Abstract
Objective
Abnormal proliferation and migration of vascular smooth muscle cells (SMCs) are the key events in the progression of neointima formation in response to vascular injury. The goal of this study is to investigate the functional role of a potent oncogene YAP in smooth muscle phenotypic modulation in vitro and in vivo.
Methods and Results
In vitro in cell culture and in vivo in both mouse and rat arterial injury models YAP expression is significantly induced and correlated with the vascular SMC synthetic phenotype. Over-expression of YAP promotes SMC migration and proliferation while attenuating smooth muscle contractile gene expression. Conversely, knocking-down endogenous YAP in SMCs up-regulates smooth muscle gene expression but attenuates SMC proliferation and migration. Consistent with this, knocking-down YAP expression in a rat carotid balloon injury model and genetic deletion of YAP specifically in vascular SMCs in mouse after carotid artery ligation injury attenuates injury-induced smooth muscle phenotypic switch and neointima formation.
Conclusions
YAP plays a novel integrative role in smooth muscle phenotypic modulation by inhibiting smooth muscle-specific gene expression while promoting smooth muscle proliferation and migration in vitro and in vivo. Blocking the induction of YAP would be a potential therapeutic approach for ameliorating vascular occlusive diseases.
Keywords: YAP, smooth muscle, phenotypic modulation, neointima formation
Introduction
The Hippo pathway has been shown to play a critical role in controlling organ size and tumorigenesis by regulating cell proliferation1. The Hippo pathway was originally discovered in Drosophila and was named after the Drosophila Hippo kinase. Later genetic and biochemical studies revealed that the components of this pathway are highly conserved from flies to mammals, including Mst1/2, Lats1/2, Yes Associated Protein (YAP)1. Strikingly these human components can functionally rescue the corresponding Drosophila mutants in vivo, suggesting a functional conservation of these genes2. In mammals, cell contact, or other unknown mechanisms, trigger Mst1/2 kinase activation with subsequent phosphorylation and activation of Lats1/2 kinase, which then directly phosphorylates YAP S1273, 4. Phosphorylated YAP binds to 14-3-3 proteins, which retain YAP in cytoplasm by hindering nuclear import, thereby inactivating YAP function3. Mutation of YAP serine 127 to alanine (S127A) renders YAP resistant to Hippo kinase-induced phosphorylation, resulting in an elevated YAP function. Furthermore, mutation of all five Lats kinase phosphorylation motifs (5SA), including S127 in YAP creates an even more powerful YAP which, unlike the YAP S127A mutant, is able to potently transform NIH-3T3 cells5. Unphosphorylated YAP translocates into the nucleus where it binds to a variety of transcription factors, to regulate gene expression required for control of cell proliferation, thereby promoting cell growth and oncogenic transformation6. In support of YAP is a potent oncoprotein, YAP is over-expressed in a spectrum of human primary tumors7. Taken together, these studies demonstrated that YAP plays an essential role in tumorigenesis and YAP phosphorylation by Hippo pathway kinases is critical for regulating YAP function.
Smooth muscle cells (SMCs) are found in most hollow organs and control the involuntary contraction of these organs. Fully differentiated or mature SMCs are quiescent with little proliferation and are almost completely programmed for contraction with high expression of a unique array of contractile proteins such as smooth muscle α-actin, calponin, Hic-58, smooth muscle myosin heavy chain (SM MHC), 130kDa myosin light chain kinase (MLCK) and SM22α9. Vascular SMCs exhibit remarkable phenotypic plasticity. In response to environmental stimuli SMCs may modulate from a contractile phenotype to a proliferative and migratory phenotype (‘synthetic phenotype’) that contributes to vascular remodeling and lesion formation9. Although tremendous progress has been made in defining specific transcriptional mechanisms involved in regulating SMC contractile gene expression10, the mechanisms that coordinate SMC contractile and synthetic phenotype switching are poorly explored.
Several studies suggest that cancer and smooth muscle related diseases such as neointimal formation during atherosclerosis or following arterial injury share several common fundamental biological mechanisms11. For example, like tumor cells, following vascular injury SMCs are highly proliferative and migratory with loss of expression of differentiation markers9. Abnormal proliferation and migration of SMCs are the key events in atherosclerosis, neointimal hyperplasia, and the response to vascular injury. The SMC proliferation and migration are critical for early plaque development and therefore plaques can be considered as benign SMC tumors of the arterial wall11–13. Given that YAP has been demonstrated to be a potent oncoprotein to promote cell proliferation and migration, we hypothesize that YAP plays an essential role in smooth muscle phenotypic switching.
In this report, we demonstrate that expression of YAP is dramatically induced during smooth muscle phenotypic modulation to the synthetic state in vitro and in vivo. We show that YAP plays a critical role in coordinating the phenotypic modulation of SMCs, by inhibiting expression of smooth muscle differentiation genes and by promoting proliferative and migratory functions. Depletion of YAP expression following arterial injury alleviates the injury-induced the smooth muscle-specific gene down-regulation and attenuates neointima formation in vivo. This study suggests that components of the Hippo-YAP pathway may be appropriate therapeutic targets for ameliorating smooth muscle-related vascular diseases.
Materials and Methods
Rat aortic SMCs were prepared as previously reported8. Mouse carotid artery ligation and rat carotid artery balloon injury were performed as described in our previous reports8, 14. Full materials and methods are detailed in the “Supplement Material”.
