Abstract
Background
A typology of cerebral vasospasm has been proposed based on distinct clinical manifestations: delayed cerebral ischemia, symptomatic ‘vasospasm’, angiographic vasospasm, and transcranial Doppler vasospasm. We examined each distinct clinical manifestation in a nonparametric genetic association study.
Aims
The purpose of this study was to examine and compare each four distinct acute clinical manifestations and test its perspectives in genetic association studies.
Methods
Two hundred forty-five Caucasian patients with sub-arachnoid hemorrhage were evaluated for these four distinct clinical manifestations along with 906 600 single-nucleotide polymorphisms across the human genome.
Results
The four clinical manifestations were significantly associated with each other as P-values ranged from 3·31 × 10−4 to 8·10 × 10−15. Transcranial Doppler vasospasm showed significant genetic association with single nucleotide polymorphism (SNP) (rs999662, P = 3·39 × 10−8). Statistical P-value of rs999662 in association with delayed cerebral ischemia, symptomatic ‘vasospasm’, and angiographic vasospasm was 0·0017, 0·0017, and 0·19, respectively.
Conclusions
Despite different criteria for each of the four clinical manifestations, they are significantly associated with each other. Our results suggest transcranial Doppler vasospasm may be an appropriate intermediate but still clinically relevant phenotype for genetic association studies. Association with SNP rs999662 indicates a potential role for the region containing the solute carrier family 12 member 3 (SLC12A3) gene in transcranial Doppler vasospasm following sub-arachnoid hemorrhage.
Keywords: brain, genetic disorders, hemorrhage, ischaemic stroke, risk factors, sub-arachnoid hemorrhage
Introduction
Cerebral vasospasm (CV), a transient constriction of the cerebral arteries, is a frequently occurring condition following a sub-arachnoid hemorrhage (SAH). The lack of consensus in the characteristic pathology and consequences of CV makes prediction difficult and treatment questionable. This inconsistency comes primarily from interchanging radiographic evidence of vasospasm with clinical features of cerebral ischemia. A typology of CV has been proposed based on distinct clinical manifestations: delayed cerebral ischemia (DCI), symptomatic vasospasm (SV), angiographic vasospasm (AV), and transcranial Doppler (TCD) vasospasm (1). An international ad hoc panel of experts in SAH supports the distinct morphological and clinical characteristics associated with vasospasm and DCI as different measures and also suggest that as outcomes, they should be separately reported (2). Following these authors’ recommendation, we operationalized these four distinct acute clinical outcomes following SAH in this study as follows:
DCI: presence of cerebral infarction on neuroimaging
symptomatic ‘vasospasm’: clinical deterioration showing focal neurological impairment
AV: moderate to severe arterial narrowing on angiography
TCD vasospasm: elevated flow velocity of vessels.
DCI occurs in ~30% of SAH patients surviving the initial hemorrhage. It is based on evidence of cerebral ischemia detected by computed tomography (CT) scan. Similar to symptomatic ‘vasospasm’, it is closely associated with poor clinical outcomes following SAH (1). It is, however, a retrospective diagnosis, and its lack of value for preemptive intervention limits its clinical use. Symptomatic ‘vasospasm’ appears to be the most clinically significant manifestation in that it is characterized by clinical worsening. It occurs in 20–40% of SAH patients and is associated with poor outcomes. It is a subjective diagnosis and difficult to be applied in poor grade cases (3). AV reportedly occurs in up to 70% of patients (4). Only a subset of AV patients results in clinical symptoms (4,5). TCD vasospasm, using TCD techniques, detects the presence of vascular hemodynamic changes within the major arteries of the brain and is frequently used in the clinical setting. But its efficacy in predicting severity of clinical worsening is inconsistent (6).
Between 5% and 20% of SAH patients report a positive family history of SAH (7) and first-degree relatives of patients with SAH have threefold to sevenfold increased risk of SAH as compared with second-degree relatives who have the same level of risk as general population (8–10). Some studies report that SNP in the promoter region (–786 T>C, a nucleotide conversion from thymine to cytosine) of endothelial nitric oxide synthase (eNOS) encoding gene is associated with symptomatic ‘vasospasm’ or AV after SAH (11,12), while others suggest no association (13). It is suggested that the DCI and CV are complex traits influenced by multiple pathways (inflammation, vascular activity, fibrinolysis). Similar to many other complex traits, multiple genetic factors may contribute subtle effects on interrelated mechanisms that act together (14) to induce phenotypic manifestations after SAH. Selection of appropriate phenotype can be critical in finding responsible genetic factors for the clinical outcome of SAH.
