Abstract
Objective
To describe the risk and risk factors for hypotony in a non-infectious uveitis cohort.
Design
Retrospective cohort study.
Participants
Patients with non-infectious uveitis seen between 1979-2007 at four academic ocular inflammation specialty clinics.
Method
Data were collected by trained, certified, expert reviewers from medical records.
Main Outcome Measures
Hypotony (<5mmHg) and low intraocular pressure (<8mmHg), each sustained for ≥2 visits spanning at least 30 days.
Results
During follow-up, 126/6785 (1.86%) developed hypotony at the rate of 0.61%(95% Confidence Interval (CI) 0.50, 0.75%)/eye-year. Cataract surgery was associated with a 7.5-fold risk (adjusted hazard ratio (aHR = 7.51, 95% CI 3.97,14.23) of incident hypotony. Phacoemulsification, the type of cataract surgery associated with the least hypotony risk still was associated with nearly five-fold higher hypotony incidence (aHR =4.87, 95% CI 2.25, 10.55). Increased risk was observed in children (aHR =2.92, 95% CI 1.20, 7.10) with respect to young adults, and duration of uveitis of >5 years (aHR =3.08, 95% CI 1.30, 7.31) with respect to uveitis of <6 month duration. ≥3+ vitreous cells, band keratopathy, exudative retinal detachment, posterior synechia and history of pars plana vitrectomy also were associated with greater hypotony incidence. With respect to anterior uveitis, intermediate uveitis (aHR = 0.17, 95% CI 0.05,0.56) and posterior uveitis (aHR =0.11, 95% CI 0.03,0.45) were associated with lower hypotony risk, whereas panuveitis (aHR=1.25, 95% CI 0.67, 2.35) was similar. Approximately five-sixths (84.1%) of eyes presenting with hypotony had a visual acuity of 20/200 or worse (aOR for visual acuity 20/200 or worse=13.85, 95% CI 7.23, 26.53). Risk factors for prevalent hypotony were similar.
Conclusions
The risk of hypotony is low among eyes with non-infectious uveitis, but is more frequently observed in cases with anterior segment inflammation. Signs of present or past inflammation were associated with higher risk, suggesting excellent inflammatory control may reduce the risk of hypotony. Prior ocular surgery also was associated with higher risk; cataract surgery in particular was associated with much higher risk of hypotony. Lower risk of hypotony with phacoemulsification than alternative cataract surgery approaches suggests the phacoemulsification approach is preferable.
Extreme alterations in intraocular pressures (IOP) are not uncomm on in the setting of uveitis. While elevated IOP is often a focus of interest and concern in uveitis, hypotony in uveitis is perhaps a more formidable problem, given limited treatment options and the potential complications of visual loss, maculopathy, keratopathy, choroidal effusion, optic nerve edema, irregular astigmatism, and, ultimately, phthisis bulbi. Although there is no consensus on how hypotony should be defined, most ophthalmologists agree that structural changes are more likely to occur in the eye when the IOP is lower than 5 mmHg.1
In different uveitic subpopulations followed for various lengths of follow-up, a range of incidence rates have been reported for hypotony; among patients with HLA-B27 associated uveitis an incidence of 0.006/eye year,2 among pediatric uveitis patients 0.03/person year3,4 and among children with uveitis associated with juvenile arthritis 0.09/eye year.5 Hypotony is commonly associated with severe vision loss and has been formally confirmed to have a significant visual impact (4.5-fold higher odds of reduced visual acuity) in childhood uveitis.3 Hypotony is a challenging and often disappointing condition to manage; currently there is no definitive treatment for hypotony except in the setting of a cyclodialysis.6
Presently, the extent of risk for hypotony is incompletely characterized, and information regarding risk factors influencing the development of hypotony is limited, as the small prior series had limited power to identify risk factors. In this report, we evaluate the incidence and factors associated with hypotony in a large retrospective cohort of uveitis patients followed at four tertiary uveitis clinics in the United States.
Methods
Study Population
This report is based on data from patients with non-infectious uveitis included in the Systemic Immunosuppresive Therapy for Eye Diseases (SITE) Cohort Study. The design of this retrospective cohort study has been described earlier.7 Patients included in this report had been seen between 1979-2007 at four academic ocular inflammation specialty clinics; patients from a fifth clinic which followed a consultative practice pattern, such that follow-up visits were infrequent and sometimes occurred in response to poor outcome, were excluded to avoid ascertainment biases. In prior reports, the cohort had included an approximate 40% random sample from one of the four centers, but by the time of this analysis 100% data were available from all of the centers. The study was performed with the approval of the four institutions’ Institutional Review Board in accordance with the Declaration of Helsinki.
Data Collection
Data collection for the SITE Cohort Study was done by trained, certified, expert reviewers who reviewed the medical records of all patients and entered information for each eye of every patient at every visit into a custom Microsoft Access database (Microsoft Corporation, Redmond, Washington, USA). The data collection system included a quality control program that required error corrections in real time. Demographic information obtained during the initial visit and details of all medications in use at every clinic visit were available. Details of ocular characteristics based on clinical evaluation using external, slit-lamp, and dilated fundus examination also were recorded. Sequelae of ocular inflammation were noted when present. Intraocular pressures that had been obtained for each eye at each visit during routine clinical care were recorded, along with the method by which IOP was measured.
Main Outcome Measures
Both the prevalence of hypotony (<5mmHg) at cohort entry and the incidence of hypotony were assessed. Prior glaucoma surgery was considered as a potential risk factor for presentation with hypotony, reflecting the scenario a consulting clinician would be faced with, but was not assessed as a potential risk factor for incident hypotony in order to better make inferences regarding pathogenesis, given the different pathophysiology of overfiltration versus hypotony absent glaucoma surgery. A similar assessment was done for low intraocular pressure (<8mmHg). To calculate the incidence of these outcomes, patients who were free of each respective outcome at the time of cohort entry and had follow up visits were followed until the first occurrence of hypotony, until the patient ceased attending the clinic or the eye had glaucoma surgery, or until completion of the study. Since a single measurement of reduced intraocular pressure is not uncommon with active inflammation, and may differ qualitatively from sustained hypotony, our hypotony case definition for the primary analysis required that an IOP below 5mmHg (or below 8 mm Hg) be observed on at least two visits spanning no less than 30 days, including all visits within 30 days of the first measurement. The following variables were assessed for their potential association with hypotony and low IOP: age, sex, race, site of uveitis, primary ocular diagnosis, presence of systemic inflammatory diseases, hypertension, diabetes, visual acuity, prior cataract surgery, inflammatory activity in the eye (anterior chamber cells, vitreous cells and vitreous haze), duration of uveitis, and the presence of other specific ocular complications of uveitis including keratic precipitates, band keratopathy, posterior synechia, exudative retinal detachment, epiretinal membrane, macular edema, snowbanking, the presence of snowballs and of retinal vascular sheathing.
Statistical Analysis
For the prevalence analysis, baseline was established as the first visit with a uveitis diagnosis. For the incidence analysis, all non-hypotonous eyes from the prevalence analysis with follow-up visits available and no prior glaucoma surgery were included; eyes with glaucoma surgery during follow-up were censored at the date of surgery. All visits within 30 days post-cataract surgery were excluded, but visits after 30 days were retained. Covariates missing at a given visit were imputed by carrying the last value forward. Visits with missing IOP measurements were censored.
Frequencies and medians of demographic variables were tabulated for patients and compared using Fisher’s exact test or one-way ANOVA (analysis of variance) for categorical and numerical data respectively. For the risk factor analyses regarding prevalence of hypotony or low IOP among eyes at cohort entry, crude and adjusted odds ratios were calculated using univariate and multivariate logistic regression. Eye-year incidence rates and 95% confidence intervals were calculated using Poisson regression. For both the prevalence and incidence analyses, the appropriate generalizations of general estimating equations (GEE) to the model in question were used to account for correlation between the eyes of individual patients.8 For the evaluation of potential risk factors for the incidence of hypotony, crude and adjusted hazard ratios were calculated using time-updated univariate and multivariate Cox proportional hazards models with a robust sandwich estimate to account for correlation between the eyes of individual patients9. 95% confidence intervals are presented by giving the lower and upper bound of the confidence intervals as subscripts before and after risk ratio estimates. All statistical computations were performed with SAS 9.2 (SAS Institute, Inc., Cary, NC).
Results
Characteristics of the study population
Among 11,119 eyes (of 6,545 patients) with uveitis, 189 (1.471.7%1.96) eyes of 161 patients presented with hypotony (intraocular pressure <5mmHg) at cohort entry. Twenty-eight patients (17.3 %) presented with bilateral hypotony. Intraocular pressure was measured by Goldmann applanation tonometer in 67.3% of eyes, 2.3% by tonopen, 30.2% by pneumotonometer and 0.2% using other methods. On visits where hypotony was diagnosed, 76.2% of measurements were made using Goldmann applanation tonometry.
By primary site of inflammation, patients with panuveitis had the highest prevalence of hypotony (4.8%) followed by anterior uveitis (1.8%), posterior uveitis (0.6%) and intermediate uveitis (0.3%). The prevalence of intraocular pressures less than 8 mmHg similarly was distributed across the various types of uveitis, with panuveitis (3.0%) and anterior uveitis (1.9%) having the highest frequency of low IOP, compared with lower frequency among intermediate (1.3%) and posterior (0.7%) uveitis cases. A total of 295 eyes with uveitis had anti-glaucoma surgery before cohort entry and 144 had anti-glaucoma surgery during follow up. Table 1, available at http://aaojournal.org, summarizes patient- and eye-specific characteristics of cases of hypotony at cohort entry.
Table 1. Characteristics of eyes with uveitis and hypotony (<5 mmHg vs 5+ mmHg) at presentation.