Results
YAP expression is induced during smooth muscle phenotypic modulation
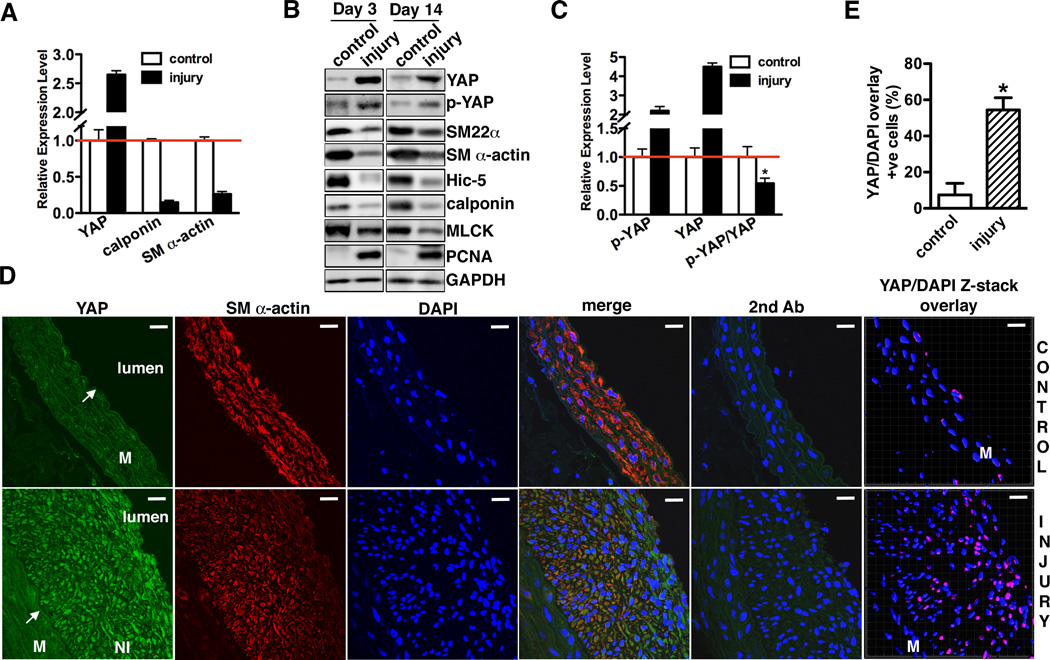
To explore the potential function of YAP in SMCs, first we examined the expression of YAP during smooth muscle phenotypic modulation. During phenotypic switch of vascular SMCs induced by cell culture, YAP expression was dramatically induced in parallel with expression of the synthetic SMC marker non-muscle MHC IIB (NMHCII-B)15, whereas expression of contractile SMC markers including SM22α, SM α-actin and Hic-5 were significantly reduced (Supplemental Figure IA). The phosphorylated YAP (p-YAP, S127) signal was elevated as well, suggesting the YAP upstream kinase pathway was activated after vascular SMC cultured in growth medium. Furthermore, immunostaining experiments revealed that YAP primarily localized in the nuclei of cultured vascular SMCs, although consistent with the observed high levels of p-YAP by Western blot, YAP signal was also seen in cytoplasm (Supplemental Figure IB). We next sought to examine YAP expression during smooth muscle phenotypic modulation in vivo. Consistent with the in vitro data we found that YAP was significantly elevated in a rat carotid artery balloon injury model that resembles angioplasty in humans16, both 3 and 14 days following injury (Figure 1A, B and D). YAP expression coincides with increased expression of proliferative smooth muscle marker, PCNA (Figure 1B) and down-regulation of smooth muscle contractile genes (Figure 1A and B). Similarly elevated YAP expression was observed 3, 7 and 21 days following mouse carotid ligation injury that is characterized by phenotypic modulation of medial SMCs17, as indicated by the data from qRT-PCR, Western blot and immunohistological staining assays (Supplemental Figure IIA, B and C). In contrast, genes for smooth muscle contractile phenotype including SM α-actin, Hic-5 and SM22α were significantly reduced (Supplemental Figure IIB). In agreement with a previous study showing arterial injury can induce and activate Mst118, an up-stream Hippo kinase of YAP, we found balloon injury increases p-YAP level (Figure 1B). However, total YAP expression increased significantly more than the p-YAP signal as the percentage of YAP phosphorylated following injury actually decreased by 50% (Figure 1C). Consistent with this finding, immunohistochemical staining demonstrated that YAP is highly expressed in the synthetic neointima SMCs where it is localized in the nuclei of about 50% of the SMCs (Figure 2D and E). Furthermore, the expression YAP was significantly increased in the nuclear fractions from balloon injured carotid arteries (Supplemental Figure III). These data suggested that the excessive induction of YAP can override the phosphorylation-induced repression of YAP mediated by upstream Hippo pathway kinases, suggesting a feed-back regulatory loop. Taken together, these in vitro and in vivo data demonstrate that YAP expression and nuclear localization is positively correlated with the “synthetic” smooth muscle phenotype.
Figure 1. YAP expression is induced in balloon injured rat carotid arteries.