Aims
No research has compared the genetic determinants among the different types of clinical manifestations across the entire human genome. The purpose of this study is to examine and compare each four distinct acute clinical manifestations and test its perspectives in genetic association studies.
Methods
Patient population
Subjects included in the final analyses were 245 Caucasians among a total of 268 aneurismal SAH patients who were admitted to the University of Pittsburgh Medical Center and provided consent for use of samples for genetic marker assessment. This study was approved by the Institutional Review Board (IRB) of University of Pittsburgh and Office of Human Subject Research (OHSR) of National Institutes of Health (NIH).
Vasospasm
Data from TCDs conducted daily from the anterior, middle, and posterior cerebral arteries were available from the study. Patients underwent conventional angiography and/or computed tomographic angiography as standard of care or in the presence of elevated TCD velocities and/or the presence of clinical neurologic deterioration. The angiography results were coded based on the location and severity of vasospasm (moderate 25–75%, severe >75% vessel narrowing). Neurologic deterioration and symptomatic ‘vasospasm’ was defined as the development of new focal neurological signs including new focal deficits, changes in level of consciousness, and/or deterioration in pupil exam. DCI was defined as the appearance of neurological deterioration associated with new perfusion deficits or new infarction on CT. AV was defined as the presence of moderate and/or severe arterial narrowing. TCD vasospasm was defined as Lindegaard ratio >3 or mean MCA velocity >120 cm/s.
Genotype data collection
The genome-wide association study (GWAS) was conducted with Affymetrix Gene Chip Assay SNP 6·0 (Affymetrix, Santa Clara, CA, USA) using 500 ng of genomic DNA extracted from peripheral blood.
Data analysis
Using SPSS 15·0 (IBM SPSS, Armonk, New York, USA), independent t-test was performed to analyze the influence of age, gender, Fisher scale, and Hunt and Hess score at the admission. Chi-squared test was performed to analyze relationship among four distinct clinical manifestations following SAH.
Quality control procedures (15) excluded 31 810 SNPs with call rates <0·9, as well as 70 866 SNPs not in Hardy–Weinberg equilibrium (P < 0·001) in the sample, yielding 803 924 SNPs available for analysis. For GWAS statistical analyses, we used INTERACTIVE TREE ANALYSIS OF HELIX TREE 6·2.0 software (Golden Helix, Bozeman, MT) with minimum number of elements in a node at 10 (16).
For multiple test correction, P = 5 × 10−8 was the threshold in this GWAS. Risk factors (age, gender, Fisher scale, Hunt and Hess score) were selected based on known epidemiologic data and pathways. Bonferroni multiple test correction was also applied to the interactions within and between the risk factors and clinical manifestations. Threshold for the statistical significance was P = 0·05 after the correction. P-values in the manuscript represent noncorrected P-values.
Results
The mean age of 245 Caucasian patients was 53·9 years (ranged from 18 to 75) and 70% were female.
Features and concordance among CV manifestations
DCI occurred in 44·5% of patients, symptomatic ‘vasospasm’ in 44·5%, AV in 25·7%, and TCD vasospasm in 78·8%. Table 1 compares the baseline clinical information by the four acute clinical manifestations. Fisher scale (P = 0·002) and Hunt and Hess (HH) score (P = 1·64 × 10−4 and 4·32 × 10−4, respectively) showed significant association with DCI and symptomatic ‘vasospasm’ but not with angiographic and TCD vasospasm. There was a significant difference in age; patients with TCD vasospasm were younger than those without TCD vasospasm (P = 2·75 × 10−5).
Table 1.