| Intraocular Pressures | Univariate | Multivariate* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <5 mmHg n=189 |
5-7 mmHg n=196 |
>7 mmHg n=10734 |
Odds Ratio (95% Confidence Interval) |
p-value | Odds Ratio (95% Confidence Interval) |
p-value | |||
| Age | <18 | 28.30% | 27.80% | 16.20% | 2.26 (1.43 - 3.58) | <.001 | 1.06 (0.52 - 2.17) | 0.63 | |
| 18-25 | 10.90% | 12.90% | 12.40% | 1.17 (0.65 - 2.11) | 0.78 (0.35 - 1.73) | ||||
| 26-35 | 17.90% | 19.10% | 20.90% | Reference | Reference | ||||
| 36-45 | 19.00% | 18.60% | 19.60% | 1.22 (0.74 - 2.02) | 1.19 (0.67 - 2.14) | ||||
| 46-55 | 9.80% | 11.30% | 14.80% | 0.82 (0.46 - 1.45) | 0.59 (0.27 - 1.32) | ||||
| 56-65 | 3.80% | 3.60% | 8.60% | 0.52 (0.22 - 1.22) | 0.69 (0.26 - 1.83) | ||||
| >65 | 10.30% | 6.70% | 7.50% | 1.58 (0.87 - 2.88) | 0.77 (0.31 - 1.92) | ||||
| Gender | Female | 67.20% | 60.70% | 64.10% | Reference | 0.53 | Reference | 0.94 | |
| Male | 32.80% | 39.30% | 35.90% | 0.90 (0.66 - 1.24) | 0.98 (0.63 - 1.53) | ||||
| Race | White | 56.60% | 56.10% | 72.10% | Reference | <.001 | Reference | 0.14 | |
| African-derived | 32.30% | 28.10% | 15.70% | 2.69 (1.93 - 3.75) | 1.58 (0.94 - 2.67) | ||||
| Other | 11.10% | 15.80% | 12.20% | 1.36 (0.84 - 2.21) | 1.55 (0.82 - 2.94) | ||||
| Contralateral uveitis |
Yes | 89.40% | 78.10% | 82.60% | Reference | 0.02 | Reference | 0.31 | |
| No | 10.60% | 21.90% | 17.40% | 0.61 (0.40 - 0.92) | 0.72 (0.39 - 1.35) | ||||
| Hypertension | No | 73.00% | 84.20% | 82.80% | Reference | 0.03 | Reference | 0.41 | |
| Yes | 27.00% | 15.80% | 17.20% | 1.48 (1.04 - 2.10) | 0.80 (0.46 - 1.37) | ||||
| Diabetes | No | 89.40% | 94.90% | 94.80% | Reference | 0.002 | Reference | 0.27 | |
| Yes | 10.60% | 5.10% | 5.20% | 2.16 (1.34 - 3.48) | 1.50 (0.73 - 3.08) | ||||
| Systemic diseases |
Any† | 36.50% | 34.70% | 24.10% | 1.80 (1.31 - 2.47) | <.001 | 0.94 (0.49 - 1.78) | 0.84 | |
| HLA-B27 and/or spondyloarthropathy | 6.30% | 12.20% | 10.40% | 0.55 (0.30 - 1.02) | 0.06 | 0.70 (0.31 - 1.57) | 0.39 | ||
| Juvenile idiopathic arthritis | 18.00% | 15.30% | 5.00% | 4.39 (2.95 - 6.55) | <.001 | 1.91 (0.93 - 3.92) | 0.08 | ||
| Sarcoidosis | 11.60% | 9.20% | 7.10% | 1.68 (1.03 - 2.76) | 0.04 | 1.83 (0.95 - 3.53) | 0.07 | ||
| Type of uveitis | Anterior Uveitis | 56.60% | 59.70% | 54.10% | Reference | <.001 | Reference | 0.04 | |
| Intermediate Uveitis | 2.60% | 12.80% | 17.30% | 0.27 (0.13 - 0.56) | 0.35 (0.14 - 0.84) | ||||
| Panuveitis | 34.40% | 20.90% | 11.60% | 2.61 (1.86 - 3.67) | 1.20 (0.72 - 2.00) | ||||
| Posterior Uveitis | 6.30% | 6.60% | 17.00% | 0.38 (0.21 - 0.69) | 0.57 (0.28 - 1.14) | ||||
| Visual Acuity | 20/40 or better | 4.20% | 31.60% | 63.30% | Reference | <.001 | Reference | <.001 | |
| >20/40 - <20/200 | 11.60% | 24.50% | 20.60% | 5.59 (3.07 - 10.19) | 3.67 (1.78 - 7.59) | ||||
| 20/200 or worse | 84.10% | 43.90% | 16.00% | 36.15 (21.51 -60.78) | 13.85 (7.23 -26.53) | ||||
| Duration of uveitis at baseline |
<6 months | 19.00% | 36.70% | 35.40% | Reference | <.001 | Reference | 0.21 | |
| 6 months to 2 years | 11.10% | 13.80% | 22.00% | 0.82 (0.48 - 1.38) | 1.04 (0.52 - 2.10) | ||||
| 2 to 5 years | 11.10% | 13.80% | 18.10% | 0.92 (0.54 - 1.59) | 1.06 (0.50 - 2.23) | ||||
| Greater than 5 years | 58.70% | 35.70% | 24.60% | 3.30 (2.28 - 4.78) | 1.71 (0.90 - 3.24) | ||||
| Prior Cataract Surgery by Type |
None | 37.60% | 65.30% | 87.60% | Reference | <.001 | Reference | <.001 | |
| Phacoemulsification | 1.10% | 4.60% | 2.30% | 1.88 (0.75 - 4.74) | 2.39 (0.76 - 7.49) | ||||
| Extracapsular cataract extraction (ECCE) | 10.10% | 8.20% | 2.60% | 7.01 (4.03 - 12.20) | 4.75 (2.10 - 10.78) | ||||
| Intracapsular cataract extraction (ICCE) | 5.80% | 2.00% | 0.50% | 24.01 (9.69 -59.47) | 36.48 (12.09 -110.06) | ||||
| Pars plana lensectomy | 7.40% | 1.50% | 0.70% | 15.10 (7.17 -31.82) | 8.78 (3.38 - 22.86) | ||||
| Unknown | 38.10% | 18.40% | 6.30% | 10.66 (7.36 -15.43) | 9.51 (5.44 - 16.60) | ||||
| Prior Glaucoma Surgery |
No | 84.70% | 86.20% | 97.00% | Reference | <.001 | Reference | <0.001 | |
| Yes | 15.30% | 13.80% | 3.00% | 4.47 (2.74 - 7.29) | 2.61 (1.50 - 4.56) | ||||
| Prior Trabeculectomy |
No | 88.90% | 89.70% | 98.10% | Reference | <.001 | Reference | 0.7 | |
| Yes | 11.10% | 10.30% | 1.90% | 5.68 (3.56 - 9.07) | 1.24 (0.42 - 3.60) | ||||
| Prior Tube- Shunt Surgery |
No | 94.70% | 95.90% | 99.40% | Reference | <.001 | Reference | 0.28 | |
| Yes | 5.30% | 4.10% | 0.60% | 5.83 (2.13 - 15.93) | 1.72 (0.64 - 4.63) | ||||
| Prior Pars Plana Vitrectomy | No | 76.20% | 90.80% | 97.10% | Reference | <.001 | Reference | <0.001 | |
| Yes | 23.80% | 9.20% | 2.90% | 6.43 (4.22 - 9.78) | 2.76 (1.57 - 4.84) | ||||
| Prior Retinal Detachment Surgery |
No | 93.10% | 96.90% | 99.10% | Reference | <.001 | Reference | 0.62 | |
| Yes | 6.90% | 3.10% | 0.90% | 5.48 (2.54 - 11.86) | 1.28 (0.48 - 3.45) | ||||
| Signs of Inflammatory Activity at Presentation |
Anterior chamber cells | Quiet | 56.10% | 32.50% | 56.20% | Reference | 0.32 | Reference | 0.21 |
| 0.5+ | 15.60% | 14.70% | 16.50% | 0.88 (0.57 - 1.37) | 0.96 (0.57 - 1.63) | ||||
| 1+ | 12.10% | 20.40% | 12.40% | 0.88 (0.53 - 1.46) | 0.80 (0.43 - 1.52) | ||||
| 2+ | 8.70% | 18.30% | 9.70% | 1.16 (0.72 - 1.86) | 1.22 (0.64 - 2.33) | ||||
| 3+ or worse | 7.50% | 14.10% | 5.20% | 1.64 (0.96 - 2.80) | 2.15 (1.04 - 4.44) | ||||
| Vitreous cells | Quiet | 58.70% | 53.60% | 57.60% | Reference | 0.008 | Reference | 0.03 | |
| 0.5+ | 11.90% | 9.80% | 12.70% | 0.79 (0.42 - 1.49) | 0.87 (0.45 - 1.69) | ||||
| 1+ | 7.90% | 16.30% | 15.30% | 0.62 (0.35 - 1.09) | 1.09 (0.53 - 2.24) | ||||
| 2+ | 12.70% | 13.10% | 10.90% | 1.37 (0.81 - 2.30) | 2.12 (1.14 - 3.92) | ||||
| 3+ or worse | 8.70% | 7.20% | 3.40% | 2.28 (1.26 - 4.12) | 2.46 (1.15 - 5.30) | ||||
| Vitreous haze | Quiet | 70.90% | 74.30% | 82.90% | Reference | <.001 | Reference | 0.02 | |
| 1+ | 17.90% | 13.50% | 10.90% | 1.86 (1.15 - 3.03) | 2.22 (1.31 - 3.74) | ||||
| 2+ | 6.00% | 8.80% | 4.40% | 1.87 (0.95 - 3.67) | 1.44 (0.59 - 3.52) | ||||
| 3+ or worse | 5.10% | 3.40% | 1.80% | 3.69 (1.67 - 8.14) | 0.49 (0.06 - 3.74) | ||||
| Keratic precipitates | No | 85.50% | 70.50% | 84.20% | Reference | 0.99 | Reference | 0.44 | |
| Yes | 14.50% | 29.50% | 15.80% | 1.00 (0.60 - 1.66) | 0.77 (0.39 - 1.52) | ||||
| Structural complications | Any† | 67.80% | 62.20% | 43.00% | 2.28 (1.66 - 3.13) | <.001 | 1.60 (1.01 - 2.54) | 0.04 | |
| Band keratopathy | 33.10% | 19.10% | 4.10% | 10.71 (7.11 -16.14) | <.001 | 3.64 (1.69 - 7.84) | 0.001 | ||
| Peripheral anterior synechia |
15.30% | 8.30% | 3.60% | 4.19 (2.54 - 6.89) | <.001 | 1.63 (0.87 - 3.03) | 0.12 | ||
| Posterior synechia | 33.30% | 30.70% | 18.00% | 1.84 (1.31 - 2.58) | <.001 | 1.81 (1.14 - 2.87) | 0.01 | ||
| Exudative detachment | 16.50% | 3.60% | 1.30% | 8.81 (4.61 - 16.84) | <.001 | 6.97 (3.40 - 14.27) | <.001 | ||
Any systemic disease includes those in the table as well as Behçet’s disease. Any structural complication includes those in the table as well as choroidal neovascularization, epiretinal membrane, exudative detachment, macular edema and retinal neovascularization
Vitreous cells and haze are never adjusted by anterior chamber cells
Adjusting for cataract surgery by type, prior glaucoma surgery, pars plana vitrectomy, retinal detachment surgery, sarcoidosis, juvenile idiopathic arthritis, type of uveitis, age, race, hypertension, diabetes, duration of uveitis at baseline, anterior chamber cells and exudative detachment
Hyperlipidemia, smoking status, epiretinal membrane, macular edema, and retinal vascular sheathing are excluded from the table because they were never found to be significant at p<0.10. Active lesions in choroid/retina, snowballs, snowbanking, Behçet's disease, choroidal neovascularization and retinal neovascularization are excluded from the table because people with these characteristics had fewer than 5 hypotony events
Risk factors for hypotony at presentation
Several risk factors for hypotony were identified (see Table 1 available at http://aaojournal.org and Table 2 which gives results obtained from the final multiple regression model). Younger age, African-derived race, and diagnosis of hypertension or diabetes mellitus all had a crude association with hypotony that was abrogated by adjustment for other factors. Eyes presenting with hypotony were less likely to have a primary diagnosis of intermediate uveitis (2.6%, adjusted OR 0.140.350.84) or posterior uveitis (6.3%, aOR 0.280.571.14) than of panuveitis (34.4%, aOR 0.721.202.00) when compared with anterior uveitis (overall p=0.04).
Table 2. Multiple Regression Model for Characteristics of Eyes with Uveitis and Hypotony (<5 mmHg vs 5+ mmHg) at Presentation.