A. qRT-PCR was performed to assess YAP and smooth muscle contractile gene mRNA expression in rat carotid arteries 14 days following balloon injury. Data are expressed relative to control uninjured vessels (set to 1) after normaliztion to RPLP0 as an internal control. N=3. B. 3 or 14 days after rat carotid artery balloon injury control or injured arterial vessels were harvested for Western blotting to assess protein expression as indicated. A representative blot is shown from 4 independent experiments. C. Quantification of immunoblot signals of p-YAP, YAP or p-YAP/YAP shown in “B” of 14 day post injury vessels. *p<0.05. D. 14 days after rat carotid artery balloon injury sections from injured and control arteries were stained for YAP (green) or SM α-actin (red). Samples treated with secondary antibody alone served as negative control. All sections were counter-stained by DAPI to visualize nuclei (blue). Co-localization of YAP and DAPI nuclear staining is shown in purple in control and injured samples by confocal microscope Z-stack scanning and reconstructed by Imaris Bitplane software (far right panel). An arrow points to internal elastic lamina. M: media. NI: neointima. Scale bar, 20um. E. Quantification of the percentage of SMCs that YAP and DAPI staining were co-localized in panel “D” (far right panel). Data were collected from 3 independent experiments. *p<0.05
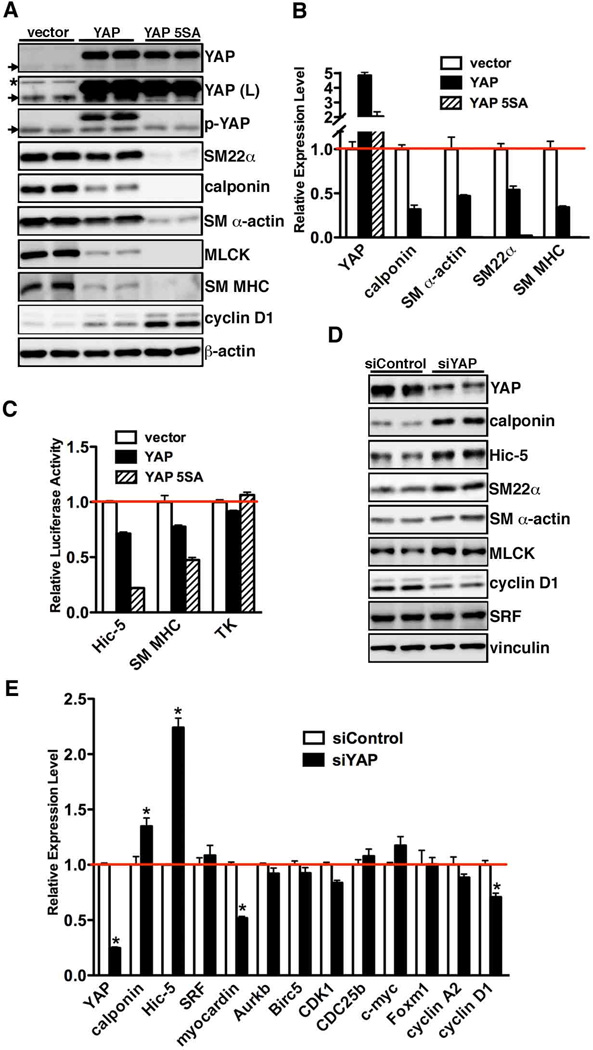
Figure 2. YAP attenuates smooth muscle gene expression.
A. Cultured rat aortic SMCs were transduced with retrovirus encoding wild-type or 5SA mutant YAP, and then harvested for Western blotting or qRT-PCR (B) to evaluate gene expression as indicated. The cells infected with empty retroviral vector served as a control. Arrows point to endogenous YAP or p-YAP signal and an asterisk denotes a non-specific signal after long exposure of the YAP blot (L). As expected, 5SA, a mutant resistant to Hippo kinase, cannot be detected by anti-pYAP (S127) antibody. C. Expression plasmids for WT YAP or YAP 5SA were transfected with either Hic-5, SM MHC or a control minimal thymidine kinase (TK) promoter-luciferase reporter genes into PAC1 SMCs and then promoter activity was measured by dual-luciferase reporter assay. Promoter activity was normalized to a renilla luciferase internal control and expressed relative to vector control transfections (set to 1, red line). Data are presented as mean±SEM of 8 samples. D. Silencing YAP or control RNA duplex was transfected into cultured rat aortic SMCs for 48 hours and then cells were harvested for Western blot or qRT-PCR (E) analysis as indicated. *p<0.05.
YAP represses smooth muscle-specific gene expression
As YAP expression is significantly induced during smooth muscle phenotypic modulation in vitro and in vivo, we next sought to investigate the function of YAP in regulating expression of smooth muscle-specific genes by gain- or loss-of-function assays. Retrovirus encoding wild-type (WT) YAP, constitutively active YAP 5SA or empty control vector were transduced into rat aortic SMCs. We first performed phalloidin staining to examine the effects of over-expression of YAP and 5SA on SMC cytoskeleton organization. Data from this experiment demonstrated that over-expression WT YAP and YAP 5SA displayed dramatic cell morphological alteration. Moreover, cells expressing YAP 5SA lost cell-cell contact inhibition and grow on top of each other (Supplemental Figure IV). To further characterize YAP functions in SMCs, protein or RNA from rat aortic SMCs that transduced by WT YAP, YAP 5SA or empty control vector were harvested and smooth muscle gene expression quantitated by Western blotting and qRT-PCR. All smooth muscle-specific genes examined were significantly down-regulated 50–90% by over-expression of WT YAP or the 5SA YAP mutant at both the protein and mRNA level (Figure 2A and B). The YAP 5SA mutant that is resistant to inhibition induced by the Hippo pathway, was more potent in abrogating smooth muscle-specific gene expression compared to WT YAP (Figure 2A and B). Moreover, data from reporter assays revealed that WT YAP or YAP 5SA significantly inhibit smooth muscle-specific Hic-5 and SM MHC promoter activity (Figure 2C), suggesting the inhibitory effect on smooth muscle-specific gene expression by WT YAP or 5SA mutant YAP is at the transcriptional level. Consistently, depletion of YAP using siRNA significantly increased expression of smooth muscle markers in rat aortic SMCs (Figure 2D and E). Finally, over-expression YAP or YAP 5SA strongly induced the expression of cyclin D1, a cell cycle regulating gene that previously shown to promote cell proliferation19 (Figure 2A) while knock-down YAP significantly down-regulated cyclin D1 expression at both protein and mRNA levels in cultured vascular SMCs (Figure 2D and E). Silencing YAP has no effects on the expression of SRF, a key regulator for smooth muscle differentiation20. Together, these data demonstrate that YAP is a potent repressor for smooth muscle-specific gene expression while YAP is a strong inducer for cell proliferation gene expression.
