) and angelylalkannin (2) on HCT-116 cells were 0.8% and 16.7%, respectively. Thus, compared to the significant antiproliferative effect of alkannin (1) and angelylalkannin (2) on colorectal cancer cells, IEC-6 cells were largely not affected.
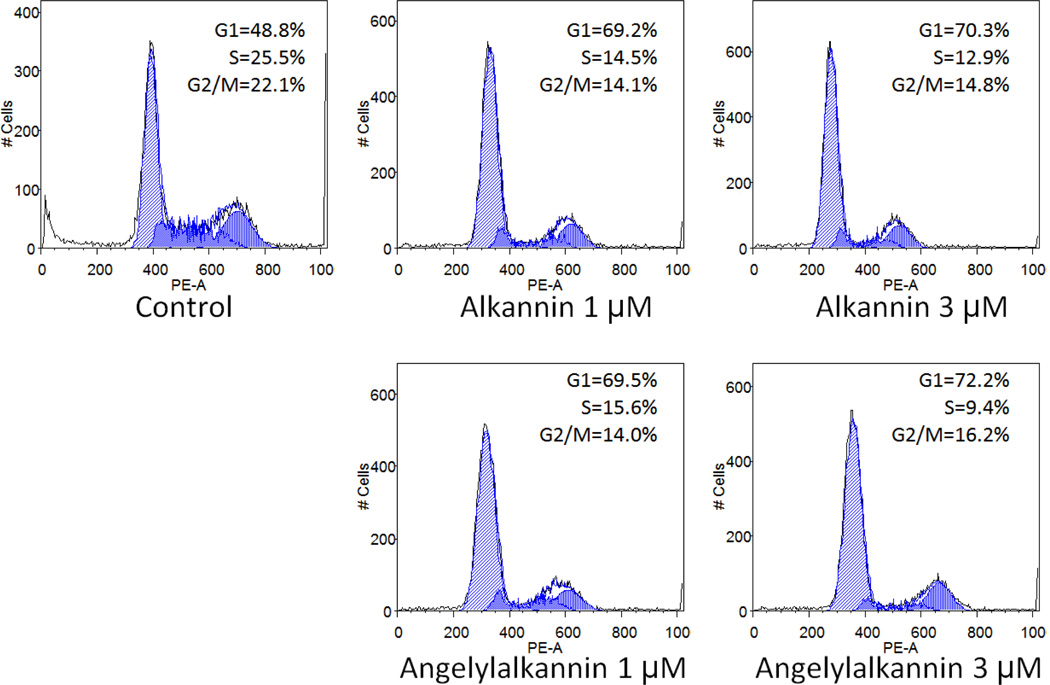
As shown in Fig. 3, compared with control (48.8% in G1 phase, 25.5% in S phase, and 22.1% in G2/M phase), treatment with alkannin (1) gradually changed the cell cycle profile of HCT-116 cells. After treatment with alkannin (1) at 1 µM, the distribution was 69.2% in G1 phase, 14.5% in S phase, and 14.1% in G2/M phase. At an alkannin concentration of 3 µM, the fractions of cells were 70.3% (G1 phase), 12.9% (S phase) and 14.8% (G2/M phase). After treatment with angelylalkannin (2) at 1 µM, the cell cycle profile was 69.5% (G1 phase), 15.6% (S phase) and 14.0% (G2/M phase), while with angelylalkannin (2) at 3 µM, the fractions of cells were 72.2% (G1 phase), 9.4% (S phase) and 16.2% (G2/M phase). Notably, both alkannin (1) and angelylalkannin (2) significantly arrested HCT-116 cells at the G1 phase at the tested concentrations.
Figure 3.
Effects of alkannin (1) and angelylalkannin (2) on HCT-116 cell cycle. HCT-116 cells were treated with 1 and 3 µM of the compounds for 48 h. Cell cycle profile was determined using flow cytometry after staining with PI/RNase. Percentages of cells in G1, S and G2/M phases are indicated.