Baseline clinical information by the four clinical manifestations
| DCI
|
Symptomatic ‘vasospasm’
|
Angiographic vasospasm
|
Transcranial Doppler vasospasm
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No spasm | Spasm | P-value | No spam | Spasm | P-value | No spasm | Spasm | P-value | No spasm | Spasm | P-value | |
| Age | 53·8 ± 0·98 | 54·0 ± 1·2 | 0·90 | 53·6 ± 1·0 | 54·0 ± 1·2 | 0·81 | 54·3 ± 0·79 | 52·9 ± 1·8 | 0·48 | 60·1 ± 1·6 | 52·3 ± 0·8 | 2·75 × 10−5* |
| Female | 73·6% | 68·8% | 0·47 | 72·9% | 68·8% | 0·57 | 69·8% | 71·4% | 0·87 | 70·2% | 71·0% | 1·0 |
| Fisher | 2·73 ± 0·06 | 3·00 ± 0·06 | 0·002* | 2·72 ± 0·06 | 2·99 ± 0·06 | 0·002* | 2·81 ± 0·05 | 3·05 ± 0·08 | 0·02 | 2·98 ± 0·12 | 2·83 ± 0·05 | 0·18 |
| HH | 2·43 ± 0·09 | 2·92 ± 0·10 | 4·32 × 10−4* | 2·40 ± 0·09 | 2·93 ± 0·10 | 1·64 × 10−4* | 2·61 ± 0·08 | 2·89 ± 0·13 | 0·08 | 2·66 ± 0·17 | 2·66 ± 0·08 | 0·98 |
Statistical significance.
Relationship of each manifestation after SAH along with genetic variations and noncorrected P-values are summarized together in Table 2. The concordance rate between DCI and symptomatic ‘vasospasm’ was 99·1%, and both were highly correlated with angiographic and TCD vasospasm. Of those without DCI, 99·2% were negative in symptomatic ‘vasospasm’ while 94·6%, and 34·4% were negative in angiographic and TCD vasospasm, respectively. Similar pattern was found in patients without symptomatic ‘vasospasm’. Among patients without AV, 69·3% did not develop symptomatic ‘vasospasm’ and DCI, while 24·9% were negative in TCD vasospasm. In patients with negative TCD vasospasm, 93·5% were negative in symptomatic ‘vasospasm’, and 93·6% were negative in DCI and AV.
Table 2.
Relationship of each manifestation along with genetic associations
| DCI
|
Symptomatic ‘vasospasm’ (SV)
|
Angiographic vasospasm (AV)
|
Transcranial Doppler (TCD) vasospasm
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No spam (%) | Spasm (%) | P-value | No spasm (%) | Spasm (%) | P-value | No spasm (%) | Spasm (%) | P-value | No spasm (%) | Spasm (%) | P-value | |
| Concordance | ||||||||||||
| DCI | 99·2 | 99·1 | 8·10 × 10−15* | 69·3 | 88·7 | 1·32 × 10−14* | 93·6 | 55·6 | 1·66 × 10 | |||
| SV | 99·2 | 99·1 | 8·10 × 10−15* | 69·3 | 88·7 | 1·32 × 10−14* | 93·5 | 55·3 | 3·70 × 10 | |||
| AV | 94·6 | 50·5 | 1·32 × 10−14* | 94·6 | 50·5 | 1·32 × 10−14* | 93·6 | 31·1 | 3·31 × 10 | |||
| TCD | 34·4 | 97·2 | 1·66 × 10−10* | 33·6 | 97·2 | 3·70 × 10−10* | 24·9 | 95·2 | 3·31 × 10−4* | |||
| SNP with P-value <10−6 | rs10967965, P = 9·01 × 10−7 | rs6805782, P = 3·07 × 10−7 rs10967965, P = 4·05 × 10−7 rs3849943, P = 5·40 × 10−7 |
rs11521116, P = 7·50 × 10−7 | rs999662, P = 3·39 × 10−8* rs4946752, P = 1·83 × 10−7 rs12417549, P = 2·40 × 10−7 rs6760279, P = 2·45 × 10−7 rs16865932, P = 8·58 × 10−7 rs6992703, P = 8·59 × 10−7 rs6742546, P = 9·47 × 10−7 rs4713864, P = 9·54 × 10−7 |
||||||||
Statistical significance.
Of those with DCI, 50·5% showed AV and 97·2% showed TCD vasospasm. Because of the high concordance rate between symptomatic ‘vasospasm’ and DCI, their relationship to angiographic and TCD vasospasm showed very similar patterns. Among the patients with AV, 88·7% showed DCI and symptomatic ‘vasospasm’, while 95·2% showed TCD vasospasm. In patients with TCD vasospasm, 55·3% showed symptomatic ‘vasospasm’, 55·6% showed DCI, while only 31·1% showed AV.
In patients with both angiographic and TCD vasospasm, 91·5% showed DCI, while 95·5% of patients with negative signs on both angiographic and TCD vasospasm did not develop DCI. When SV was combined with angiographic and TCD vasospasm, 100% of DCI was predicted.