YAP promotes SMC proliferation
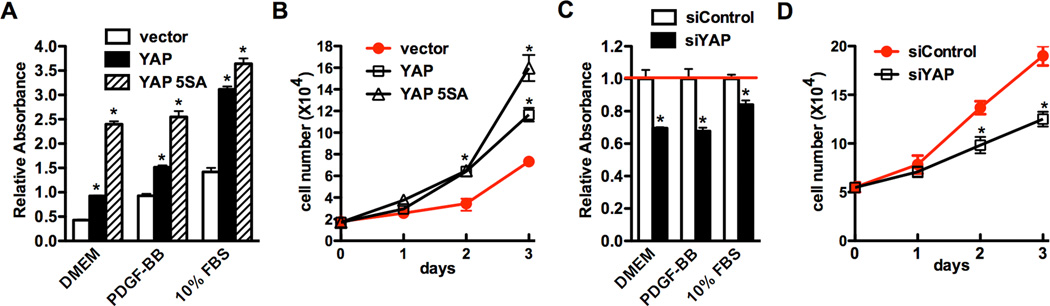
Proliferation of vascular SMCs plays a key role in the development of neointima thickening. Since YAP is induced in cell culture and following arterial injury where modulated SMCs are known to be proliferative and migratory, next we directly tested whether YAP is able to affect SMC proliferation by over-expressing YAP or 5SA mutant in rat aortic SMCs (Figure 3A, B) and silencing endogenous YAP with siRNA (Figure 3C and D). Data from these experiments revealed that the rates of SMC proliferation were stimulated by YAP over-expression while inhibited by silencing endogenous YAP expression, supporting a positive role for the YAP in regulating vascular SMC proliferation.
Figure 3. YAP promotes SMC proliferation.
A. Retrovirus encoding wild type or 5SA mutant YAP or empty vector were transduced into cultured rat aortic SMCs and then proliferation was measured using a cell proliferation WST-1 kit (Roche) in the different culture medium as indicated. *p<0.05. B. Virus transduced rat aortic SMCs were plated at equal density in 10% FBS medium and then cells were collected and counted at each time point as indicated. *p<0.05. C. Control or YAP silencing duplex were transfected into rat aortic SMCs and cell proliferation was measured using a WST-1 kit or cells were counted at each time point as described above (D). *p<0.05.
YAP promotes SMC migration
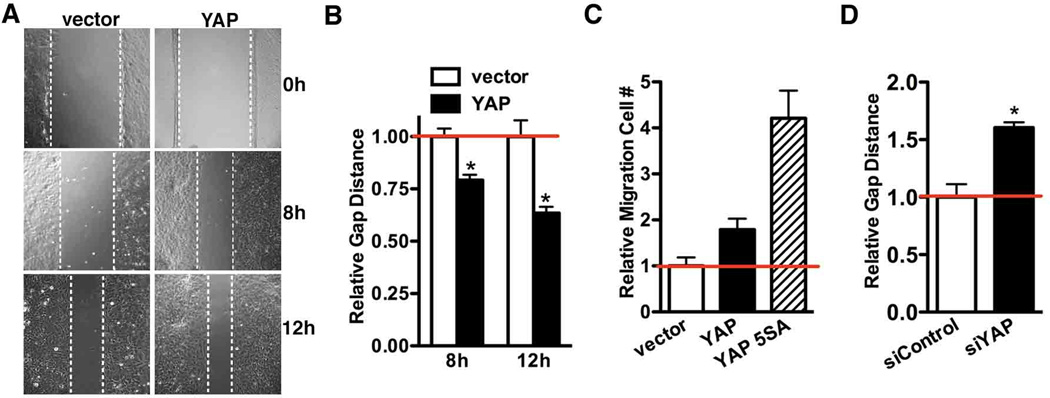
In response arterial injury SMC migration from medial layer is another key event to build neointima. Using a scratch wound healing assay, we found that over-expression of YAP in rat aortic SMCs significantly promotes “wound” closure which is known to be dependent primarily on cell migration over this time period (Figure 4A and B). Boyden chamber assays further confirmed YAP can promote SMC migration whereas 5SA mutant has a stronger effect (Figure 4C). In contrast, silencing endogenous YAP impaired wound closure (Figure 4D), suggesting YAP plays an important role in SMC migration.
Figure 4. YAP promotes SMC migration.
A. Rat aortic SMCs transduced with retrovirus encoding YAP or control vector were seeded in 6-well plates at confluent density. Cell migration was assayed 8 or 12-hour after scratching a ‘wound’ across the cell monolayer and the relative closure distance were measured (B). The gap distance in vector control cells was normalized and set to 1. Data presented are means±SE for 2 independent experiments with 5 independent fields counted in each experiment. C. Quantification of Boyden chamber assay to assess cell migration after 5h plating of virus transduced rat aortic SMCs. The cell numbers of control vector group were set to 1. D. Control or YAP silencing duplex were transfected into rat aortic SMCs over-night and the wound healing assay was performed to measure cell migration. The closure distance was measured 12hrs after wounding and plotted as described in “A” and “B”. *p<0.05.