| Intraocular Pressures | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| <5 mmHg >n=189 |
5-7 mmHg n=196 |
>7 mmHg n=10734 |
Odds Ratio (95% Confidence Interval) |
p-value | Odds Ratio (95% Confidence Interval) |
p-value | ||
| Age | <18 | 28.30% | 27.80% | 16.20% | 2.26 (1.43 - 3.58) | <.001 | 1.06 (0.52 - 2.17) | 0.63 |
| 18-25 | 10.90% | 12.90% | 12.40% | 1.17 (0.65 - 2.11) | 0.78 (0.35 - 1.73) | |||
| 26-35 | 17.90% | 19.10% | 20.90% | Reference | Reference | |||
| 36-45 | 19.00% | 18.60% | 19.60% | 1.22 (0.74 - 2.02) | 1.19 (0.67 - 2.14) | |||
| 46-55 | 9.80% | 11.30% | 14.80% | 0.82 (0.46 - 1.45) | 0.59 (0.27 - 1.32) | |||
| 56-65 | 3.80% | 3.60% | 8.60% | 0.52 (0.22 - 1.22) | 0.69 (0.26 - 1.83) | |||
| >65 | 10.30% | 6.70% | 7.50% | 1.58 (0.87 - 2.88) | 0.77 (0.31 - 1.92) | |||
| Race | White | 56.60% | 56.10% | 72.10% | Reference | <.001 | Reference | 0.14 |
| African-derived | 32.30% | 28.10% | 15.70% | 2.69 (1.93 - 3.75) | 1.58 (0.94 - 2.67) | |||
| Other | 11.10% | 15.80% | 12.20% | 1.36 (0.84 - 2.21) | 1.55 (0.82 - 2.94) | |||
| Hypertension | No | 73.00% | 84.20% | 82.80% | Reference | 0.03 | Reference | 0.41 |
| Yes | 27.00% | 15.80% | 17.20% | 1.48 (1.04 - 2.10) | 0.80 (0.46 - 1.37) | |||
| Diabetes | No | 89.40% | 94.90% | 94.80% | Reference | 0.002 | Reference | 0.27 |
| Yes | 10.60% | 5.10% | 5.20% | 2.16 (1.34 - 3.48) | 1.50 (0.73 - 3.08) | |||
| Systemic diseases |
Juvenile idiopathic arthritis |
18.00% | 15.30% | 5.00% | 4.39 (2.95 - 6.55) | <.001 | 1.91 (0.93 - 3.92) | 0.08 |
| Sarcoidosis | 11.60% | 9.20% | 7.10% | 1.68 (1.03 - 2.76) | 0.04 | 1.83 (0.95 - 3.53) | 0.07 | |
| Type of uveitis |
Anterior Uveitis | 56.60% | 59.70% | 54.10% | Reference | <.001 | Reference | 0.04 |
| Intermediate Uveitis | 2.60% | 12.80% | 17.30% | 0.27 (0.13 - 0.56) | 0.35 (0.14 - 0.84) | |||
| Panuveitis | 34.40% | 20.90% | 11.60% | 2.61 (1.86 - 3.67) | 1.20 (0.72 - 2.00) | |||
| Posterior Uveitis | 6.30% | 6.60% | 17.00% | 0.38 (0.21 - 0.69) | 0.57 (0.28 - 1.14) | |||
| Duration of uveitis at baseline |
<6 months | 19.00% | 36.70% | 35.40% | Reference | <.001 | Reference | 0.21 |
| 6 months to 2 years | 11.10% | 13.80% | 22.00% | 0.82 (0.48 - 1.38) | 1.04 (0.52 - 2.10) | |||
| 2 to 5 years | 11.10% | 13.80% | 18.10% | 0.92 (0.54 - 1.59) | 1.06 (0.50 - 2.23) | |||
| Greater than 5 years | 58.70% | 35.70% | 24.60% | 3.30 (2.28 - 4.78) | 1.71 (0.90 - 3.24) | |||
| Prior Cataract Surgery by Type |
None | 37.60% | 65.30% | 87.60% | Reference | <.001 | Reference | <.001 |
| Phacoemulsification | 10% | 4.60% | 2.30% | 1.88 (0.75 - 4.74) | 2.39 (0.76 - 7.49) | |||
| Extracapsular cataract extraction (ECCE) |
10.10% | 8.20% | 2.60% | 7.01 (4.03 - 12.20) | 4.75 (2.10 - 10.78) | |||
| Intracapsular cataract extraction (ICCE) |
5.80% | 2.00% | 0.50% | 24.01 (9.69 - 59.47) | 36.48 (12.09 - 110.06) |
|||
| Pars plana lensectomy |
7.40% | 1.50% | 0.70% | 15.10 (7.17 - 31.82) | 8.78 (3.38 - 22.86) | |||
| Unknown | 38.10% | 18.40% | 6.30% | 10.66 (7.36 - 15.43) | 9.51 (5.44 - 16.60) | |||
| Prior Glaucoma Surgery |
No | 84.70% | 86.20% | 97.00% | Reference | <.001 | Reference | <0.001 |
| Yes | 15.30% | 13.80% | 3.00% | 4.47 (2.74 - 7.29) | 2.61 (1.50 - 4.56) | |||
| Prior Pars Plana Vitrectomy |
No | 76.20% | 90.80% | 97.10% | Reference | <.001 | Reference | <0.001 |
| Yes | 23.80% | 9.20% | 2.90% | 6.43 (4.22 - 9.78) | 2.76 (1.57 - 4.84) | |||
| Prior Retinal Detachment Surgery |
No | 93.10% | 96.90% | 99.10% | Reference | <.001 | Reference | 0.62 |
| Yes | 6.90% | 3.10% | 0.90% | 5.48 (2.54 - 11.86) | 1.28 (0.48 - 3.45) | |||
| Anterior chamber cells |
Quiet | 56.10% | 32.50% | 56.20% | Reference | 0.32 | Reference | 0.21 |
| 0.5+ | 15.60% | 14.70% | 16.50% | 0.88 (0.57 - 1.37) | 0.96 (0.57 - 1.63) | |||
| 1+ | 12.10% | 20.40% | 12.40% | 0.88 (0.53 - 1.46) | 0.80 (0.43 - 1.52) | |||
| 2+ | 8.70% | 18.30% | 9.70% | 1.16 (0.72 - 1.86) | 1.22 (0.64 - 2.33) | |||
| 3+ or worse | 7.50% | 14.10% | 5.20% | 1.64 (0.96 - 2.80) | 2.15 (1.04 - 4.44) | |||
| Structural complications |
Exudative detachment |
16.50% | 3.60% | 1.30% | 8.81 (4.61 - 16.84) | <.001 | 6.97 (3.40 - 14.27) | <.001 |
Presentation with hypotony was associated strongly with diminished visual acuity. Approximately five-sixths (84.1%) of eyes presenting with hypotony had a visual acuity of 20/200 or worse (aOR 7.2313.8526.53), while 11.6% more presented with a visual acuity worse than 20/40 but better than 20/200 (aOR 1.783.677.59) when compared to only 4.2 % presenting with a visual acuity of 20/40 or better.
Eyes of patients presenting with hypotony were more likely to have had cataract surgery (62.4% vs 12.4% , aOR 6.0410.6918.91) and/or glaucoma surgery (15.3% vs 3.0%, aOR 1.502.614.56) than those without hypotony. Patients with pars plana vitrectomy (23.8.% vs 2.9%, aOR 1.572.764.84) also had more hypotony than patients without the surgery. Patients with hypotony were more likely to have had retinal detachment surgery previously, but adjustment for other factors abrogated this crude association.
Eyes presenting with hypotony also had more signs of past and current inflammation, including the presence of band keratopathy or posterior synechiae; high grades of anterior chamber cells, vitreous cells, and vitreous haze; and exudative retinal detachment. Inflammatory signs typical of intermediate and posterior uveitis were not associated with hypotony.
A similar pattern of association was present for presentation with low IOP (less than 8 mm Hg) with respect to normal levels of IOP (Table 3, available at http://aaojournal.org). After adjusting for prior cataract and glaucoma surgery, pars plana vitrectomy, retinal detachment surgery, sarcoidosis, juvenile idiopathic arthritis, type of uveitis, age, race, hypertension, diabetes, duration of uveitis at baseline, anterior chamber cells and exudative retinal detachment, the estimated multivariate adjusted odds ratios of eyes with <8mmHg vs 8+ mmHg, having similar references as in Table 1, available at http://aaojournal.org, were 1.091.913.33 for juvenile idiopathic arthritis, 1.081.823.07 for sarcoidosis, 0.060.230.96 for Bechet’s disease, 3.214.696.85 for visual acuity of 20/200 or worse, 4.096.249.15 for prior cataract surgery, 1.742.714.22 for prior glaucoma surgery, 1.582.564.14 for pars plana vitrectomy, 1.893.085.04 for 3+ or worse anterior chamber cells, 1.652.915.15 for 3+ or worse vitreous cells, 1.362.494.56 for band keratopathy, 1.051.482.09 for posterior synechia and 2.745.019.13 for exudative retinal detachment.
Table 3. Characteristics of eyes with uveitis and hypotony (<8 mmHg vs 8+ mmHg) at presentation.
| Intraocular Pressures | Univariate | Multivariate* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <5 mmHg n=189 |
5-7 mmHg n=196 |
>7 mmHg n=10734 |
Odds Ratio (95% Confidence Interval) |
p- value |
Odds Ratio (95% Confidence Interval) |
p-value | |||
| Age | <18 | 28.30% | 27.80% | 16.20% | 1.91 (1.34 - 2.72) |
<.001 | 1.15 (0.69 - 1.93) |
0.53 | |
| 18-25 | 10.90% | 12.90% | 12.40% | 1.06 (0.68 - 1.64) |
0.88 (0.50 - 1.56) |
||||
| 26-35 | 17.90% | 19.10% | 20.90% | Reference | Reference | ||||
| 36-45 | 19.00% | 18.60% | 19.60% | 1.03 (0.70 - 1.51) |
1.07 (0.67 - 1.69) |
||||
| 46-55 | 9.80% | 11.30% | 14.80% | 0.78 (0.50 - 1.20) |
0.73 (0.42 - 1.30) |
||||
| 56-65 | 3.80% | 3.60% | 8.60% | 0.49 (0.26 - 0.94) |
0.61 (0.30 - 1.26) |
||||
| >65 | 10.30% | 6.70% | 7.50% | 1.32 (0.83 - 2.10) |
0.67 (0.34 - 1.33) |
||||
| Gender | Female | 67.20% | 60.70% | 64.10% | Reference | 0.8287 | Reference | 0.1 | |
| Male | 32.80% | 39.30% | 35.90% | 1.03 (0.81 - 1.31) |
1.30 (0.96 - 1.77) |
||||
| Race | White | 56.60% | 56.10% | 72.10% | Reference | <.001 | Reference | 0.11 | |
| African-derived | 32.30% | 28.10% | 15.70% | 2.40 (1.83 - 3.14) |
1.35 (0.89 - 2.06) |
||||
| Other | 11.10% | 15.80% | 12.20% | 1.41 (0.98 - 2.01) |
1.54 (0.98 - 2.42) |
||||
| Contralateral uveitis |
Yes | 89.40% | 78.10% | 82.60% | Reference | 0.5944 | Reference | 0.35 | |
| No | 10.60% | 21.90% | 17.40% | 0.93 (0.70 - 1.23) |
