Abstract
Objective
The regulation of vascular permeability, leukocyte trafficking, and the integrity of endothelial cell-cell contacts are closely linked by a complex mechanism of inter-regulation. Here we investigate the role of KRIT1, an adherens junction accessory protein required for cell-cell junction stability, in the regulating these vascular functions.
Methods and Results
Krit1+/− mice exhibited an enhanced edematous response to the complex inflammatory stimuli found in the passive K/BxN model of inflammatory arthritis and the murine air pouch model, yet leukocyte infiltration was unchanged. Correspondingly, reduced KRIT1 expression increased baseline arteriole and venule permeability 2-fold over that of wildtype littermates, as measured by intravital microscopy of the intact cremaster muscle vascular network, but this increase was not accompanied by increased leukocyte extravasation or activation. Direct stimulation with tumor necrosis factor–α induced increased permeability in wildtype mice, but surprisingly, no increase over baseline levels was observed in Krit1+/− mice, despite extensive leukocyte activation. Finally, adoptive transfer of Krit1+/− bone marrow failed to increase permeability in wildtype mice. However, reduced expression of KRIT1 in the hematopoietic lineage dampened the differences observed in baseline permeability.
Conclusions
Taken together, our data indicate an integral role for KRIT1 in microvessel homeostasis and the vascular response to inflammation.
Keywords: permeability, vascular biology, arthritis, inflammation, CCM1
Vascular permeability is an important function of our cardiovascular system, controlling fluid balance and governing the movement of protein and cells into tissue. Permeability is cooperatively regulated by many inputs, including those from circulating blood cells, neighboring endothelial and smooth muscle cells, and the surrounding tissue. Central to our current understanding of vascular permeability is the regulation of endothelial cell-cell contacts, which contain the structural components, i.e. the cadherin-based adherens junctions and tight junctions, which form the functional barrier of the vessel wall. Much of what is currently understood about the regulation of endothelial cell-cell contacts, and by extension microvessel permeability, is derived from studies in which reversible increases in permeability are induced by inflammatory stimuli 1. Importantly, many of the signals transduced by a variety of inflammatory signals functionally regulate endothelial permeability by altering the stability and localization of the adherens junction protein VE-cadherin 2, 3, suggesting that this may be the final step required for permeability changes, though other adherens and tight junction molecules may play a role. Based on findings in inflammation models, it is assumed that the endothelial cell-cell contact is also the site of permeability regulation under non-inflammatory, or homeostatic, conditions. However, direct manipulation of these contacts in vivo, for example by genetically deleting or mutating VE-cadherin or the junctional molecules p120 or β–catenin, leads to developmental vascular defects that prevent the study of microvessel permeability in situ 4-7. Thus, our understanding of how changes in the stability of the endothelial junction complex affect microvessel permeability is hampered by a lack of appropriate animal models.
KRIT1, an 80kDa scaffolding protein, is a member of a multiprotein complex that promotes endothelial adherens junction stability in vitro 8. In the human population, heterozygous loss-of-function mutations of KRIT1 cause cerebral cavernous malformation (CCM), a vascular malformation characterized by defective endothelial cell junctions and hemorrhage 9. Though global or endothelial specific deletion of Krit1 is embryonic lethal during early vascular development (E9.5) 10, heterozygous mice (Krit1+/−) are viable and model the genetic alteration found in human CCM patients. Our previous studies established a role for KRIT1 in endothelial cells in vitro, where the protein promotes the association of p120 and β–catenin with the cytoplasmic tail of VE-cadherin, thus stabilizing adherens junctions and promoting barrier function 11. Even partial (~50-60%) loss of KRIT1 in vitro is sufficient to increase flux of small molecules across an endothelial monolayer 8, and reduces binding of β– and p120-catenin to the cytoplasmic tail of VE-cadherin 11. In vivo studies using Krit1 heterozygous mice have demonstrated increased Evans Blue extravasation (Miles assay) in lung and brain tissues, particularly in response to lipopolysaccharide (LPS, 12). While these findings point to an increase in protein and/or fluid flux across the vascular wall in the partial absence of Krit1, an important caveat to the Miles assay is that dye flux reflects the combined contribution of hydrostatic forces and vessel permeability. Thus the role of KRIT1 in the context of vascular permeability regulation – i.e. barrier function– in normal vessel physiology warrants further investigation in vivo.
The contribution of KRIT1 to barrier function in vitro suggests that alterations in Krit1 expression could modify physiological responses that require complex changes in vascular permeability by affecting the stability of endothelial cell-cell contacts. Herein we report that decreased KRIT1 expression results in a heightened response to inflammatory stimuli in mouse models of inflammatory arthritis and edema. This response correlates with increased baseline microvessel permeability to albumin in the intact vascular networks in situ. We further explore the relative contribution of Krit1 expression in endothelial and hematopoietic cell populations to the observed defects in barrier function, revealing an unexpected role for KRIT1 in permeability regulation by hematopoietic cells.