Knock-down or knock-out of YAP in vivo prevents arterial injury-induced smooth muscle phenotypic modulation and attenuates neointima formation
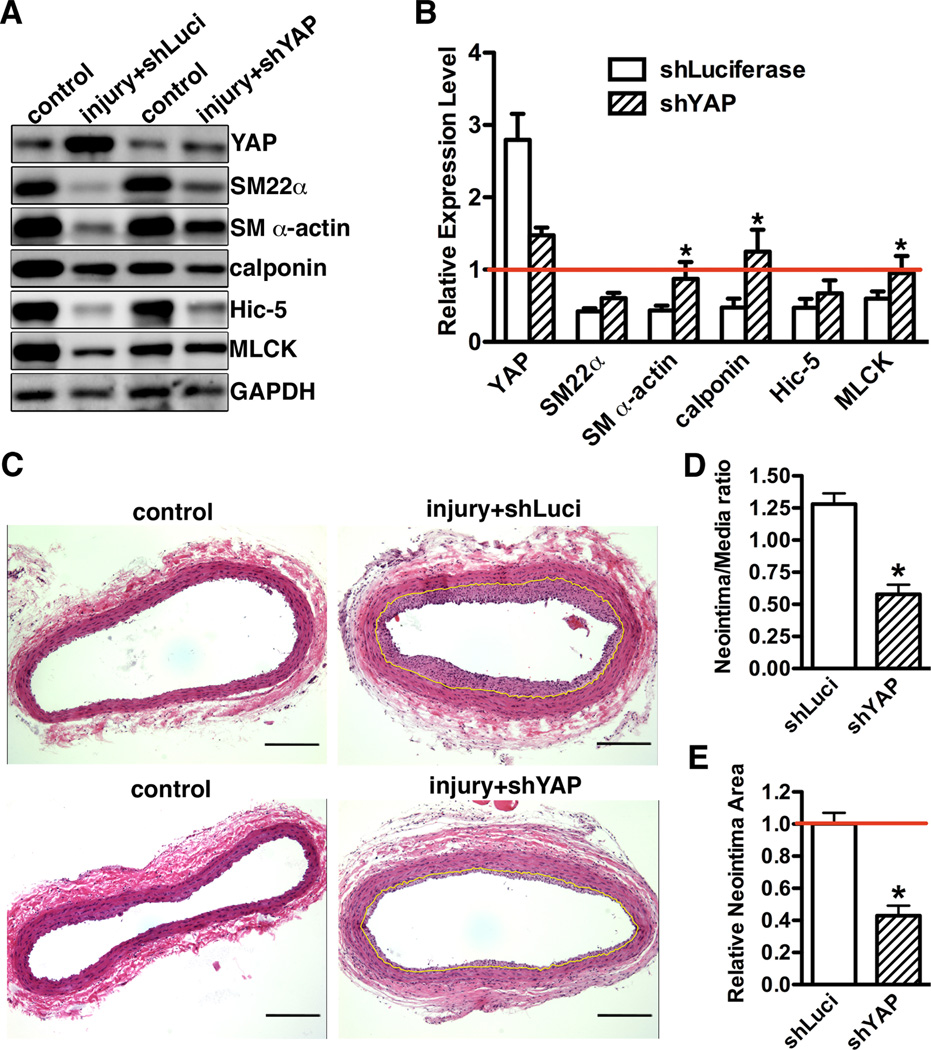
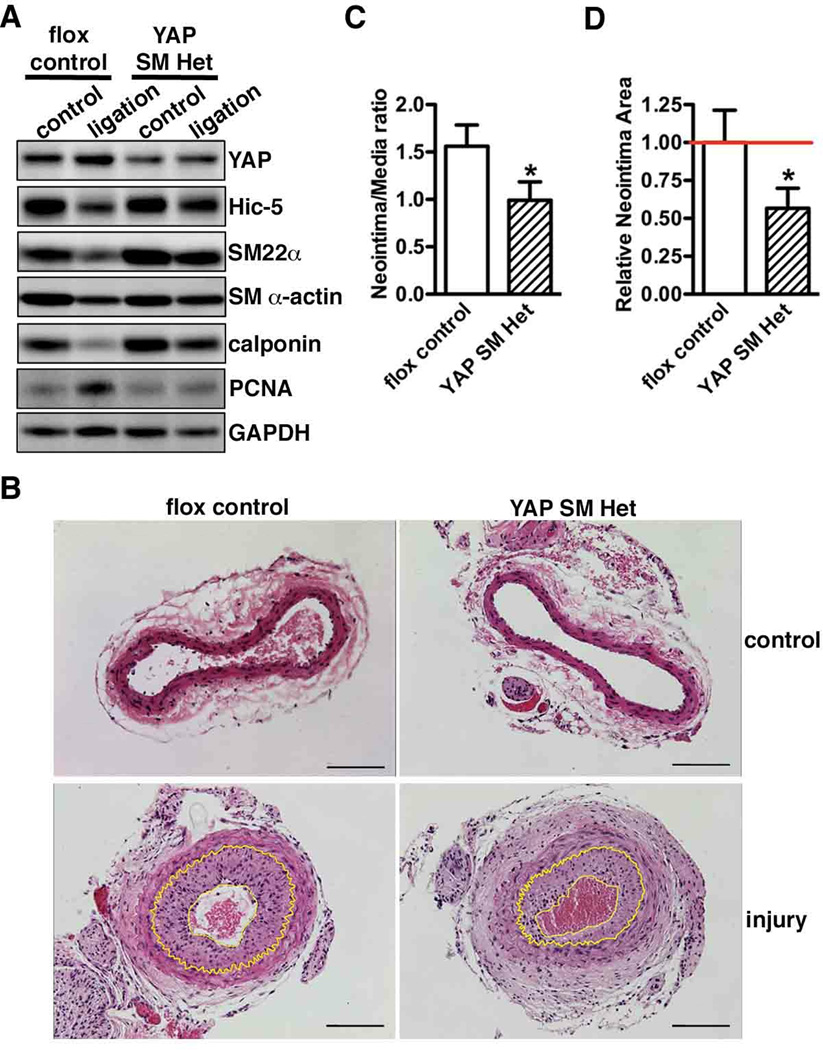
We next sought to determine the role of YAP in smooth muscle phenotypic modulation and neointima hyperplasia in vivo by transduction with lentivirus encoding short hairpin RNA against YAP (shYAP) to attenuate YAP gene up-regulation following rat carotid artery balloon injury. We first confirmed that shYAP lentivirus efficiently knock-down expression of YAP in vitro (Supplemental Figure VA) and that the lentivirus efficiently transduced into the injured vascular wall following arterial injury (Supplemental Figure VB). Short hairpin luciferase lentivirus-transduced animals were served as controls. We chose D14 post-injury to assess the effects of silencing YAP on smooth muscle gene expression and neointima formation as previously study has shown that the highest SMC fraction within neointima was seen at this time point16. Data from Western blotting and immuno-staining revealed that in vivo knock-down of YAP following rat carotid balloon injury significantly rescued injury-induced down-regulation of smooth muscle-specific genes (Figure 5A and B), including the most restrict smooth muscle marker, SM MHC (Supplemental Figure VI). Treatment with shYAP significantly decreased the neointima/media ratio and attenuated neointima area as compared to control shLuciferase virus transduced carotid arteries (Figure 5C, D and E). Moreover, knock-down of YAP significantly decreased neointima SMC proliferation by 50% as indicated by Ki67 staining (Supplemental Figure VII). Taken together, these data demonstrated that blocking YAP induction following rat carotid artery balloon injury can reduce the arterial injury-induced decrease in expression of smooth muscle-specific genes and attenuate neointima formation by inhibiting SMC proliferation. To further determine the specific contribution of SMC-derived YAP on neointima formation, a carotid artery ligation injury was performed on SMC-specific YAP heterozygote knockout mice. For these experiments we could not use homozygous SMC-specific YAP knockout mice as they die perinatally (data not shown). First we demonstrated that YAP expression in SMC-specific YAP heterozygotes (SM22α-Cre+/0/YAPf/wt, YAP SM Het) was reduced in SMCs by 50%, with no change of expression in other non-smooth muscle tissues such as brain (Supplemental Figure VIII). 21 days following ligation injury there was an attenuated reduction of smooth muscle-specific gene expression and decreased SMC proliferation in YAP heterozygous mice (Figure 6A). This resulted in a 40–50% decrease in neointima formation in the smooth muscle-specific heterozygote YAP mice (Figure 6B, C and D). Taken together, these data demonstrated that inactivation of YAP within SMCs is sufficient to up-regulate smooth muscle-specific gene expression and attenuate neointima formation following arterial ligation injury.