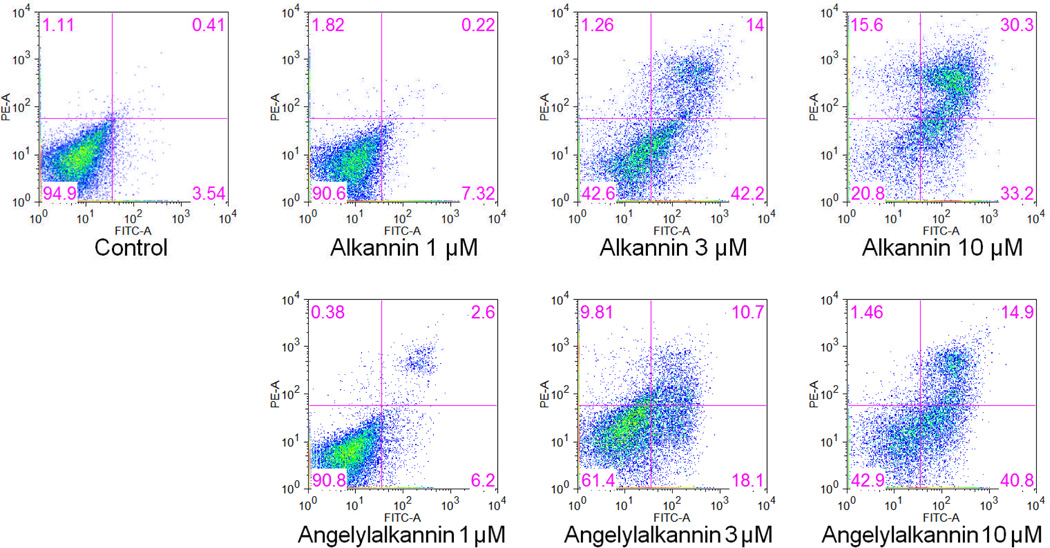
To further characterize the anticancer mechanisms of the two compounds, we performed an apoptotic assay (Fig. 4). After treatment for 48 h, the percentage of apoptotic cells (early and late apoptosis) induced by alkannin (1) at 1, 3, and 10 µM were 7.5%, 56.2% and 63.5%. The percentage of apoptotic cells induced by angelylalkannin (2) at concentrations of 1, 3, and 10 µM were 8.8%, 28.8 to 55.7%, respectively. Compared to control (4.0%), treatment with 3 µM or 10 µM of the two compounds significantly induced cancer cell apoptosis. These results suggested that the antiproliferative effects of alkannin (1) and angelylalkannin (2) could be mediated by the induction of apoptosis.
Figure 4.
Effects of alkannin (1) and angelylalkannin (2) on HCT-116 cell apoptosis. HCT-116 cells were treated with 1, 3, and 10 µM of the compounds for 48 h. Apoptosis was quantified using flow cytometry after staining with annexin V/PI.
Botanicals have been the major source of therapy in many traditional medical systems and have been used clinically for the treatment of a variety of diseases (Huang, 1999; Katoh et al., 2011). Botanical ingredients in natural products contain bioactive constituents with potential health benefits (Balunas and Kinghorn, 2005). The World Health Organization (WHO) reported that 65–80% of the world’s healthcare services consist of traditional medicines (Akerele, 1993).
Botanicals have been a significant resource for the discovery and use of efficacious chemotherapeutic agents (Mann, 2002). In addition to low cost, novel natural products could be effective against cancer, and their toxicity could be lower than other commonly used chemotherapeutic drugs. A. tinctoria could contain several effective anticancer compounds that may be used alone or as adjuncts to existing chemotherapy to improve efficacy and reduce drug-induced toxicity.
Alkannin (1) and angelylalkannin (2) are principle components of the red pigments from the roots of A. tinctoria along with some minor derivatives reported to date. Previous studies have shown that alkannin derivatives possess antiproliferative effects on several cancer cell lines, e.g. K562, GLC-82, CNE2, and Bel-7402 (Wu et al., 2005; Deng, 2010; Bogurcu et al., 2011). Recently, there have been reports on molecular mechanisms of their cytotoxic actions regarding apoptosis induction in K562 leukemia, GLC-82 adenocarcinoma, and Hep3B hepatocarcinoma cell lines, respectively (Deng, 2010; Bogurcu et al., 2011). The present study was the first to evaluate the therapeutic potential of the components from A. tinctoria against the human colorectal cancer.