1.20 (0.82 - 1.74) |
||||
| Hypertension | No | 73.00% | 84.20% | 82.80% | Reference | 0.0954 | Reference | 0.97 | |
| Yes | 27.00% | 15.80% | 17.20% | 1.28 (0.96 - 1.70) |
0.99 (0.66 - 1.50) |
||||
| Diabetes | No | 89.40% | 94.90% | 94.80% | Reference | 0.051 | Reference | 0.83 | |
| Yes | 10.60% | 5.10% | 5.20% | 1.54 (1.00 - 2.38) |
1.07 (0.57 - 2.01) |
||||
| Systemic diseases | Any† | 36.50% | 34.70% | 24.10% | 1.72 (1.35 - 2.20) |
<.001 | 1.15 (0.72 - 1.85) |
0.56 | |
| HLA-B27 and/or spondyloarthropathy |
6.30% | 12.20% | 10.40% | 0.94 (0.65 - 1.36) |
0.7307 | 1.34 (0.84 - 2.13) |
0.22 | ||
| Juvenile idopathic arthritis |
18.00% | 15.30% | 5.00% | 3.77 (2.71 - 5.23) |
<.001 | 1.91 (1.09 - 3.33) |
0.02 | ||
| Sarcoidosis | 11.60% | 9.20% | 7.10% | 1.48 (0.98 - 2.23) |
0.0602 | 1.82 (1.08 - 3.07) |
0.03 | ||
| Type of uveitis | Anterior Uveitis | 56.60% | 59.70% | 54.10% | Reference | <.001 | Reference | 0.053 | |
| Intermediate Uveitis | 2.60% | 12.80% | 17.30% | 0.43 (0.28 - 0.66) |
0.59 (0.34 - 1.01) |
||||
| Panuveitis | 34.40% | 20.90% | 11.60% | 2.18 (1.65 - 2.88) |
1.07 (0.70 - 1.62) |
||||
| Posterior Uveitis | 6.30% | 6.60% | 17.00% | 0.35 (0.22 - 0.57) |
0.54 (0.31 - 0.95) |
||||
| Visual Acuity | 20/40 or better | 4.20% | 31.60% | 63.30% | Reference | <.001 | Reference | <.001 | |
| >20/40 - <20/200 | 11.60% | 24.50% | 20.60% | 2.85 (2.04 - 3.98) |
2.13 (1.43 - 3.17) |
||||
| 20/200 or worse | 84.10% | 43.90% | 16.00% | 11.65 (8.73 - 15.54) |
4.69 (3.21 - 6.85) |
||||
| Duration of uveitis at baseline |
<6 months | 19.00% | 36.70% | 35.40% | Reference | <.001 | Reference | 0.16 | |
| 6 months to 2 years | 11.10% | 13.80% | 22.00% | 0.73 (0.50 - 1.07) |
0.80 (0.50 - 1.29) |
||||
| 2 to 5 years | 11.10% | 13.80% | 18.10% | 0.85 (0.58 - 1.26) |
0.79 (0.48 - 1.30) |
||||
| Greater than 5 years | 58.70% | 35.70% | 24.60% | 2.34 (1.77 - 3.10) |
1.23 (0.82 - 1.86) |
||||
| Prior Cataract Surgery by Type |
None | 37.60% | 65.30% | 87.60% | Reference | <.001 | Reference | <.001 | |
| Phacoemulsification | 1.10% | 4.60% | 2.30% | 2.06 (1.13 - 3.76) |
2.35 (1.11 - 4.95) |
||||
| Extracapsular cataract extraction |
10.10% | 8.20% | 2.60% | 5.13 (3.33 - 7.92) |
3.76 (2.06 - 6.85) |
||||
| Intracapsular cataract extraction |
5.80% | 2.00% | 0.50% | 11.60 (5.03 - 26.76) |
12.61 (4.43 - 35.92) |
||||
| Pars plana lensectomy | 7.40% | 1.50% | 0.70% | 7.83 (4.03 - 15.18) |
3.78 (1.55 - 9.22) |
||||
| Unknown | 38.10% | 18.40% | 6.30% | 6.63 (4.94 - 8.90) |
6.25 (4.15 - 9.39) |
||||
| Prior Glaucoma Surgery |
No | 84.70% | 86.20% | 97.00% | Reference | <.001 | Reference | <.001 | |
| Yes | 15.30% | 13.80% | 3.00% | 4.20 (2.83 - 6.23) |
2.71 (1.74 - 4.22) |
||||
| Prior Trabeculectomy |
No | 88.90% | 89.70% | 98.10% | Reference | <.001 | Reference | 0.44 | |
| Yes | 11.10% | 10.30% | 1.90% | 5.40 (3.54 - 8.24) |
1.40 (0.60 - 3.31) |
||||
| Prior Tube-Shunt Surgery |
No | 94.70% | 95.90% | 99.40% | Reference | 0.0002 | Reference | 0.56 | |
| Yes | 5.30% | 4.10% | 0.60% | 5.10 (2.19 - 11.90) |
1.29 (0.55 - 3.03) |
||||
| Prior Pars Plana Vitrectomy |
No | 76.20% | 90.80% | 97.10% | Reference | <.001 | Reference | <.001 | |
| Yes | 23.80% | 9.20% | 2.90% | 4.97 (3.50 - 7.08) |
2.56 (1.58 - 4.14) |
||||
| Prior Retinal Detachment Surgery |
No | 93.10% | 96.90% | 99.10% | Reference | 0.0001 | Reference | 0.66 | |
| Yes | 6.90% | 3.10% | 0.90% | 4.04 (1.99 - 8.21) |
1.22 (0.50 - 2.98) |
||||
| Signs of Inflammatory Activity at Presentation |
Anterior chamber cells |
Quiet | 56.10% | 32.50% | 56.20% | Reference | <.001 | Reference | <.001 |
| 0.5+ | 15.60% | 14.70% | 16.50% | 0.97 (0.69 - 1.38) |
1.08 (0.72 - 1.62) |
||||
| 1+ | 12.10% | 20.40% | 12.40% | 1.49 (1.07 - 2.08) |
1.30 (0.85 - 2.00) |
||||
| 2+ | 8.70% | 18.30% | 9.70% | 1.65 (1.16 - 2.35) |
1.97 (1.25 - 3.10) |
||||
| 3+ or worse |
7.50% | 14.10% | 5.20% | 2.49 (1.72 - 3.62) |
3.08 (1.89 - 5.04) |
||||
| Vitreous cells |
Quiet | 58.70% | 53.60% | 57.60% | Reference | 0.0019 | Reference | 0.004 | |
| 0.5+ | 11.90% | 9.80% | 12.70% | 0.88 (0.57 - 1.34) |
0.99 (0.61 - 1.59) |
||||
| 1+ | 7.90% | 16.30% | 15.30% | 0.93 (0.65 - 1.35) |
1.39 (0.91 - 2.13) |
||||
| 2+ | 12.70% | 13.10% | 10.90% | 1.12 (0.73 - 1.71) |
1.53 (0.94 - 2.50) |
||||
| 3+ or worse |
8.70% | 7.20% | 3.40% | 2.35 (1.51 - 3.67) |
2.91 (1.65 - 5.15) |
||||
| Vitreous haze |
Quiet | 70.90% | 74.30% | 82.90% | Reference | 0.0004 | Reference | 0.03 | |
| 1+ | 17.90% | 13.50% | 10.90% | 1.54 (1.05 - 2.26) |
1.59 (1.03 - 2.45) |
||||
| 2+ | 6.00% | 8.80% | 4.40% | 2.04 (1.24 - 3.36) |
2.13 (1.17 - 3.86) |
||||
| 3+ or worse |
5.10% | 3.40% | 1.80% | 2.72 (1.44 - 5.12) |
1.08 (0.31 - 3.77) |
||||
| Keratic precipitates |
No | 85.50% | 70.50% | 84.20% | Reference | 0.0132 | Reference | 0.69 | |
| Yes | 14.50% | 29.50% | 15.80% | 1.49 (1.09 - 2.04) |
0.91 (0.59 - 1.42) |
||||
| Structural complications |
Any† | 67.80% | 62.20% | 43.00% | 2.09 (1.66 - 2.62) |
<.001 | 1.50 (1.09 - 2.05) |
0.01 | |
| Band keratopathy | 33.10% | 19.10% | 4.10% | 7.71 (5.55 - 10.72) |
<.001 | 2.49 (1.36 - 4.56) |
0.003 | ||
| Peripheral anterior synechia |
15.30% | 8.30% | 3.60% | 3.32 (2.19 - 5.04) |
<.001 | 1.52 (0.91 - 2.53) |
0.11 | ||
| Posterior synechia | 33.30% | 30.70% | 18.00% | 1.89 (1.48 - 2.41) |
<.001 | 1.48 (1.05 - 2.09) |
0.03 | ||
| Exudative detachment | 16.50% | 3.60% | 1.30% | 6.50 (3.83 - 11.04) |
<.001 | 5.01 (2.74 - 9.13) |
<.001 | ||
Any systemic disease includes those in the table as well as Behçet’s disease. Any structural complication includes those in the table as well as choroidal neovascularization, epiretinal membrane, exudative detachment, macular edema and retinal neovascularization
Vitreous cells and haze are never adjusted by anterior chamber cells
Adjusting for cataract surgery by type, prior glaucoma surgery, pars plana vitrectomy, retinal detachment surgery, sarcoidosis, juvenile idiopathic arthritis, type of uveitis, age, race, hypertension, diabetes, duration of uveitis at baseline, anterior chamber cells and exudative detachment
Hyperlipidemia, smoking status, epiretinal membrane, macular edema, and retinal vascular sheathing are excluded from the table because they were never found to be significant at p<0.10. Active lesions in choroid/retina, snowballs, snowbanking, Behçet’s disease, choroidal neovascularization and retinal neovascularization are excluded from the table because people with these characteristics had fewer than 5 hypotony events
Incidence of hypotony (IOP<5mmHg for at least 30 days)
A total of 126 out of 6,785 uveitic eyes (1.86%) initially free of hypotony and followed sufficiently long to be at risk developed sustained hypotony during 20,972.27 eye-years of observation, an incidence rate of 0.006 per person-year (95% CI 0.005-0.007). Of the 101 patients who developed hypotony in at least one eye, 76 developed unilateral and 25 developed bilateral hypotony. Risk factors for incident hypotony are given as Table 4, available at http://aaojournal.org and the final Cox regression model results are shown in Table 5. Children were more likely to develop hypotony than adults (adjusted hazard ratio (aHR) 1.202.927.10 with respect to adults ages 26-35). Early adults (age 18-25 years) also tended to develop hypotony more often than adults in the 26-35 year-old age range (aHR 0.982.275.27). As in the prevalence analysis, eyes of non-white and diabetic patients were more likely to develop hypotony, but the association was abrogated after adjusting for other factors in the multiple regression analysis; hypertension tended to be associated greater risk (aHR 0.992.044.20). Uveitic eyes of patients with juvenile idiopathic arthritis also had a strong crude association with incident hypotony, but the association was abrogated by adjustment for other factors (aHR0.792.045.31). Sarcoidosis was not associated with increased risk of hypotony in the incidence analysis.
Table 4. Hazard ratio for Intraocular Pressure <5mmHg, sustained for at least 30 days.