Figure 5. Knock-down YAP after rat carotid artery balloon injury rescues smooth muscle phenotypic switch and attenuates neointima formation.
A. 14 days after injury, rat control or balloon injured carotid arteries that infected with either shLuc or shYAP lentivirus during injury were harvested for Western blotting. B. Quantification of protein expression in panel “A” from 3 independent injury experiments. Expression was normalized to loading control GAPDH then expressed relative to its corresponding uninjured control artery (set to 1, red line). N=3. *p<0.05. C. HE staining of carotid artery sections from either control or balloon-injured vessels that were transduced with shLuciferase (shLuci), or shYAP lentivirus during injury. Tissues were harvested 14 days post-injury. The internal elastic lamina was highlighted in yellow. Scale bar, 100um. D. Statistical analysis of neointima/media layer ratio and relative neointima area (E) in panel “C” using ImageJ software. N=4. *p<0.05.
Figure 6. YAP reduction specifically in SMCs alleviates smooth muscle phenotypic modulation and abrogates neointima formation following carotid artery ligation injury.
A. Carotid artery ligation-induced vascular injury was performed in YAP flox control and smooth muscle-specific YAP heterozygous mice (SM YAP Het). 21 days after ligation, carotid arteries were harvested for Western blot analysis or for HE staining (B). A representative blot or HE staining photo is shown from 3 pairs of littermates per genotype. Neointima lesions are outlined with yellow lines. Scale bar, 100um. C. Statistical analysis of neointima/media layer ratio and relative neointima area (D) in panel “B” using ImageJ software. N=3 pair mice per genotype. *p<0.05.
Discussion
The Hippo signaling pathway is evolutionarily conserved from Drosophila to mammals and plays a critical role in controlling organ size and tumorigenesis by regulating cell proliferation1. In this report we demonstrated that YAP plays a novel role in vascular SMCs. We found that over-expression of YAP promotes SMC migration and proliferation while attenuating smooth muscle contractile gene expression (Figure 2, 3 and 4). Conversely, knocking-down endogenous YAP in SMCs up-regulates smooth muscle-specific gene expression while attenuating SMC proliferation and migration (Figure 2, 3 and 4) in vitro. During the preparation of the manuscript, we noticed that a recent study showed similar results demonstrating YAP is induced upon stimuli of smooth muscle phenotypic modulation and knock-down YAP can increase smooth muscle-specific gene expression in vitro21. However, this study was performed in cell culture settings while YAP function in vascular SMCs in vivo is completely unknown. In the current report, we provides the first evidence demonstrating that knock-down YAP expression in vivo in rats and mice is sufficient to prevent vascular injury-induced decreases in smooth muscle-specific genes and attenuates neointima formation (Figure 5 and 6).