In summary, two naphthoquinone compounds, namely alkannin (1) and angelylalkannin (2), were isolated and identified from the root of A. tinctoria. Both of these compounds presented significant inhibitory effects on the proliferation of the human colon cancer cell lines HCT-116 and SW-480. A cell cycle assay showed that they arrested the cell cycle at the G1 phase. They also showed apoptotic induction activity. Thus, the observed anticancer activities of these two compounds were related to cell cycle arrest and induction of apoptosis. These results provide promising information for the potential use of A. tinctoria, as well as some of the isolated compounds, in the treatment of colorectal cancer.
Acknowledgements
This work was supported in part by the NIH grants AT004418 and AT005362. One of the authors (N.H.T.) is grateful to the Japan Society for the Promotion of Science for a Postdoctoral Research Fellowship at Nagasaki International University. The authors acknowledge Prof. Tibor Wenger, Semmelweis University, Budapest, Hungary for the donation of A. tinctoria roots.
REFERENCES
- Akerele O. Nature's medicinal bounty: don't throw it away. World Health Forum. 1993;14:390–395. [PubMed] [Google Scholar]
- Ali A, Assimopoulou AN, Papageorgiou VP, Kolodziej H. Structure/antileishmanial activity relationship study of naphthoquinones and dependency of the mode of action on the substitution patterns. Planta Med. 2011;77:2003–2012. doi: 10.1055/s-0031-1280092. [DOI] [PubMed] [Google Scholar]
- Assimopoulou AN, Papageorgiou VP. Radical scavenging activity of Alkanna tinctoria root extracts and their main constituents, hydroxynaphthoquinones. Phytother Res. 2005;19:141–147. doi: 10.1002/ptr.1645. [DOI] [PubMed] [Google Scholar]
- Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Bogurcu N, Sevimli-Gur C, Ozmen B, Bedir E, Korkmaz KS. ALCAPs induce mitochondrial apoptosis and activate DNA damage response by generating ROS and inhibiting topoisomerase I enzyme activity in K562 leukemia cell line. Biochem Biophys Res Commun. 2011;409:738–744. doi: 10.1016/j.bbrc.2011.05.078. [DOI] [PubMed] [Google Scholar]
- Bemis DL, Capodice JL, Costello JE, Vorys GC, Katz AE, Buttyan R. The use of herbal and over-the-counter dietary supplements for the prevention of prostate cancer. Curr Urol Rep. 2006;7:166–174. doi: 10.1007/s11934-006-0017-x. [DOI] [PubMed] [Google Scholar]
- Deng R, Tang J, Xie BF, Feng GK, Huang YH, Liu ZC, Zhu XF. SYUNZ-16, a newly synthesized alkannin derivative, induces tumor cells apoptosis and suppresses tumor growth through inhibition of PKB/AKT kinase activity and blockade of AKT/FOXO signal pathway. Int J Cancer. 2010;127:220–229. doi: 10.1002/ijc.25032. [DOI] [PubMed] [Google Scholar]
- Engstrom PF, Benson AB, Chen YJ, Choti MA, Dilawari RA, Enke CA, Fakih MG, Fuchs C, Kiel K, Knol JA, Leong LA, Ludwig KA, Martin EW, Rao S, Saif MW, Saltz L, Skibber JM, Venook AP, Yeatman TJ National Comprehensive Cancer Network. Colon cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2005;3:468–491. doi: 10.6004/jnccn.2005.0024. [DOI] [PubMed] [Google Scholar]
- Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 2007;5:25–37. doi: 10.2310/7200.2006.029. [DOI] [PubMed] [Google Scholar]
- HemaIswarya S, Doble M. Potential synergism of natural products in the treatment of cancer. Phytother Res. 2006;20:239–249. doi: 10.1002/ptr.1841. [DOI] [PubMed] [Google Scholar]