| Univariate n=6,785 events=126 |
Multivariate** n=6,540 event=97 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Events/ At Risk |
Hazard Ratio (95% Confidence Interval) |
p- value |
Events/ At Risk |
Hazard Ratio (95% Confidence Interval) |
p- value |
|||
| Age | <18 | 45/973 | 4.97 (2.58 - 9.57) | <.001 | 36/933 | 2.92 (1.20 - 7.10) | 0.15 | |
| 18-25 | 22/897 | 2.50 (1.21 - 5.15) | 19/878 | 2.27 (0.98 - 5.27) | ||||
| 26-35 | 15/1483 | Reference | 12/1458 | Reference | ||||
| 36-45 | 15/1300 | 1.19 (0.53 - 2.65) | 11/1275 | 0.99 (0.40 - 2.43) | ||||
| 46-55 | 13/983 | 1.39 (0.63 - 3.08) | 9/963 | 1.00 (0.35 - 2.85) | ||||
| 56-65 | 6/556 | 1.14 (0.39 - 3.35) | 5/548 | 0.79 (0.22 - 2.82) | ||||
| >65 | 7/497 | 1.87 (0.69 - 5.07) | 5/485 | 0.84 (0.24 - 2.97) | ||||
| Gender | Male | 34/2388 | Reference | 0.12 | 23/2305 | Reference | 0.49 | |
| Female | 92/4397 | 1.45 (0.91 - 2.30) | 74/4235 | 1.22 (0.70 - 2.14) | ||||
| Race | White | 73/4755 | Reference | 0.002 | 55/4629 | Reference | 0.74 | |
| African-derived | 42/1208 | 2.22 (1.42 - 3.46) | 32/1148 | 1.25 (0.71 - 2.22) | ||||
| Other | 11/822 | 1.10 (0.52 - 2.30) | 10/763 | 1.03 (0.49 - 2.14) | ||||
| Contralateral uveitis |
No | 8/1170 | Reference | 0.02 | 8/1144 | Reference | 0.41 | |
| Yes | 118/5615 | 2.35 (1.14 - 4.87) | 89/5396 | 1.39 (0.63 - 3.07) | ||||
| Hypertension | No | 86/5547 | Reference | 0.006 | 70/5351 | Reference | 0.052 | |
| Yes | 40/1238 | 1.89 (1.20 - 2.97) | 27/1189 | 2.04 (0.99 - 4.20) | ||||
| Diabetes | No | 121/6398 | Reference | 0.37 | 95/6169 | Reference | 0.16 | |
| Yes | 5/387 | 0.66 (0.26 - 1.65) | 2/371 | 0.34 (0.08 - 1.52) | ||||
| Systemic Diseases | Any† | 57/1846 | 1.80 (1.20 - 2.72) | 0.005 | 43/1775 | 0.79 (0.42 - 1.49) | 0.46 | |
| HLA-B27 and/or spondyloarthropathy |
16/801 | 1.05 (0.58 - 1.88) | 0.88 | 12/784 | 1.43 (0.61 - 3.32) | 0.41 | ||
| Juvenile idiopathic arthritis | 33/329 | 6.13 (3.83 - 9.81) | <.001 | 25/297 | 2.04 (0.79 - 5.31) | 0.14 | ||
| Sarcoidosis | 14/576 | 1.05 (0.53 - 2.07) | 0.88 | 11/555 | 0.97 (0.38 - 2.48) | 0.96 | ||
| Type of Uveitis | Anterior Uveitis | 74/3609 | Reference | <.001 | 61/3459 | Reference | <.001 | |
| Intermediate Uveitis | 6/1197 | 0.20 (0.07 - 0.58) | 4/1169 | 0.17 (0.05 - 0.56) | ||||
| Posterior Uveitis | 3/1112 | 0.10 (0.03 - 0.31) | 2/1087 | 0.11 (0.03 - 0.45) | ||||
| Panuveitis | 43/867 | 1.93 (1.24 - 3.01) | 30/825 | 1.25 (0.67 - 2.35) | ||||
| Duration of uveitis entation |
<6 months | 18/2749 | Reference | <.001 | 10/2613 | Reference | 0.002 | |
| 6 months to 2 years | 15/1401 | 1.46 (0.67 - 3.17) | 14/1382 | 1.52 (0.59 - 3.94) | ||||
| 2 to 5 years | 27/1134 | 3.36 (1.68 - 6.69) | 26/1112 | 4.53 (1.94 - 10.61) |
||||
| Greater than 5 years | 66/1501 | 6.13 (3.33 - 11.26) |
47/1433 | 3.08 (1.30 - 7.31) | ||||
| Prior Cataract Surgery by Type |
None | 32/* | Reference | <.001 | 19/* | Reference | <.001 | |
| Phacoemulsification | 13/* | 4.21 (2.15 - 8.27) | 13/* | 4.87 (2.25 - 10.55) |
||||
| Extracapsular cataract surgery | 17/* | 8.00 (4.34 - 14.73) |
16/* | 6.50 (3.14 - 13.45) |
||||
| Intracapsular cataract surgery | 11/* | 22.20 (9.32 - 52.89) |
8/* | 12.19 (3.78 - 39.30) |
||||
| Pars plana lensectomy | 19/* | 25.63 (13.86 - 47.40) |
18/* | 8.51 (3.12 - 23.21) |
||||
| Unknown | 34/* | 13.98 (8.10 - 24.13) |
23/* | 7.28 (3.31 - 16.00) |
||||
| Prior Pars Plana Vitrectomy |
No | 89/* | Reference | <.001 | 63/* | Reference | 0.008 | |
| Yes | 37/* | 5.58 (3.61 - 8.62) | 34/* | 2.03 (1.21 - 3.42) | ||||
| Prior Retinal Detachment Surgery |
No | 118/* | Reference | <.001 | 91/* | Reference | 0.88 | |
| Yes | 8/* | 4.87 (2.24 - 10.59) |
6/* | 0.92 (0.29 - 2.89) | ||||
| Signs of Inflammatory Activity |
Anterior chamber cells |
Quiet | 72/* | Reference | 0.03 | 58/* | Reference | 0.45 |
| 0.5+ | 26/* | 1.94 (1.20 - 3.14) | 19/* | 0.86 (0.46 - 1.58) | ||||
| 1+ | 14/* | 1.76 (0.93 - 3.35) | 13/* | 1.24 (0.63 - 2.46) | ||||
| 2+ | 4/* | 0.78 (0.28 - 2.14) | 3/* | 0.31 (0.08 - 1.14) | ||||
| 3+ or worse |
5/* | 1.87 (0.76 - 4.63) | 4/* | 0.97 (0.32 - 2.94) | ||||
| Vitreous cells |
Quiet | 74/* | Reference | <.001 | 63/* | Reference | 0.005 | |
| 0.5+ | 11/* | 1.02 (0.54 - 1.92) | 10/* | 0.97 (0.46 - 2.04) | ||||
| 1+ | 8/* | 0.80 (0.38 - 1.69) | 7/* | 0.72 (0.27 - 1.90) | ||||
| 2+ | 7/* | 1.23 (0.55 - 2.75) | 7/* | 1.61 (0.65 - 3.94) | ||||
| 3+ or worse |
10/* | 6.07 (2.99 -12.32) | 6/* | 4.73 (2.08 -10.76) | ||||
| Vitreous haze |
Quiet | 75/* | Reference | <.001 | 64/* | Reference | 0.006 | |
| 1+ | 20/* | 2.97 (1.81 - 4.86) | 19/* | 2.48 (1.32 - 4.67) | ||||
| 2+ | 8/* | 4.65 (2.05 -10.56) | 8/* | 3.87 (1.60 - 9.32) | ||||
| 3+ or worse |
5/* | 6.69 (2.29 - | 2/* | 1.32 (0.26 - 6.78) | ||||
| Keratic precipitates |
No | 104/* | Reference | 0.18 | 80/* | Reference | 0.22 | |
| Yes | 14/* | 1.52 (0.83 - 2.78) | 13/* | 1.60 (0.75 - 3.42) | ||||
| Structural complications |
Any† | 85/* | 2.65 (1.77 - 3.96) | <.001 | 70/* | 1.78 (1.06 - 2.99) | 0.03 | |
| Band keratopathy | 38/* | 12.24 (7.90 -18.98) | <.001 | 29/* | 3.14 (1.42 - 6.98) | 0.005 | ||
| Peripheral anterior synechia | 7/* | 3.38 (1.55 - 7.37) | 0.002 | 5/* | 1.16 (0.42 - 3.14) | 0.78 | ||
| Posterior synechia | 31/* | 1.88 (1.22 - 2.89) | 0.005 | 23/* | 2.05 (1.21 - 3.45) | 0.007 | ||
| Exudative detachment | 9/* | 14.15 (6.77 -29.55) |
<.001 | 9/* | 5.25 (1.86 - 14.80) |
0.002 | ||
Any systemic disease includes those in the table as well as Behçet’s disease. Any structural complication includes those in the table as well as choroidal neovascularization, epiretinal membrane, exudative detachment, macular edema and retinal neovascularization
The number at risk for time-varying characteristics changes over followup, and is therefore not reported as a number of patients
Adjusting for age, race, hypertension, juvenile idiopathic arthritis, systemic diseases†, type of uveitis, presence of contralateral uveitis, duration of uveitis at presentation, prior cataract surgery by type, prior pars plana vitrectomy, prior retinal detachment surgery, anterior chamber cells and presence of exudative retinal detachment. However, ‡ Vitreous cells and haze are never adjusted by anterior chamber cells
Hyperlipidemia, smoking status, epiretinal membrane, and macular edema were studied but are not included in the table because they were never found to be significant at p<0.10. Retinal vascular sheathing, presence of active lesions in choroid/retina, snowballs, snowbanking, Behçes Disease, choroidal neovascularization and retinal neovascularization are excluded from the table because eyes with these characteristics had fewer than 5 hypotony events, and there was no suggestion of a protective pattern.
Table 5. Cox Regression Model of Hazard Ratio for IOP<5mmHg, sustained for at least 30 days.
| Univariate n=6,785 events=126 |
Multivariate n=6,540 event=97 |
||||||
|---|---|---|---|---|---|---|---|
| Events/ At Risk |
Hazard Ratio (95% Confidence Interval) |
p- value |
Events/ At Risk |
Hazard Ratio (95% Confidence Interval) |
p- value |
||
| Age | <18 | 45/973 | 4.97 (2.58 - 9.57) | <.001 | 36/933 | 2.92 (1.20 - 7.10) | 0.15 |
| 18-25 | 22/897 | 2.50 (1.21 - 5.15) | 19/878 | 2.27 (0.98 - 5.27) | |||
| 26-35 | 15/1483 | Reference | 12/1458 | Reference | |||
| 36-45 | 15/1300 | 1.19 (0.53 - 2.65) | 11/1275 | 0.99 (0.40 - 2.43) | |||
| 46-55 | 13/983 | 1.39 (0.63 - 3.08) | 9/963 | 1.00 (0.35 - 2.85) | |||
| 56-65 | 6/556 | 1.14 (0.39 - 3.35) | 5/548 | 0.79 (0.22 - 2.82) | |||
| >65 | 7/497 | 1.87 (0.69 - 5.07) | 5/485 | 0.84 (0.24 - 2.97) | |||
| Race | White | 73/4755 | Reference | 0.002 | 55/4629 | Reference | 0.74 |
| African-derived | 42/1208 | 2.22 (1.42 - 3.46) | 32/1148 | 1.25 (0.71 - 2.22) | |||
| Other | 11/822 | 1.10 (0.52 - 2.30) | 10/763 | 1.03 (0.49 - 2.14) | |||
| Contralateral uveitis | No | 8/1170 | Reference | 0.02 | 8/1144 | Reference | 0.41 |
| Yes | 118/5615 | 2.35 (1.14 - 4.87) | 89/5396 | 1.39 (0.63 - 3.07) | |||
| Hypertension | No | 86/5547 | Reference | 0.006 | 70/5351 | Reference | 0.052 |
| Yes | 40/1238 | 1.89 (1.20 - 2.97) | 27/1189 | 2.04 (0.99 - 4.20) | |||
| Systemic Diseases | Behçet’s disease, HLA-B27, spondyloarthropathy, and/or Sarcoidosis |
57/1846 | 1.80 (1.20 - 2.72) | 0.005 | 43/1775 | 0.79 (0.42 - 1.49) | 0.46 |
| Juvenile idiopathic arthritis |
33/329 | 6.13 (3.83 - 9.81) | <.001 | 25/297 | 2.04 (0.79 - 5.31) | 0.14 | |
| Type of Uveitis | Anterior Uveitis | 74/3609 | Reference | <.001 | 61/3459 | Reference | <.001 |
| Intermediate Uveitis | 6/1197 | 0.20 (0.07 - 0.58) | 4/1169 | 0.17 (0.05 - 0.56) | |||
| Posterior Uveitis | 3/1112 | 0.10 (0.03 - 0.31) | 2/1087 | 0.11 (0.03 - 0.45) | |||
| Panuveitis | 43/867 | 1.93 (1.24 - 3.01) | 30/825 | 1.25 (0.67 - 2.35) | |||
| Duration of uveitis at presentation |
<6 months | 18/2749 | Reference | <.001 | 10/2613 | Reference | 0.002 |
| 6 months to 2 years | 15/1401 | 1.46 (0.67 - 3.17) | 14/1382 | 1.52 (0.59 - 3.94) | |||
| 2 to 5 years | 27/1134 | 3.36 (1.68 - 6.69) | 26/1112 | 4.53 (1.94 - 10.61) | |||
| Greater than 5 years | 66/1501 | 6.13 (3.33 - 11.26) | 47/1433 | 3.08 (1.30 - 7.31) | |||
| Prior Cataract Surgery by Type |
None | 32/* | Reference | <.001 | 19/* | Reference | <.001 |
| Phacoemulsification | 13/* | 4.21 (2.15 - 8.27) | 13/* | 4.87 (2.25 - 10.55) | |||
| Extracapsular cataract extraction (ECCE) |
17/* | 8.00 (4.34 - 14.73) | 16/* | 6.50 (3.14 - 13.45) | |||
| Intracapsular cataract extraction (ICCE) |
11/* | 22.20 (9.32 - 52.89) | 8/* | 12.19 (3.78 - 39.30) | |||
| Pars plana lensectomy | 19/* | 25.63 (13.86 - 47.40) |
18/* | 8.51 (3.12 - 23.21) | |||
| Unknown | 34/* | 13.98 (8.10 - 24.13) | 23/* | 7.28 (3.31 - 16.00) | |||
| Prior Pars Plana Vitrectomy |
No | 89/* | Reference | <.001 | 63/* | Reference | 0.008 |
| Yes | 37/* | 5.58 (3.61 - 8.62) | 34/* | 2.03 (1.21 - 3.42) | |||
| Prior Retinal Detachment Surgery |
No | 118/* | Reference | <.001 | 91/* | Reference | 0.88 |
| Yes | 8/* | 4.87 (2.24 - 10.59) | 6/* | 0.92 (0.29 - 2.89) | |||
| Anterior chamber cells | Quiet | 72/* | Reference | 0.03 | 58/* | Reference | 0.45 |
| 0.5+ | 26/* | 1.94 (1.20 - 3.14) | 19/* | 0.86 (0.46 - 1.58) | |||
| 1+ | 14/* | 1.76 (0.93 - 3.35) | 13/* | 1.24 (0.63 - 2.46) | |||
| 2+ | 4/* | 0.78 (0.28 - 2.14) | 3/* | 0.31 (0.08 - 1.14) | |||
| 3+ or worse | 5/* | 1.87 (0.76 - 4.63) | 4/* | 0.97 (0.32 - 2.94) | |||
| Structural complications | Exudative detachment | 9/* | 14.15 (6.77 - 29.55) | <.001 | 9/* | 5.25 (1.86 - 14.80) | 0.002 |
The number at risk for time-varying characteristics changes over followup, and is therefore not reported as a number of patients
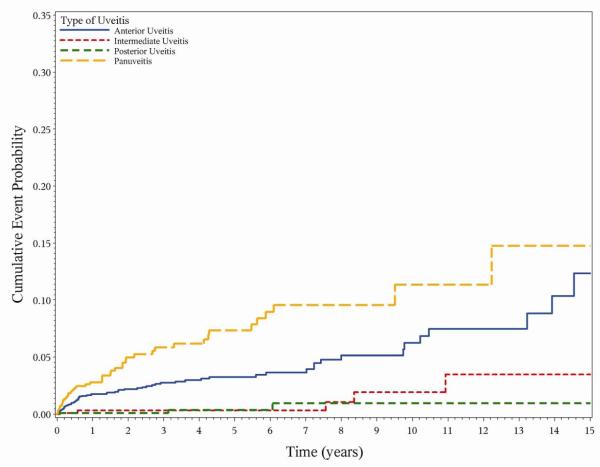
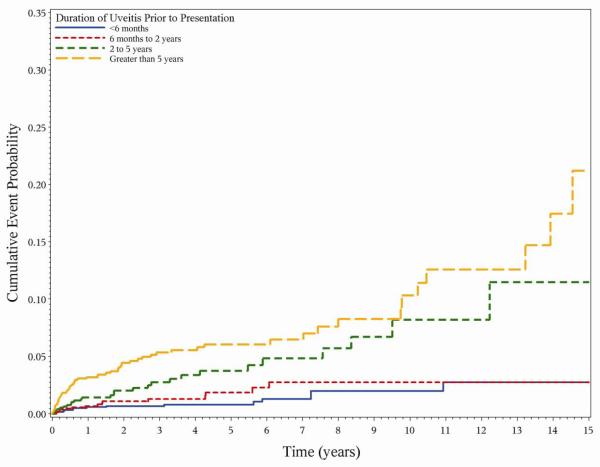
As in the prevalence analysis, both intermediate uveitis (aHR 0.050.170.56) and posterior uveitis (aHR 0.030.110.45) were associated with a lower risk of hypotony than panuveitis (aHR 0.671.252.35) or anterior uveitis (reference group) (Figure 1). Greater duration of uveitis at cohort entry also was associated with increased risk of hypotony, with longstanding cases (≥5 years at presentation) having three-fold higher risk (aHR 1.303.087.31) with respect to newly diagnosed cases (≤6 months at presentation) (Figure 2). Several signs of present inflammatory activity also were associated with chronic hypotony. While anterior chamber cells did not show an association with hypotony, higher levels of vitreous cells and vitreous haze (which persist longer after flareups of inflammation) were associated with a higher risk of hypotony. As in the prevalence analysis, exudative retinal detachment was associated with a particularly high risk of hypotony (aHR 1.865.2514.80). Increased risk of hypotony also was associated with band keratopathy (aHR 1.423.146.98), and posterior synechia (aHR 1.212.053.45), but not with the other ocular complications of uveitis studied.