Methods
Mice
KRIT1 heterozygous (Krit1+/−) mice were obtained from Dr. Dean Li (University of Utah). These mice are on a clean C57BL/6 background after having been backcrossed 10 generations to C57BL/6NCrl (Charles River Labs, stock #027). B6.SJL-Ptprca Pepcb/BoyJ mice (CD45.1 B6) were obtained from Jackson Laboratory. KRN T cell receptor (TCR) transgenic mice were a gift from Drs. D. Mathis and C. Benoist (Harvard Medical School, Boston, MA and Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France), and were maintained on a C57BL/6N background (K/B) 13. Arthritic mice were obtained by crossing K/B with NOD/Lt (N) animals (K/BxN). Mice were bred and maintained under standard conditions in the University of California, San Diego (UCSD) animal facility (KRN, NOD/Lt, K/BxN and some Krit1+/−) or the University of Rochester animal facility (Krit1+/− and CD45.1 B6), which are accredited by the American Association for Accreditation of Laboratory Animal Care. All protocols were approved by the respective institutional review board. All mice were used between 8 and 12 weeks of age; littermate controls were used in all experiments.
Statistics
Statistical analysis was performed using PRISM software (version 4.0, GraphPad Software Inc., La Jolla, CA). Significance was set at α=0.05.
Serum transfer and arthritis scoring
Arthritic adult K/BxN mice were bled and their sera were pooled. Age-matched male Krit1+/− or Krit1+/+ littermates were injected with 50μl i.p. on Day 0 and Day 2 (n=9 mice/group) or 150 μl i.p. on Day 0 (n=20 mice/group). Clinical arthritis scores were evaluated using a scale of 0–4 for each paw (0, normal; 1, minimal erythema and mild swelling; 2, moderate erythema and mild swelling; 3, marked erythema and severe swelling, digits not yet involved; 4, maximal and swelling, digits involved) for a maximum score of 16. Ankle thickness in mm was measured with a caliper (Manostat, Switzerland). Change in ankle thickness was calculated as increase in size relative to baseline taken prior to serum administration. Statistical analysis was performed using 2-way ANOVA and Bonferroni post-hoc testing.
Miles Assay
Male Krit1+/− or Krit1+/+ littermates (n=8 mice/group) were injected with either K/BxN serum or normal saline, 200μl i.p.: after 15 minutes mice 1mg/ml Evan’s Blue dye (Sigma, St. Louis, MO) was injected via tail vein14. Fifteen minutes following dye injection the mice were bled into EDTA coated capillary tubes and serum collected by centrifugation. The mice were then perfused with PBS for 3 minutes to eliminate circulating dye. The wrists and ankles were harvested individually and extracted with formamide (Sigma). Supernatants were transferred to a 96 well flat bottom plate for absorbance measurement (600nm). Values were normalized to serum, and statistical analysis was performed using ANOVA and Tukey post-hoc testing.
Histology
Whole hind paws (n=8 mice/group) were fixed in 10% formalin, decalcified, trimmed and embedded. Sectioned tissue was stained with H&E and Safranin O (HistoTox, Boulder CO). Joints of arthritic mice were given scores of 0-4 for inflammation, according to the following criteria: 0 = normal; 1 = minimal infiltration of inflammatory cells in periarticular area; 2 = mild infiltration; 3 = moderate infiltration; and 4 = marked infiltration. Cartilage depletion was identified by the presence of diminished Safranin O staining of the matrix and was scored on a scale of 0-4, where 0 = no cartilage destruction (full staining with Safranin O), 1 = localized cartilage erosions, 2 = more extended cartilage erosions, 3= severe cartilage erosions and 4 = depletion of entire cartilage. Histologic analyses were performed in a blinded manner.
Intravital Microscopy
Male wildtype (Krit1+/+) or KRIT1 heterozygous null (Krit1+/−) mice were anesthetized with sodium pentobarbital (65 mg/kg by i.p.) and maintained on supplemental anesthetic via a jugular catheter as needed. The cremaster muscle was prepared, superfused and visualized using established methods 15, 16. Permeability to bovine serum albumin (BSA) was measured as described elsewhere 16. The vascular wall Ps is calculated from the measured flux (Js, mol/sec) of BSA into the tissue 17, with corrections for the source volume and surface area due to the confocal slice. In selected animals, the tissue was activated by intrascrotal injection of mouse recombinant tumor necrosis factor-α (TNF-⟨, 0.5μg in 0.25 ml saline, Sigma) or interleukin-1-β (IL-1β, 30ng in 0.25ml saline, Sigma) 3 hours before beginning the surgical preparation. Alternatively, 30μM histamine (Sigma) was added to the superfusate 5 min prior to image acquisition. On completion of the protocols, animals were euthanized by anesthetic overdose. For all permeability experiments, images were acquired from at least 9 vessel sites, which are the appropriate determinant for n in these experiments. Power analysis appropriate for ANOVA (F-test power) indicates that n=9, with a effect size of 0.2, and significance level of 0.05, results in a power level of 0.90. Data were analyzed using one-way ANOVA with Bonferroni post-hoc testing.