YAP 5SA, a mutant that insensitive to Hippo inhibition more dramatically abrogated smooth muscle gene expression in vitro and had a stronger ability to promote SMC proliferation and migration (Figure 2, 3 and 4). Furthermore, arterial injury and cell culture activates the endogenous Hippo kinase cascade to inactivate a portion of YAP by retaining it in the cytoplasm (Supplemental Figure I and Figure 1). This activated Hippo signaling thus can be viewed as a natural protective response to the detrimental stimuli to prevent excessive SMC proliferation and lesion formation. Although, both cell culture and arterial injury stimulate activation of YAP upstream kinases to phosphorylate and inactivate YAP, the excessive induction of total YAP expression overwhelms this regulatory system resulting in unphospholylated YAP translocating into SMC nuclei. This nuclear YAP promotes SMC migration and proliferation while inhibits smooth muscle differentiation by attenuating smooth muscle-specific gene expression. In support of the notion that the Hippo pathway plays a critical role in the vasculature following injury, a previous study showed that arterial injury induces and activates Mst1 kinase and infection with adenovirus encoding Mst1 in balloon-injured rat carotid artery suppressed neointimal formation18. Additionally, cardiac-specific deletion of Mst kinase associated activator salvador, or deletion of Hippo kinases Mst1/2 and Lats1/2 in mouse, led to activated YAP, resulting in large hearts with elevated cardiomyocyte proliferation22. While the endogenous ligands stimulating this pathway are poorly characterized in mammals, one recognized stimulus is cell contact, a stimulus that is known to activate Hippo kinases but negatively affect SMC proliferation and promote differentiation23, 24. Taken together, these studies suggested that Hippo signaling is crucial for regulating YAP function in cardiomyocytes and vascular SMCs. Although our data have shown that YAP phosphorylation is elevated during smooth muscle phenotypic modulation, it is unknown which Hippo signaling components are involved in this process. Future studies are wanted to explore the precise mechanisms by which the Hippo pathway is activated in response to arterial injury and screen which stimuli involved in YAP induction during smooth muscle phenotypic modulation.
It is interesting to speculate on how YAP may promote smooth muscle cell proliferation while inhibiting differentiation. YAP lacks a DNA binding domain but functions as a potent transcriptional co-factor through interaction with TEADs or PPxY (PY) motif containing transcription factors via its N-terminal TEAD binding domain (TBD) or through a C-terminus WW domain, respectively6. Both TBD and WW domains have been shown to be required for YAP-mediated oncogenic activity25. For instance, TEAD binding element within cyclin D1 gene promoter is crucial for YAP mediated malignant mesothelioma cell proliferation19. Consistent with this, we found cylcin D1 is significantly induced by over-expression YAP while knock-down YAP decreases cyclin D1 expression, suggesting the positive effects of YAP on SMC proliferation is at least partially through induction of cell cycle regulating genes. Previous studies also have shown that TEAD binding elements within the SM α-actin promoter, that bind TEAD proteins, are required for activity of the promoter in embryonic SMCs but not in adult SMCs26, 27. TEAD1 has also been shown to directly interact with SRF (Serum Response Factor)28, 29, a transcription factor required for expression of most smooth muscle-specific genes20. SRF also plays an important role in regulating SMC migration and proliferation by interaction with a variety of cofactors30. The Hippo pathway may thus regulate expression of smooth muscle-specific genes and affect smooth muscle proliferation and migration through modulating the activity of SRF. Further studies are needed to investigate this possibility. Furthermore, YAP also physically and functionally interacts with PY motif-containing transcription factors such as SMAD7, p73, Runx1 and Runx2 through its WW domain6. Interestingly we also identified a conserved PY motif in the myocardin family proteins (data not shown), a group of factors are potent activators of smooth muscle-specific genes through interacting with SRF30. Consistent with this, YAP can interact with myocardin and disrupt myocardin binding to SRF thereby down-regulating smooth muscle-specific gene expression21. Together these findings suggest that YAP may regulate the phenotype of vascular SMCs by altering the activity of the SRF axis. Additionally, it has been shown that TGFβ and Notch signaling are involved in the smooth muscle differentiation9, 31, further investigation is wanted to test the possible cross talk between Hippo-YAP pathway with TGFβ and Notch signaling.
Elucidating the mechanisms controlling smooth muscle phenotypic modulation is critical for understanding the etiology and progression of many vascular diseases and ultimately identifying new therapeutic targets to treat these diseases. A number of serious clinical conditions including atherosclerosis, intimal hyperplasia associated with restenosis and vein graft stenosis are largely dependent upon SMC phenotype modulation contributing to progression of intimal lesions, resulting in occlusions of vessels9. These phenotypically switched SMCs in the neointima in turn, exacerbate lesion development by increasing production of growth factors and extracellular matrix32. In this report we demonstrate that preventing YAP induction following arterial injury can significantly attenuate neointima formation in vivo at least in part through inhibiting SMC proliferation and migration (Figure 5 and 6). As neointimal formation after arterial injury includes multiple cellular processes such as SMC death, matrix production and endothelial regeneration, it will be intriguing to investigate whether YAP can affect these aspects. In summary, this exciting study not only provides completely novel insights into the mechanisms controlling SMC phenotypic modulation but also identifies YAP as a potential therapeutic target for ameliorating vascular diseases.
Supplementary Material
Acknowledgements
We thank Drs. Paul Herring and Harold Singer for a critical reading of the manuscript. We also thank Dr. Duojia Pan for sharing YAP flox mice. We are thankful to Ms. Christina Rotondi at the AMC Histology Core for her excellent technical support with immunohistochemistry staining.