Figure 1.
Incident hypotony and type of uveitis
Figure 2.
Incident hypotony and duration of uveitis at presentation
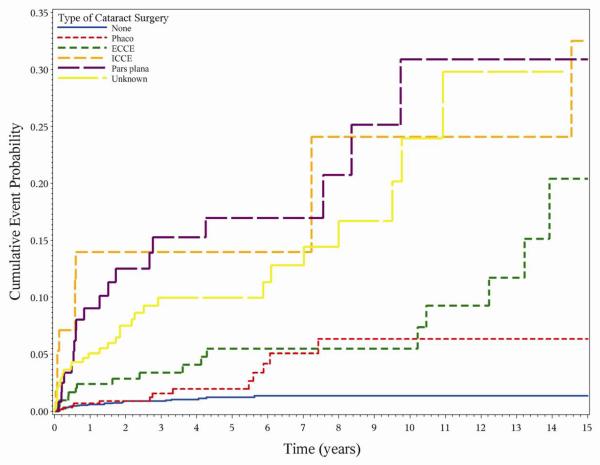
Prior cataract surgery was associated with remarkable differences in the incidence of hypotony. Eyes with uveitis had a 7.5-fold higher risk (aHR = 3.977.5114.23) of incident hypotony following cataract surgery compared to patients who had no cataract surgery, despite censoring the first 30 postoperative days from analysis. Compared to eyes which never had a cataract surgery, eyes with prior phacoemulsification (aHR 2.254.8710.55), extracapsular cataract extraction (ECCE) (aHR 3.146.5013.45), intracapsular cataract extraction (ICCE) (aHR 3.7812.1939.30), pars plana lensectomy (aHR 3.128.5123.21) and unknown types of cataract surgery (aHR 3.317.2816.00) all had a greater risk of developing hypotony than unoperated eyes (Figure 3). Although the relative risk of hypotony associated with cataract surgery was high, the absolute risk was relatively low (1.31.72.1% per eye-year). By Kaplan-Meier analysis, an estimated 91.693.494.8 % of eyes were free of hypotony five years after cataract surgery, vs. 98.298.899.2 % of phakic eyes five years after presentation (log-rank p-value <0.0001). Among the 1618 eyes that had previously undergone cataract surgery, 1,143 (70.6%) were pseudophakic, 461 (28.5%) were aphakic; for 6 (0.37%) no information on intraocular lens status was available. When lens status was substituted for type of cataract surgery, both aphakia (aHR 5.5811.1722.37 ) and pseudophakia (aHR 2.104.138.12) were associated with a higher risk of hypotony when compared with eyes without any cataract surgery. Compared to pseudophakia, aphakia had a higher risk (1.452.705.04) of hypotony. Aphakia was more common in eyes that had undergone ICCE (n=52/58, 89.7%) or pars plana lensectomy (n=118/131, 90.1%) than in eyes which underwent phacoemulsification (n=57/677, 8.4%) or ECCE (n=105/217, 32.6%).
Figure 3.
Incident hypotony and type of cataract surgery. Phaco=Phocoemulsification, ECCE=Extracapsular cataract extraction, ICCE=Intracapsular cataract extraction and pars plana= Pars plana lensectomy
Following pars plana vitrectomy, eyes also had increased risk of hypotony (aHR 1.212.033.42) than eyes which had not undergone vitrectomy, but the effect was not as large as that of cataract surgery. As in the prevalence analysis, eyes that had undergone retinal detachment repair had higher crude risk of hypotony, but the risk was not sustained following adjustment for other factors.
The analogous analyses evaluating the incidence of sustained low IOP (less than 8 mm Hg) were performed, and yielded similar results (see Table 6, available at http://aaojournal.org)
Table 6. Hazard ratio for Intraocular Pressure <8mmHg, sustained for at least 30 days.
| Univariate n=6785 events=241 |
Multivariate** n=6524 events=189 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Events/ At Risk |
Hazard Ratio (95% Confidence Interval) |
p- value |
Events/ At Risk |
Hazard Ratio (95% Confidence Interval) |
p- value |
|||
| Age | <18 | 79/973 | 3.49 (2.22 - 5.51) | <.001 | 58/924 | 1.74 (0.95 - 3.18) | 0.35 | |
| 18-25 | 43/897 | 2.01 (1.21 - 3.33) | 36/876 | 1.61 (0.90 - 2.88) | ||||
| 26-35 | 37/1483 | Reference | 31/1455 | Reference | ||||
| 36-45 | 31/1300 | 1.01 (0.58 - 1.76) | 25/1273 | 1.02 (0.57 - 1.84) | ||||
| 46-55 | 25/983 | 1.06 (0.61 - 1.85) | 20/963 | 1.11 (0.57 - 2.16) | ||||
| 56-65 | 10/556 | 0.78 (0.33 - 1.85) | 9/548 | 0.74 (0.29 - 1.90) | ||||
| >65 | 12/497 | 1.23 (0.60 - 2.49) | 10/485 | 0.79 (0.32 - 1.97) | ||||
| Gender | Male | 66/2388 | Reference | 0.03 | 48/2299 | Reference | 0.41 | |
| Female | 175/4397 | 1.42 (1.03 - 1.97) | 141/4225 | 1.17 (0.81 - 1.70) | ||||
| Race | White | 137/4755 | Reference | <.001 | 112/4624 | Reference | 0.06 | |
| African-derived | 82/1208 | 2.35 (1.70 - 3.26) | 60/1139 | 1.56 (1.03 - 2.36) | ||||
| Other | 22/822 | 1.10 (0.65 - 1.85) | 17/761 | 0.89 (0.51 - 1.54) | ||||
| Contralateral uveitis |
No | 18/1170 | Reference | 0.005 | 16/1142 | Reference | 0.28 | |
| Yes | 223/5615 | 2.01 (1.24 - 3.28) | 173/5382 | 1.35 (0.78 - 2.31) | ||||
| Hypertension | No | 183/5547 | Reference | 0.17 | 147/5336 | Reference | 0.47 | |
| Yes | 58/1238 | 1.29 (0.90 - 1.84) | 42/1188 | 1.20 (0.73 - 1.98) | ||||
| Diabetes | No | 228/6398 | Reference | 0.63 | 180/6153 | Reference | 0.69 | |
| Yes | 13/387 | 0.86 (0.48 - 1.56) | 9/371 | 0.86 (0.42 - 1.78) | ||||
| Systemic Diseases | Any† | 100/1846 | 1.59 (1.17 - 2.15) | 0.003 | 71/1764 | 0.73 (0.48 - 1.13) | 0.16 | |
| HLA-B27 and/or spondyloarthropathy |
24/801 | 0.79 (0.50 - 1.24) | 0.3 | 19/783 | 1.35 (0.71 - 2.56) | 0.36 | ||
| Juvenile idopathic arthritis | 57/329 | 5.59 (3.91 - 7.98) | <.001 | 37/288 | 3.09 (1.56 - 6.14) | 0.001 | ||
| Sarcoidosis | 26/576 | 1.06 (0.66 - 1.69) | 0.82 | 20/553 | 1.25 (0.60 - 2.57) | 0.55 | ||
| Type of Uveitis | Anterior Uveitis | 124/3609 | Reference | <.001 | 97/3448 | Reference | 0.02 | |
| Intermediate Uveitis | 25/1197 | 0.51 (0.30 - 0.85) | 22/1168 | 0.72 (0.40 - 1.28) | ||||
| Posterior Uveitis | 19/1112 | 0.39 (0.23 - 0.68) | 16/1086 | 0.67 (0.36 - 1.27) | ||||
| Panuveitis | 73/867 | 2.01 (1.42 - 2.83) | 54/822 | 1.61 (1.01 - 2.58) | ||||
| Duration of uveitis at presentation |
<6 months | 55/2749 | Reference | <.001 | 41/2610 | Reference | 0.03 | |
| 6 months to 2 years | 34/1401 | 1.10 (0.66 - 1.83) | 28/1378 | 0.87 (0.50 - 1.50) | ||||
| 2 to 5 years | 43/1134 | 1.76 (1.10 - 2.82) | 40/1110 | 1.59 (0.94 - 2.70) | ||||
| Greater than 5 years | 109/1501 | 3.42 (2.33 - 5.00) | 80/1426 | 1.70 (1.05 - 2.74) | ||||
| Prior Cataract Surgery by Type |
None | 94/* | Reference | <.001 | 65/* | Reference | <.001 | |
| Phaco | 25/* | 2.97 (1.81 - 4.89) | 21/* | 2.78 (1.62 - 4.79) | ||||
| Extracapsular cataract extraction |
36/* | 6.58 (4.31 - 10.04) | 34/* | 5.47 (3.35 - 8.92) | ||||
| Intracapsular cataract extraction |
15/* | 11.82 (5.74 - 24.35) |
12/* | 7.03 (2.84 - 17.40) | ||||
| Pars plana lensectomy | 26/* | 14.54 (9.15 - 23.11) |
25/* | 5.49 (2.79 - 10.82) | ||||
| Unknown | 45/* | 6.42 (4.26 - 9.70) | 32/* | 3.79 (2.20 - 6.53) | ||||
| Prior Pars Plana Vitrectomy |
No | 182/* | Reference | <.001 | 137/* | Reference | 0.008 | |
| Yes | 59/* | 4.76 (3.37 - 6.73) | 52/* | 1.89 (1.24 - 2.90) | ||||
| Prior Retinal Detachment Surgery |
No | 231/* | Reference | <.001 | 181/* | Reference | 0.52 | |
| Yes | 10/* | 3.26 (1.65 - 6.45) | 8/* | 0.73 (0.29 - 1.88) | ||||
| Signs of Inflammatory Activity |
Anterior chamber cells |
Quiet | 128/* | Reference | <.001 | 101/* | Reference | 0.06 |
| 0.5+ | 47/* | 1.92 (1.35 - 2.74) | 38/* | 1.06 (0.68 - 1.66) | ||||
| 1+ | 23/* | 1.64 (1.01 - 2.66) | 20/* | 1.20 (0.72 - 1.99) | ||||
| 2+ | 19/* | 2.07 (1.24 - 3.45) | 15/* | 1.42 (0.80 - 2.52) | ||||
| 3+ or worse |
18/* | 3.73 (2.24 - 6.19) | 15/* | 2.57 (1.34 - 4.91) | ||||
| Vitreous cells |
Quiet | 132/* | Reference | 0.004 | 114/* | Reference | 0.04 | |
| 0.5+ | 28/* | 1.43 (0.92 - 2.24) | 26/* | 1.25 (0.77 - 2.02) | ||||
| 1+ | 22/* | 1.18 (0.73 - 1.90) | 21/* | 1.20 (0.71 - 2.03) | ||||
| 2+ | 14/* | 1.35 (0.76 - 2.38) | 14/* | 1.55 (0.81 - 2.95) | ||||
| 3+ or worse |
11/* | 3.73 (1.90 - 7.31) | 8/* | 2.98 (1.49 - 5.95) | ||||
| Vitreous haze |
Quiet | 155/* | Reference | <.001 | 137/* | Reference | 0.006 | |
| 1+ | 32/* | 2.31 (1.51 - 3.52) | 31/* | 1.97 (1.23 - 3.15) | ||||
| 2+ | 12/* | 3.30 (1.75 - 6.23) | 11/* | 2.46 (1.24 - 4.90) | ||||
| 3+ or worse |
7/* | 4.38 (1.86 - 10.31) | 5/* | 1.60 (0.65 - 3.95) | ||||
| Keratic precipitates |
No | 198/* | Reference | 0.03 | 156/* | Reference | 0.8 | |
| Yes | 28/* | 1.59 (1.04 - 2.42) | 23/* | 1.07 (0.64 - 1.77) | ||||
| Structural complications |
Any† | 164/* | 2.70 (1.99 - 3.65) | <.001 | 127/* | 1.58 (1.11 - 2.26) | 0.01 | |
| Band keratopathy | 57/* | 9.71 (6.98 - 13.50) | <.001 | 38/* | 2.79 (1.57 - 4.94) | <.001 | ||
| Peripheral anterior synechia |
12/* | 2.95 (1.62 - 5.39) | <.001 | 8/* | 1.29 (0.60 - 2.73) | 0.51 | ||
| Posterior synechia | 77/* | 2.68 (1.97 - 3.63) | <.001 | 52/* | 2.45 (1.66 - 3.61) | <.001 | ||
| Exudative detachment | 13/* | 12.32 (6.54 - 23.21) |
<.001 | 13/* | 4.84 (2.23 - 10.49) | <.001 | ||
Any systemic disease includes those in the table as well as Behçet’s disease. Any structural complication includes those in the table as well as choroidal neovascularization, epiretinal membrane, exudative detachment, macular edema and retinal neovascularization
The number at risk for time-varying characteristics changes over followup, and is therefore not reported as a number of patients