Chi-squared test for manifestations after SAH revealed that four clinical manifestations were significantly associated with each other as P-values ranged from 3·31 × 10−4 to 8·10 × 10−15 (Table 2).
Genetic association
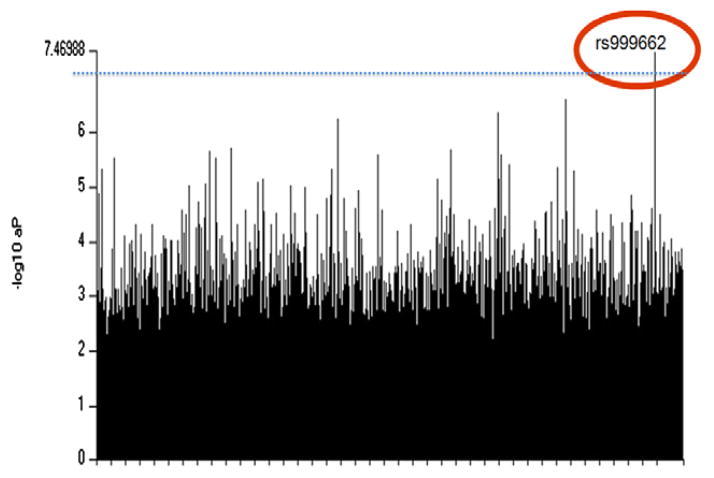
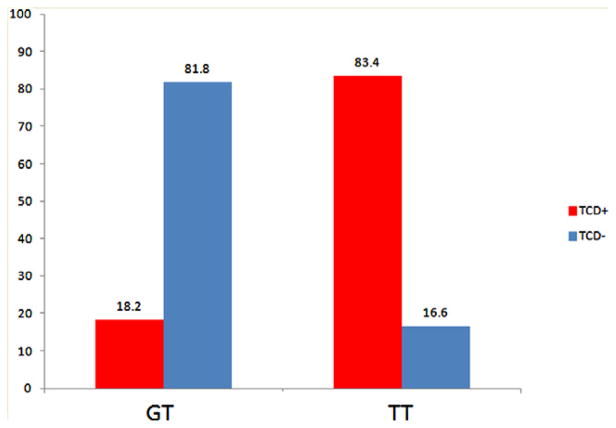
TCD vasospasm showed significant genetic association with SNP (rs999662) with P = 3·39 × 10−8 (Fig. 1, corrected P = 2·68 × 10−2). Table 3 summarized the association between rs999662 and four distinct clinical manifestations. Among 245 patients, 95·5% were genotyped at rs999662 as thymine-thymine (TT) allele and 4·5% as guanine-thymine (GT) allele, while no subject was genotyped as guanine-guanine (GG) allele. Due to phenotypically undetermined patients, final sample size for association analyses of rs999662 is slightly different for four distinct clinical manifestations (Table 3). Odds ratio of TT of rs999662 compared with GT was 22·62 in TCD vasospasm (P = 8·69 × 10−6) (Fig. 2). None of the GT developed DCI or SV, while 48·0% of TT developed DCI (P = 0·001). There was no difference in AV by rs999662. Statistical P-value of rs999662 in association with DCI, SV, and AV was 0·0017, 0·0017, and 0·19, respectively.
Fig. 1.
Plot of statistical significance (−log10P) value of genotyped SNPs across the whole genome Threshold, P = 7·20.
Table 3.
Genetic associations between rs999662 and four distinct clinical manifestations of cerebral vasospasm
| Genotypes | Number of patients
|
|||
|---|---|---|---|---|
| TCD+/TCD− | DCI+/DCI− | SV+/SV− | AV+/AV− | |
| TT | 191/38 | 109/118 | 109/118 | 62/172 |
| GT | 2/9 | 0/11 | 0/11 | 1/10 |
| Interactive tree analysis | 3·39 × 10−8* | 0·0017 | 0·0017 | 0·19 |
Statistical significance.
TCD, transcranial Doppler; DCI, delayed cerebral ischemia; SV, symptomatic vasospasm; AV, angiographic vasospasm.
Fig. 2.
Odds ratio of TT of rs999662 compared with GT was 22·62 in transcranial Doppler vasospasm.