Source of funding
The project described was supported by a grant (1R01HL109605-01A1) from the National Heart, Lung, and Blood Institute, NIH to J. Z. and a Postdoctoral Fellowship from AHA to X.W.. The content is solely the responsibility of the authors and does not necessarily represent the official views the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edgar BA. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124:267–273. doi: 10.1016/j.cell.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 5.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20:638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Hu G, Betts C, Harmon EY, Keller RS, Van De Water L, Zhou J. Transforming growth factor-beta1-induced transcript 1 protein, a novel marker for smooth muscle contractile phenotype, is regulated by serum response factor/myocardin protein. J Biol Chem. 2011;286:41589–41599. doi: 10.1074/jbc.M111.250878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 10.Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res. 2007;100:633–644. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- 11.Doherty TM, Shah PK, Rajavashisth TB. Cellular origins of atherosclerosis: towards ontogenetic endgame? FASEB J. 2003;17:592–597. doi: 10.1096/fj.02-0913hyp. [DOI] [PubMed] [Google Scholar]
- 12.Penn A. International Commission for Protection Against Environmental Mutagens and Carcinogens. ICPEMC Working Paper 7/1/1. Mutational events in the etiology of arteriosclerotic plaques. Mutat Res. 1990;239:149–162. doi: 10.1016/0165-1110(90)90003-t. [DOI] [PubMed] [Google Scholar]
- 13.Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973;70:1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Halligan KE, Zhang X, Bisaillon JM, Gonzalez-Cobos JC, Motiani RK, Hu G, Vincent PA, Zhou J, Barroso M, Singer HA, Matrougui K, Trebak M. Orai1-mediated I (CRAC) is essential for neointima formation after vascular injury. Circ Res. 2011;109:534–542. doi: 10.1161/CIRCRESAHA.111.246777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuro-o M, Nagai R, Nakahara K, Katoh H, Tsai RC, Tsuchimochi H, Yazaki Y, Ohkubo A, Takaku F. cDNA cloning of a myosin heavy chain isoform in embryonic smooth muscle and its expression during vascular development and in arteriosclerosis. J Biol Chem. 1991;266:3768–3773. [PubMed] [Google Scholar]
- 16.Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Lab Invest. 1983;49:208–215. [PubMed] [Google Scholar]
- 17.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 18.Ono H, Ichiki T, Ohtsubo H, Fukuyama K, Imayama I, Hashiguchi Y, Sadoshima J, Sunagawa K. Critical role of Mst1 in vascular remodeling after injury. Arterioscler Thromb Vasc Biol. 2005;25:1871–1876. doi: 10.1161/01.ATV.0000174588.50971.1a. [DOI] [PubMed] [Google Scholar]
- 19.Mizuno T, Murakami H, Fujii M, Ishiguro F, Tanaka I, Kondo Y, Akatsuka S, Toyokuni S, Yokoi K, Osada H, Sekido Y. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene. 2012 doi: 10.1038/onc.2012.5. In press. [DOI] [PubMed] [Google Scholar]
- 20.Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577–593. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- 21.Xie C, Guo Y, Zhu T, Zhang J, Ma PX, Chen YE. Yap1 protein regulates vascular smooth muscle cell phenotypic switch by interaction with myocardin. J Biol Chem. 2012;287:14598–14605. doi: 10.1074/jbc.M111.329268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blank RS, Thompson MM, Owens GK. Cell cycle versus density dependence of smooth muscle alpha actin expression in cultured rat aortic smooth muscle cells. J Cell Biol. 1988;107:299–306. doi: 10.1083/jcb.107.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueki N, Sobue K, Kanda K, Hada T, Higashino K. Expression of high and low molecular weight caldesmons during phenotypic modulation of smooth muscle cells. Proc Natl Acad Sci U S A. 1987;84:9049–9053. doi: 10.1073/pnas.84.24.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao B, Kim J, Ye X, Lai ZC, Guan KL. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res. 2009;69:1089–1098. doi: 10.1158/0008-5472.CAN-08-2997. [DOI] [PubMed] [Google Scholar]
- 26.Swartz EA, Johnson AD, Owens GK. Two MCAT elements of the SM alpha-actin promoter function differentially in SM vs. non-SM cells. Am J Physiol. 1998;275:C608–C618. doi: 10.1152/ajpcell.1998.275.2.C608. [DOI] [PubMed] [Google Scholar]
- 27.Gan Q, Yoshida T, Li J, Owens GK. Smooth muscle cells and myofibroblasts use distinct transcriptional mechanisms for smooth muscle alpha-actin expression. Circ Res. 2007;101:883–892. doi: 10.1161/CIRCRESAHA.107.154831. [DOI] [PubMed] [Google Scholar]
- 28.Anbanandam A, Albarado DC, Nguyen CT, Halder G, Gao X, Veeraraghavan S. Insights into transcription enhancer factor 1 (TEF-1) activity from the solution structure of the TEA domain. Proc Natl Acad Sci U S A. 2006;103:17225–17230. doi: 10.1073/pnas.0607171103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta M, Kogut P, Davis FJ, Belaguli NS, Schwartz RJ, Gupta MP. Physical interaction between the MADS box of serum response factor and the TEA/ATTS DNA-binding domain of transcription enhancer factor-1. J Biol Chem. 2001;276:10413–10422. doi: 10.1074/jbc.M008625200. [DOI] [PubMed] [Google Scholar]
- 30.Pipes GC, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 2006;20:1545–1556. doi: 10.1101/gad.1428006. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Kennard S, Lilly B. NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ Res. 2009;104:466–475. doi: 10.1161/CIRCRESAHA.108.184846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiological Reviews. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.