Adjusting for age, race, hypertension, juvenile idiopathic arthritis, systemic diseases†, type of uveitis, presence of contralateral uveitis, duration of uveitis at presentation, prior cataract surgery by type, prior pars plana vitrectomy, prior retinal detachment surgery, anterior chamber cells and presence of exudative retinal detachment. However, ‡ Vitreous cells and haze are never adjusted by anterior chamber cells
Hyperlipidemia, smoking status, epiretinal membrane, and macular edema were studied but are not included in the table because they were never found to be significant at p<0.10. Retinal vascular sheathing, presence of active lesions in choroid/retina, snowballs, snowbanking, Behçes Disease, choroidal neovascularization and retinal neovascularization are excluded from the table because eyes with these characteristics had fewer than 5 hypotony events, and there was no suggestion of a protective pattern.
Outcome of hypotony
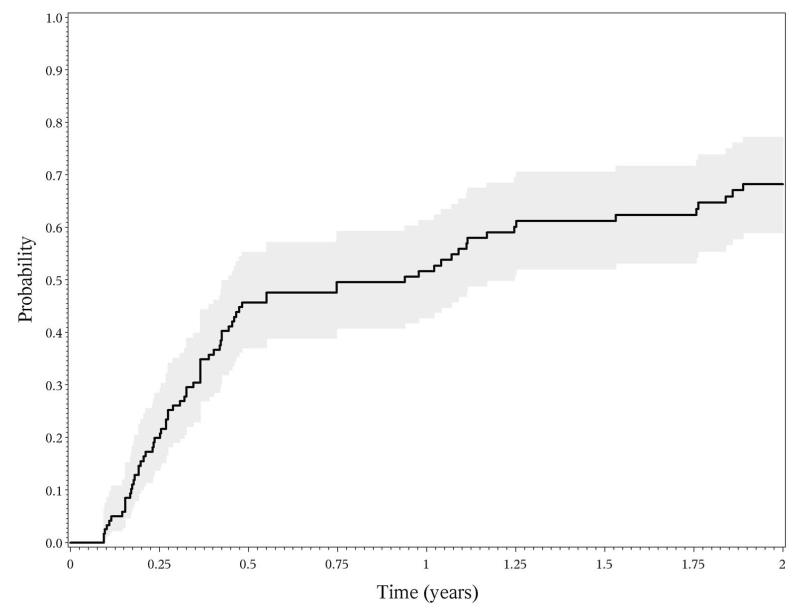
The 126 eyes observed to develop sustained hypotony <5mmHg over visits spanning at least 30 days were followed for a mean of 1.34 years (from 2 days to 10.35 years) after their first hypotonous measurement. During this time the mean IOP measurement was 4.26 (SD 3.89); 84 (66.6%) had at least one follow-up visit with an IOP ≥5. The Kaplan-Meier estimated median recovery time (first IOP≥5 in eyes with IOP ≥5 sustained for at least 30 days) was 0.440.941.25 years. The proportion with a visit with IOP ≥5 mmHg was 3746%63 by 6 months, 4352%61 by 1 year and 5968%78 by 2 years (Figure 4). Visual acuity in hypotonous eyes had a 0.0060.0800.154, (p=0.0465) logMAR increase in visual acuity as compared to a 0.0030.0040.011 logMAR increase in non-hypotonous eyes (p=0.047) with a difference of 0.0010.0760.150 during this time. A higher grade of anterior chamber cells at the time of hypotony were associated with more frequent recovery from hypotony, eyes with 2 + or worse having a 88.9% recovery compared to a 65.3% recovery in eyes with grade zero cells (1.252.113.56 p<0.01). Cataract surgery status was not significantly associated with recovery (p=0.639).
Figure 4.
Recovery of incident hypotony.
Sensitivity Analysis
A sensitivity analysis using only IOP measurements by Goldmann applanation tonometry yielded similar results to the above.
Discussion
Our study of a large cohort of eyes of patients with uveitis demonstrated that hypotony is an uncommon adverse outcome which is strongly associated with poor visual acuity. Prior studies evaluating the risk of hypotony in the context of uveitis have been small, uncontrolled case series or anecdotal cases. The 0.006/eye year incident rate of hypotony in our study is lower than that reported among pediatric uveitis patients (0.03/person year)4 and among children with uveitis associated with juvenile arthritis (0.09/eye year),5 the latter reporting a group of patients from one of our participating centers (most of whom would have been included in the present study). Restricting our population to pediatric uveitis patients and juvenile idiopathic arthritis cases, the incident rates of hypotony were 0.0110.0150.022/eye-year and 0.0190.02810.043/eye-year, respectively, still somewhat less than the former estimates from smaller series. Since our uveitis patients, and those in the other reports, were drawn from tertiary uveitis clinics, it is likely that our estimate of the risk of hypotony is higher than that which would be found in a primary ophthalmology setting where average disease severity likely is lower, but probably is reasonably representative of tertiary centers. Our cohort also did not contain infectious uveitis cases, some of which have been reported to develop hypotony. 10, 11
Young age was associated with both hypotony as well as low intraocular pressure. Children with uveitis in our cohort had a three-fold risk of developing hypotony than young adults. Hypotony maculopathy has been reported to be associated with young age,12, 13 which might reflect a similar underlying association. Hypotheses regarding the potential cause of this strong association include potentially greater average severity of cases of uveitis presenting early in life and/or possible adverse effects of uveitis on development of the ciliary body or outflow pathways (when uveitis occurs in very young children).
We excluded from analysis those patients who had anti-glaucoma surgery from the point when surgery was performed given the fundamentally different pathophysiology of hypotony resulting from overfiltration. We also excluded the early post-operative period after cataract surgery, during which it is expected that some patients will have transient hypotony.14
Our results demonstrated a striking association between cataract surgery and subsequent hypotony (a 7.5-fold higher risk), which was lowest with phacoemulsification, which still was associated with four-fold higher risk of hypotony. A similar pattern of association was seen in association with hypotony at presentation and with incident low intraocular pressure (less than 8 mm Hg). We were not able to evaluate whether the practice of operating on eyes that had been free of inflammation for three months or longer, or the use of perioperative pre-emptive corticosteroid therapy, mitigated the effect of cataract surgery on hypotony risk, although it was the usual practice of the centers to employ these approaches to all kinds of intraocular surgery. Several reasons have been cited in the ophthalmic literature for non-uveitic patients developing hypotony after cataract surgery. The association of cataract surgery and hypotony among patients with uveitis could have similar reasons in some cases: wound leak, not apparent with slit lamp examination but diagnosed through ultrasound bio-microscopy;15,16 inadvertent cyclodialysis clefts; 17,18rotation of intraocular lens haptic into a cyclodialysis cleft 19; and the haptic of intraocular lens causing cilio-choroidal irritation.20 We did not have information on the occurrence of such events in this cohort.
One possible explanation of the strong association between cataract surgery and hypotony is that cataract developed in patients with more severe uveitis which drove both the incidence of cataract requiring surgery and the incidence of hypotony. However, adjustment for variables directly measuring the current activity of uveitis and past sequelae of inflammation only slightly attenuated the association with cataract surgery. Also, pars plana vitrectomy was associated with a substantially lesser effect on the incidence of hypotony than cataract surgery, whereas intuitively we might expect eyes requiring vitrectomy to be more severely affected by uveitis. Another possibility is that cataract surgery’s known effect in shifting the distribution of IOP downward by a small amount substantially increases the small proportion of cases in the tail of the distribution below 5 mm Hg. In mostly healthy eyes of a broad range of patients undergoing cataract surgery, it is well-known that the IOP tends to be slightly lower post-operatively both a few months following surgery,21 and even five years later.22 Finally, we could speculate that there may be some biological effect of cataract surgery on the ciliary body or other anterior segment structures, perhaps a result of reduced tension from the zonules on the ciliary body having physiological effects on aqueous secretion. It seems unlikely that IOL chafing is a major factor, given that pseudophakic eyes had less hypotony. While the mechanism of the effect will require further evaluation, it seems clear that cataract surgery is associated with a large increase in the relative risk of hypotony, which may be important to consider when contemplating cataract surgery on eyes already with low IOP. Fortunately the absolute risk of hypotony is low after cataract surgery, suggesting that the large majority of patients who stand to benefit from such surgery are unlikely to develop this complication. The observation that phacoemulsification is associated with the lowest risk of cataract surgery among the various cataract surgery techniques provides further support of preference for this surgical technique, although it is possible that surgeons tended to select this technique in simpler cases or in more recent times when clinical care generally was more successful than in former years. The pattern of association of phacoemulsification relative to other forms of cataract surgery persisted after adjusting for various factors indicative of severity of uveitis (duration of uveitis, cells in the anterior and posterior chambers, visual acuity and the type of uveitis). While observational data are limited for purposes of causal inference regarding treatments, the available data suggest that phacoemulsification is preferable, if feasible.