Discussion
All four types of clinical manifestations were associated with clinical severity of SAH based on the admission Fisher scale and HH scores, known predictors for clinical outcomes (17,18). SV and DCI had the strongest association with clinical severity measures, suggesting that they are the most clinically relevant manifestations after SAH, followed by AV and TCD vasospasm. All four types of CV manifestations showed close relationship to each other, suggesting that they may share similar underlying mechanisms. Even though angiographic and TCD vasospasm showed significant association, the level was relatively weak compared with the association with SV or DCI. This may suggest additional underlying factors.
Because of the prognostic value of DCI to long-term outcomes, it was used as a gold standard to evaluate the sensitivity and specificity of the remaining three types of vasospasm after SAH. SV showed highest sensitivity and specificity to DCI, while AV showed lowest sensitivity, and TCD vasospasm showed lowest specificity. When combined together, angiographic and TCD vasospasm significantly increased the sensitivity and specificity, which suggests this combination can be clinically useful in an unconscious patient whose neurologic symptoms cannot easily be evaluated.
TCD vasospasm is the only manifestation affected by age, which is consistent with other reports (1). It had the weakest association with other types of manifestations. TDC vasospasm was the only type with a statistically significant genetic association. This specific characteristic of TCD vasospasm suggests that it may be an intermediate phenotype involving similar biological pathways as the other types of manifestations but may be less susceptible to confounding factors. While the other relations show TCD vasospasm’s clinical relevance, the genetic association highlights TCD vasospasm’s relevance to the gene’s actions (19).
The SNP of significant association with TCD vasospasm is rs999662 located in chromosome 16q13. If we chose SNPs, which passed multiple-test correction of GWAS (rs999662) with the intermediate phenotype (TCD vasospasm) and then tested its association with clinically relevant phenotypes (SV or DCI), the statistical significance could be considered significant (as P = 0·0011). Because its effect size is subtle with additional confounding factors related to more clinical phenotypes, the association might be lost by the extensively strict multiple-test correction (Bonferroni correction). For genetic association study, it may be critical to choose an adequate intermediate phenotype for its success. Our results suggest TCD vasospasm may be an appropriate intermediate but still clinically relevant phenotype for the genetic association studies. Considering the close relationship with other important clinical manifestation along with genetic association, recent criticism on TCD vasospasm (2) may need to be revisited.
The rs999662 SNP associated with TCD vasospasm is located in the intron region of a gene encoding solute carrier family 12 member 3 (SLC12A3). This gene encodes a sodium chloride cotransporter, which is important for electrolyte homeostasis and plays a critical role in regulating the salt/water balance and blood pressure. An association of a SNP in an exon of SLC12A3 with myocardial infarction was reported (20) as well as of the mutations with Gitelman syndrome (21,22), although direct association of genetic variations in the gene with hypertension was not detected in one Asian population (23). Even though genetic association within the introns of genes are common (24), it remains unclear how the nucleotide changes in the intron region of this gene induce functional consequences. Many genes have multiple splicing variants along with multiple functions, most of which are still unknown. It has been previously demonstrated that SNP in an intron of SLC12A3 resulted in a novel cryptic exon in the mRNA (21). It is also estimated that 85% of the human genome sequence is transcribed into some kind of functional elements such as small interference RNA. Therefore, SNPs in any region of the human genome can be meaningful in biological process (14). It is also possible that these SNPs are in linkage disequilibrium with real causative genetic variants in neighboring exons. Dense genotyping around the associated SNP regions are required.
We identified additional loci that were marked by high-signal SNPs, P-value <10−6, in association with four clinical manifestations, though they did not reach genome-wide significance (Table 2). These SNPs in protein coding genes as well as some SNPs in intergenic regions may also be worth further investigation, considering modified P-value of 2·6 to 4·2 × 10−7 was suggested as the threshold of GWAS with ~500 000 markers by a Bayesian formula to attenuate the risk of false negative errors (15,25). Dense genotyping along with linkage disequilibrium analysis for genes located near the high-signal SNP are required.
Interestingly, we found no association with SNPs within the area of eNOS, one of the few alleles found to be associated with vasospasm or other clinical manifestations. It is probably caused by nonpolymorphic minor allele frequency of eNOS SNPs in the microarray platform used in our study. A total of eight SNPs of eNOS were tested, but only three had enough number of minor allele frequency (the smallest n should be bigger than 10 of a group). And the three SNPs (rs3918188, rs891512, and s3793342) did not show association even at the noncorrected P-value threshold 0·05. Closest two SNPs in the flanking regions of rs2070744 (−786 T>C) did not show polymorphism, which suggests rs2070744 is also nonpolymorphic and it is consistent with HapMap data of European Americans.