- Huang KC. The Pharmacology of Chinese Herbs. Boca Raton, FL: CRC Press; 1999. [Google Scholar]
- Katoh A, Fukuda S, Fukusaki E, Hashimoto T, Hayasaki T, Kanaya S, Komura H, Nomoto K, Shojo M, Takeno KJ. Systems biology in a commercial quality study of the Japanese Angelica radix: toward an understanding of traditional medicinal plants. Am J Chin Med. 2011;39:757–777. doi: 10.1142/S0192415X11009172. [DOI] [PubMed] [Google Scholar]
- Lee TI, Chen HH, Yeh ML. Effects of chan-chuang qigong on improving symtom and psychological distress in chemotherapy patients. Am J Chin Med. 2006;34:37–46. doi: 10.1142/S0192415X06003618. [DOI] [PubMed] [Google Scholar]
- Li H, Tan G, Jiang X, Qiao H, Pan S, Jiang H, Kanwar JR, Sun X. Therapeutic effects of matrine on primary and metastatic breast cancer. Am J Chin Med. 2010;38:1115–1130. doi: 10.1142/S0192415X10008512. [DOI] [PubMed] [Google Scholar]
- Li XL, Wang CZ, Sun S, Mehendale SR, Du W, He TC, Yuan CS. American ginseng berry enhances chemopreventive effect of 5-FU on human colorectal cancer cells. Oncol Rep. 2009;22:943–952. [PubMed] [Google Scholar]
- Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer. 2002;2:143–148. doi: 10.1038/nrc723. [DOI] [PubMed] [Google Scholar]
- Papageorgiou VP. A new pigment of Alkanna tinctoria having naphthaquinone structure. Planta Med. 1977;31:390–394. [Google Scholar]
- Papageorgiou VP, Winkler A, Sagredos AN, Digenis GA. Studies on the relationship of structure to antimicrobial properties of naphthaquinones and other constituents of Alkanna tinctoria. Planta Med. 1979;35:56–60. doi: 10.1055/s-0028-1097184. [DOI] [PubMed] [Google Scholar]
- Papageorgiou VP, Digenis GA. Isolation of two new Alkannin esters from Alkanna tinctoria. Planta Med. 1980;39:81–84. [Google Scholar]
- Plyta ZF, Li T, Papageorgiou VP, Mellidis AS, Assimopoulou AN, Pitsinos EN, Couladouros EA. Inhibition of topoisomerase I by naphthoquinone derivatives. Bioorg Med Chem Lett. 1998;23:3385–3390. doi: 10.1016/s0960-894x(98)00600-3. [DOI] [PubMed] [Google Scholar]
- Schnell FM. Chemotherapy-induced nausea and vomiting: the importance of acute antiemetic control. Oncologist. 2003;8:187–198. doi: 10.1634/theoncologist.8-2-187. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Tian JH, Liu LS, Shi ZM, Zhou ZY, Wang L. A randomized controlled pilot trial of "Feiji Recipe" on quality of life of non-small cell lung cancer patients. Am J Chin Med. 2010;38:15–25. doi: 10.1142/S0192415X10007646. [DOI] [PubMed] [Google Scholar]
- Venook A. Crictical evaluation of current treatments in metastatic colorectal cancer. Oncologist. 2005;10:250–261. doi: 10.1634/theoncologist.10-4-250. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Luo X, Zhang B, Song WX, Ni M, Mehendale S, Xie JT, Aung HH, He TC, Yuan CS. Notoginseng enhances anti-cancer effect of 5-fluorouracil on human colorectal cancer cells. Cancer Chemother Pharmacol. 2007;60:69–79. doi: 10.1007/s00280-006-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HQ, Huang ZS, Bu XZ, Shen YD, Zhang ZL, Xie BF, Liu ZC, GU LQ, Chan AS. The molecular mechanisms involved in the cytotoxicity of alkannin derivatives. Eur J Med Chem. 2005;40:1341–1345. doi: 10.1016/j.ejmech.2005.05.004. [DOI] [PubMed] [Google Scholar]