Whether or not to insert an IOL into an eye with uveitis has been a topic of debate in the past, but more recently most eyes with uveitis undergoing cataract surgery receive IOLs. Although our data suggest a lower risk of hypotony in pseudophakic than aphakic eyes, these results are subject to confounding from the surgical approach and treatment selection bias-cases with worse uveitis may have been left aphakic more often. Nevertheless, these results do not suggest there should be a change in the current practice of using IOLs in most situations.
Our results generally support the thesis that more severe inflammation is associated with greater risk of hypotony, as indicated by the greater risk of hypotony with longer duration (persistence) of inflammatory disease, and with several measures of current inflammatory activity. Strong association with the forms of uveitis likely to affect the ciliary body and occurrence in association with anterior segment complications of severe (iris synechiae) or chronic (band keratopathy) cases suggest that severe anterior segment inflammation is likely an important cause of hypotony, and imply that hypotony potentially is preventable with effective management. Less strong association with anterior chamber cells than with vitreous cells or haze probably reflects the more rapid resolution of anterior chamber cellular activity than of vitreous cells and haze may take longer to resolve. Exudative retinal detachment likely causes retinal detachment via ciliary body detachment and/or through the cluster of mechanisms whereby retinal detachment tends to cause low IOP.
Juvenile idiopathic arthritis-associated uveitis has been thought to be associated with a particularly high risk of hypotony. In one such series, 40% of the eyes had hypotony lasting from 1 week to four months.23 We did observe a substantially higher risk of hypotony in this group, but the risk was substantially reduced (and rendered non-significant in the incidence analysis) by adjustment for other factors, suggesting that associated factors such as younger age (earlier onset of uveitis), longer duration of disease, and chronicity of inflammation as indicated by band keratopathy may be important factors in causing hypotony in these patients. A tendency to perform vitrectomy and pars plana lensectomy in these cases, and adjustment for these factors in multiple regression, also have contributed to the abrogation of the “effect” of JIA on hypotony in the incidence analysis. In an observational study, it is difficult to determine whether the conditions that led surgeons to take that approach or the approach itself (or both) contributed to increased risk. Other systemic disease associations with uveitis, including sarcoidosis and spondyloarthropathy, were not observed to be associated with an increased risk of hypotony.
In summary, uveitic eyes of patients being treated for uveitis developed hypotony at the rate of 0.61%/eye-year. Apart from prior glaucoma surgery, risk factors associated with hypotony include younger age, long duration of uveitis, more severe current inflammation, presence of complications of severe (iris synechiae) or chronic (band-shaped keratopathy) inflammation, exudative retinal detachment and prior intraocular surgery. Vitrectomy surgery was associated with a 2-fold and cataract surgery was associated with a 7.5-fold increased risk of developing hypotony, with the least increase in risk (~5-fold) among cases managed by phacoemulsification. Avoidance of cataract or vitrectomy surgery is not always possible, but fortunately the absolute risk of hypotony is low following these procedures. While not addressed in this report, obtaining consistent control of inflammation prior to intraocular surgery and use of pre-emptive perioperative corticosteroids might reduce the risk of hypotony following surgery.24, 25 Considerations of hypotony risk suggest a preference for the phacoemulsification approach to cataract surgery, or for extracapsular cataract extraction if phacoemulsification is not feasible. Steps to prevent or remediate hypotony in uveitis cases center on obtaining and maintaining excellent control of inflammation (and remission if possible) without excessive use of corticosteroids, so as to spare eyes as much as possible from the inflammation and inflammatory sequelae that are associated with a higher risk of hypotony.
Supplementary Material
Acknowledgments
Financial Support:
This study was supported primarily by National Eye Institute Grant EY014943 (JHK). Additional support was provided by Research to Prevent Blindness and the Paul and Evanina Mackall Foundation. JHK was an RPB James S Adams Special Scholar Award recipient, JET was an RPB Harrington Special Scholar Award recipient, and DAJ and JTR were Research to Prevent Blindness Senior Scientific Investigator Award recipients during the course of the study. GAL-C was previously supported by and RBN continues to be supported by intramural funds of the National Eye Institute. EBS receives support from the Department of Veterans’ Affairs. None of the sponsors had any role in the design and conduct of the report; collection, management, analysis, and interpretation of the data; or in the preparation, review, and approval of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
Financial Disclosure(s):
The author(s) have made the following disclosure(s): C. Stephen Foster: (equity owner) Eyegate, (consultant, lecturer) Allergan; (consultant, lecturer) Bausch & Lomb; (consultant) Sirion; (lecturer) Alcon; (lecturer) Inspire; (lecturer) Ista; (lecturer) Centocor; Douglas A. Jabs: (consultant) Roche; (consultant) Genzyme Corporation; (consultant) Novartis; (consultant) Allergan; (consultant) Glaxo Smith Kline; (consultant) Applied Genetic Technologies Corporation; (consultant) The Emmes Corporation; (consultant) The Johns Hopkins Dana Center for Preventive Ophthalmology; John H. Kempen: (consultant) Lux Biosciences; (consultant) Allergan; (consultant) Alcon; (consultant) Sanofi-Pasteur; (consultant) Allergan; (consultant) Harbor BioSciences; James Rosenbaum: (equity owner) Amgen, (consultant) Abbott; (consultant), ESBATech, (consultant) Lux Biosciences, (consultant) Centocor, (consultant) Genentech.
Institutional Review Board Approval
The project was conducted in accordance with the principles of the Declaration of Helsinki, with the approval of the governing Institutional Review Boards of each institution, each of which has granted waiver of consent, allowing all living and deceased patients to be included.
Online Material: This article contains online-only material. The following should appear online-only: Tables 1,3, 4 and 6.
Meeting Presentation: Presented as a poster at ARVO, May 2011.
References
- 1.Schubert HD. Postsurgical hypotony: relationship to fistulization, inflammation, chorioretinal lesions, and the vitreous. Surv Ophthalmol. 1996;41:97–125. doi: 10.1016/s0039-6257(96)80001-4. [DOI] [PubMed] [Google Scholar]
- 2.Loh AR, Acharya NR. Incidence rates and risk factors for ocular complications and vision loss in HLA-B27-associated uveitis. Am J Ophthalmol. 2010;150:534–42. doi: 10.1016/j.ajo.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JA, Mackensen F, Sen HN, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009;116:1544–51. doi: 10.1016/j.ophtha.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg KD, Feuer WJ, Davis JL. Ocular complications of pediatric uveitis. Ophthalmology. 2004;111:2299–306. doi: 10.1016/j.ophtha.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Thorne JE, Woreta F, Kedhar SR, et al. Juvenile idiopathic arthritis-associated uveitis: incidence of ocular complications and visual acuity loss. Am J Ophthalmol. 2007;143:840–6. doi: 10.1016/j.ajo.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 6.Kapur R, Birnbaum AD, Goldstein DA, et al. Treating uveitis-associated hypotony with pars plana vitrectomy and silicone oil injection. Retina. 2010;30:140–5. doi: 10.1097/IAE.0b013e3181b32f06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. Ophthalmic Epidemiol. 2008;15:47–55. doi: 10.1080/09286580701585892. [DOI] [PubMed] [Google Scholar]
- 8.Zeger SC, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]
- 9.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84:1065–73. [Google Scholar]
- 10.Accorinti M, Ciapparoni V, Pirraglia MP, Pivetti-Pezzi P. Treatment of severe ocular hypotony in AIDS patients with cytomegalovirus retinitis and cidofovir-associated uveitis. Ocul Immunol Inflamm. 2001;9:211–7. doi: 10.1076/ocii.9.3.211.3968. [DOI] [PubMed] [Google Scholar]
- 11.Bainbridge JW, Raina J, Shah SM, et al. Ocular complications of intravenous cidofovir for cytomegalovirus retinitis in patients with AIDS. Eye (Lond) 1999;13:353–6. doi: 10.1038/eye.1999.89. [DOI] [PubMed] [Google Scholar]
- 12.Stamper RL, McMenemy MG, Lieberman MF. Hypotonous maculopathy after trabeculectomy with subconjunctival 5-fluorouracil. Am J Ophthalmol. 1992;114:544–53. doi: 10.1016/s0002-9394(14)74481-2. [DOI] [PubMed] [Google Scholar]
- 13.Fannin LA, Schiffman JC, Budenz DL. Risk factors for hypotony maculopathy. Ophthalmology. 2003;110:1185–91. doi: 10.1016/S0161-6420(03)00227-6. [DOI] [PubMed] [Google Scholar]
- 14.Shingleton BJ, Rosenberg RB, Teixeira R, O’Donoghue MW. Evaluation of intraocular pressure in the immediate postoperative period after phacoemulsification. J Cataract Refract Surg. 2007;33:1953–57. doi: 10.1016/j.jcrs.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 15.Sicco thoe Schwartzenberg GW, Pavlin CJ. Occult wound leak diagnosed by ultrasound biomicroscopy in patients with postoperative hypotony. J Cataract Refract Surg. 2001;27:549–54. doi: 10.1016/s0886-3350(00)00647-7. [DOI] [PubMed] [Google Scholar]
- 16.Machemer HF, Roters S. Ultrasound biomicroscopy of chronic hypotony after cataract extraction. J Cataract Refract Surg. 2001;27:327–9. doi: 10.1016/s0886-3350(00)00604-0. [DOI] [PubMed] [Google Scholar]
- 17.Mushtaq B, Chiang MY, Kumar V, et al. Phacoemulsification, persistent hypotony, and cyclodialysis clefts. J Cataract Refract Surg. 2005;31:1428–32. doi: 10.1016/j.jcrs.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 18.Maffett MJ, O’Day DM. Cyclodialysis cleft following a scleral tunnel incision. Ophthalmic Surg. 1994;25:387–8. [PubMed] [Google Scholar]
- 19.Lee YC, Chung FL, Chen CC. Intraocular pressure and foveal thickness after phacoemulsification. Am J Ophthalmol. 2007;144:203–8. doi: 10.1016/j.ajo.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Jahn CE. Reduced intraocular pressure after phacoemulsification and posterior chamber intraocular lens implantation. J Cataract Refract Surg. 1997;23:1260–4. doi: 10.1016/s0886-3350(97)80325-2. [DOI] [PubMed] [Google Scholar]
- 21.Davenport WH, Brown RH, Lynch MG. Hypotony after rotation of an intraocular lens haptic into a cyclodialysis cleft. Am J Ophthalmol. 1986;101:736–7. doi: 10.1016/0002-9394(86)90782-8. [DOI] [PubMed] [Google Scholar]
- 22.Kayikcioglu O, Yagci A, Akkin C. Hypotony: an unusual consequence of intraocular lens malposition. J Cataract Refract Surg. 1997;23:1425–7. doi: 10.1016/s0886-3350(97)80128-9. [DOI] [PubMed] [Google Scholar]
- 23.Flynn HW, Jr, Davis JL, Culbertson WW. Pars plana lensectomy and vitrectomy for complicated cataracts in juvenile rheumatoid arthritis. Ophthalmology. 1988;95:1114–9. doi: 10.1016/s0161-6420(88)33051-4. [DOI] [PubMed] [Google Scholar]
- 24.Foster CS, Fong LP, Singh G. Cataract surgery and intraocular lens implantation in patients with uveitis. Ophthalmology. 1989;96:281–8. doi: 10.1016/s0161-6420(89)32898-3. [DOI] [PubMed] [Google Scholar]
- 25.Okhravi N, Lightman SL, Towler HM. Assessment of visual outcome after cataract surgery in patients with uveitis. Ophthalmology. 1999;106:710–22. doi: 10.1016/S0161-6420(99)90155-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.