Considering the relatively small sample size of our study for GWAS, replication of our findings with a second but independent cohort is necessary. It is challenging and resource-intensive to collect large cohorts of life-threatening diseases such as SAH. Even after confirming with replication, validation with the functional genomic assay for associated SNP is required. However, current functional genomic assays cannot be applied for SNPs in the intronic region. New technologies are required to decipher the underlying mechanism of genetic variants located in nonexonic area such as introns and intergenic regions.
In summary, clinical data collected at the time of admission such as Fisher scale and Hunt and Hess score are useful predictors for the probability of clinical manifestations after SAH, specifically symptomatic ‘vasospasm’ and DCI with age and gender as additional risk factors for developing TCD vasospasm.
Despite different criteria for each of the four clinical manifestations, they were found to be significantly associated with each other. Considering that DCI is the most meaningful manifestation for prognosis than other manifestations of CV (1), SV showed highest sensitivity (99·1%) and specificity (99·2%). Even though AV has higher specificity than TCD vasospasm (94·6% vs. 34·4%), its sensitivity is lower than TCD vasospasm (50·5% vs. 97·2%). When the patients showed AV and TCD vasospasm, 91·5% develop DCI while 95·5% of patients with both negative did not develop DCI. When combined with symptomatic ‘vasospasm’, angiographic and TCD vasospasm 100% of the DCI can be predicted.
Among those four clinical manifestations after SAH, only TCD vasospasm showed genetic association with one SNP across the entire human genome. Our results suggest TCD vasospasm may be an appropriate intermediate but still a clinically relevant phenotype for the genetic association studies. It is likely that this genetic variation, rs999662, would influence the vascular reactivity in the brain after SAH and eventually affect on the prognosis of SAH, though the functional consequence of the nucleotides change in intron is unclear. Even with the value of TCD as the intermediate phenotype in genetic association study, its low concordance with other vasospasm phenotypes, along with lack of association with SAH severity scales (as opposed to DCI and SV), requires further studies to evaluate its clinical significance.
Acknowledgments
Funding: This research was funded by National Institutes of Health.
Footnotes
Conflict of interest: None declared.
References
- 1.Frontera JA, Fernandez A, Schmidt JM, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke. 2009;40:1963–8. doi: 10.1161/STROKEAHA.108.544700. [DOI] [PubMed] [Google Scholar]
- 2.Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5. doi: 10.1161/STROKEAHA.110.589275. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt JM, Wartenberg KE, Fernandez A, et al. Frequency and clinical impact of asymptomatic cerebral infarction due to vasospasm after subarachnoid hemorrhage. J Neurosurg. 2008;109:1052–9. doi: 10.3171/JNS.2008.109.12.1052. [DOI] [PubMed] [Google Scholar]
- 4.Vora YY, Suarez-Almazor M, Steinke DE, Martin ML, Findlay JM. Role of transcranial Doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1999;44:1237–47. discussion 47–8. [PubMed] [Google Scholar]
- 5.Nomura Y, Kawaguchi M, Yoshitani K, et al. Retrospective analysis of predictors of cerebral vasospasm after ruptured cerebral aneurysm surgery: influence of the location of subarachnoid blood. J Anesth. 2010;24:1–6. doi: 10.1007/s00540-009-0836-2. [DOI] [PubMed] [Google Scholar]
- 6.Chen F, Wang X, Wu B. Neuroimaging research on cerebrovascular spasm and its current progress. Acta Neurochir Suppl. 2011;110:233–7. doi: 10.1007/978-3-7091-0356-2_42. [DOI] [PubMed] [Google Scholar]
- 7.Schievink WI. Genetics of intracranial aneurysms. Neurosurgery. 1997;40:651–62. doi: 10.1097/00006123-199704000-00001. discussion 62–3. [DOI] [PubMed] [Google Scholar]
- 8.Bromberg JE, Rinkel GJ, Algra A, et al. Subarachnoid haemorrhage in first and second degree relatives of patients with subarachnoid haemorrhage. BMJ. 1995;311:288–9. doi: 10.1136/bmj.311.7000.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Braekeleer M, Perusse L, Cantin L, Bouchard JM, Mathieu J. A study of inbreeding and kinship in intracranial aneurysms in the Saguenay Lac-Saint-Jean region (Quebec, Canada) Ann Hum Genet. 1996;60:99–104. doi: 10.1111/j.1469-1809.1996.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 10.Gaist D, Vaeth M, Tsiropoulos I, et al. Risk of subarachnoid haemorrhage in first degree relatives of patients with subarachnoid haemorrhage: follow up study based on national registries in Denmark. BMJ. 2000;320:141–5. doi: 10.1136/bmj.320.7228.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko NU, Rajendran P, Kim H, et al. Endothelial nitric oxide synthase polymorphism (−786T->C) and increased risk of angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2008;39:1103–8. doi: 10.1161/STROKEAHA.107.496596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starke RM, Kim GH, Komotar RJ, et al. Endothelial nitric oxide synthase gene single-nucleotide polymorphism predicts cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2008;28:1204–11. doi: 10.1038/jcbfm.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song MK, Kim MK, Kim TS, et al. Endothelial nitric oxide gene T-786C polymorphism and subarachnoid hemorrhage in Korean population. J Korean Med Sci. 2006;21:922–6. doi: 10.3346/jkms.2006.21.5.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H, Clark D, Dionne R. Genetic contributions to clinical pain and analgesia: avoiding pitfalls in genetic research. J Pain. 2009;10:663–93. doi: 10.1016/j.jpain.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lencz T, Morgan TV, Athanasiou M, et al. Converging evidence for a pseudoautosomal cytokine receptor gene locus in schizophrenia. Mol Psychiatry. 2007;12:572–80. doi: 10.1038/sj.mp.4001983. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Ramsay E, Lee H, Wahl S, Dionne RA. Genome-wide association study of acute post-surgical pain in humans. Pharmacogenomics. 2009;10:171–9. doi: 10.2217/14622416.10.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Long XA, Luo B, Karuna T, Duan CZ. Factors responsible for poor outcome after intraprocedural rerupture of ruptured intracranial aneurysms: identification of risk factors, prevention and management on 18 cases. Eur J Radiol. 2012;81:e77–85. doi: 10.1016/j.ejrad.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Ha SK, Lim DJ, Kang SH, Kim SH, Park JY, Chung YG. Analysis of multiple factors affecting surgical outcomes of proximal middle cerebral artery aneurysms. Clin Neurol Neurosurg. 2011;113:362–7. doi: 10.1016/j.clineuro.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Huang GH, Hsieh CC, Chen CH, Chen WJ. Statistical validation of endophenotypes using a surrogate endpoint analytic analogue. Genet Epidemiol. 2009;33:549–58. doi: 10.1002/gepi.20407. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Pare G, Parker AN, Zee RY, Miletich JP, Chasman DI. Polymorphism in the CETP gene region, HDL cholesterol, and risk of future myocardial infarction: genome-wide analysis among 18 245 initially healthy women from the Women’s Genome Health Study. Circ Cardiovasc Genet. 2009;2:26–33. doi: 10.1161/CIRCGENETICS.108.817304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nozu K, Iijima K, Nozu Y, et al. A deep intronic mutation in the SLC12A3 gene leads to Gitelman syndrome. Pediatr Res. 2009;66:590–3. doi: 10.1203/PDR.0b013e3181b9b4d3. [DOI] [PubMed] [Google Scholar]
- 22.Coto E, Arriba G, Garcia-Castro M, et al. Clinical and analytical findings in Gitelman’s syndrome associated with homozygosity for the c. 1925 G>A SLC12A3 mutation. Am J Nephrol. 2009;30:218–21. doi: 10.1159/000218104. [DOI] [PubMed] [Google Scholar]
- 23.Chen LY, Zhao WH, Tian W, et al. STK39 is an independent risk factor for male hypertension in Han Chinese. Int J Cardiol. 2012;154:122–7. doi: 10.1016/j.ijcard.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Sinici I, Atalar E, Kepez A, et al. Intron 4 VNTR polymorphism of eNOS gene is protective for cardiac syndrome X. J Investig Med. 2010;58:23–7. doi: 10.2310/JIM.0b013e3181c6197f. [DOI] [PubMed] [Google Scholar]
- 25.Freimer N, Sabatti C. The use of pedigree, sib-pair and association studies of common diseases for genetic mapping and epidemiology. Nat Genet. 2004;36:1045–51. doi: 10.1038/ng1433. [DOI] [PubMed] [Google Scholar]