Cell culture and transfection
Human dermal microvascular endothelial cells were obtained from Lonza (Walkerville, PA), maintained in endothelial growth medium-2 (Lonza), and used prior to passage 6. Mouse dermal microvascular endothelial cells were obtained from Cell Biologics (Chicago, IL), maintained in mouse endothelial cell medium (Cell Biologics), supplemented with 10% fetal bovine serum, 10% Krit1+/+ mouse serum, or 10% Krit1+/− mouse serum. Mouse serum was obtained by bleeding into EDTA coated capillary tubes followed by centrifugation. Cells were transfected with non-targeting negative control siRNA and anti-KRIT1 siRNA (Ambion/Invitrogen; ID#146530) as previously reported 8.
RNA isolation and semi-quantitative RT-PCR
RNA was isolated using the RNeasy Micro kit according to the manufacturer’s instructions (Qiagen, Germantown, MD). Complementary DNA was obtained with Superscript III reverse transcriptase using random hexamers (Invitrogen, Grand Island, NY). Taqman assays for human ICAM-1 (Assay ID: Hs00164932_m1), human Krit-1 (Hs00184988_m1), human β-actin (401846) and human GAPDH (Hs99999905_m1) were purchased from Applied Biosystems (Invitrogen). VEGF primers are listed in supplemental methods. Amplifications were run in a 7000 Real-Time PCR system (Applied Biosystems/Invitrogen). Each value was calculated using the comparative Ct method 18 and normalized to internal controls. All samples were run in at least triplicate, and each experiment was performed from 3 separate cell/RNA preparations (n=3). Data were analyzed using 2-way ANOVA with Bonferroni post-hoc testing.
Adoptive bone marrow transfer
Bone marrow recipient adult male B6.SJL-Ptprca Pepcb/BoyJ and Krit1+/− mice (8mice/group) were irradiated (475 Rads) twice 4 hours apart, using the Gammacell40 Cesium irradiator (Theratronics, Ottowa, ON). Immediately following the irradiations, mice were anesthetized with isofluorane (Baxter International, Deerfield, IL) and injected i.v. with 1 × 106 bone marrow cells in 200 μl of HBSS (Mediatech, Manassas, VA). Mice were rested for 8 weeks to allow for complete immune system reconstitution. To evaluate the effectiveness of the bone marrow transplant, peripheral blood cells were isolated from the chimeric mice and analyzed by flow cytometry using antibodies specific for mouse CD45.1 (BD Biosciences, San Jose, CA), CD45.2 (eBioscience) and B220 (BD Biosciences). Data files containing live (propidium iodide negative) cells were collected on an Accuri C6 flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star, Ashland, OR).
Air pouch
The murine air pouch model was performed as described in 19. Briefly, sequential subcutaneous injections of 5ml and 3ml sterile air were performed 72 hours apart on age- and sex- matched isoflurane anesthetized Krit1+/+ or Krit1+/− mice (n=9/group). Seventy-two hours later, the mice were again anesthetized, and the air pouch was filled with 1.1ml sterile 1% carageenan dissolved in PBS (Sigma), or PBS alone. 24 hours later, the exudate was removed using an 18-gauge needle, and the volume and number of cells in the exudate quantified. Cell type distribution was quantified by visual counting of Giemsa stained cells. Statistical analysis was performed using ANOVA with Bonferroni post-hoc testing.
CBC
Complete blood counts and differential blood cell analysis were performed by the UCSD Animal Care Program Diagnostic Laboratory, n=3.
Leukocyte interactions
Bright-field images used to track leukocyte interactions with the vessel wall were acquired with a CCD camera (Dage-MTI CD72). Delivered leukocytes (cells translating in proximity to the vessel wall but not necessarily in continuous contact) were counted per 40 sec time interval. For each experimental group, at least 17 vessel sites were analyzed. Rolling leukocytes were defined as any leukocytes observed translating along the vessel wall continuously in contact with the endothelium for 1 sec or greater as defined in 15, 20. Rolling leukocyte velocity was measured by tracking the distance rolled by individual cells for a period of time greater than 0.15 sec (at least 10 frames) within a 50μm length of vessel. All cells meeting our imaging criteria were analyzed from 17 vessel sites/group. Firmly adhered leukocytes (those remaining stationary for ≥ 30 sec) were counted in 10 vessel sites/group. Cell extravasation was measured as the number of cells in the tissue within 40μm of the vessel wall along a defined vessel length; for each condition, ≥ 9 vessel sites were analyzed. Data were analyzed using one-way ANOVA with Bonferroni post-hoc testing.
Supplemental Methods
TNF-α levels in air-pouch exudates were measured using the mouse TNF-a ELISA Ready-Set-Go assay from eBioscience (San Diego, CA). NFκB reporter assays were performed using the Dual-Glo luciferase assay system (Promega, Madison, WI) according to the manufacturer’s instructions. Cell surface expression of 6 hematopoetic cell markers was measured by antibody labeling, followed by FACS analysis. Serum levels of inflammatory cytokines/chemokines were measured in duplicate using a multiplex bead assay (Eve Technologies, Calgary). Additional information is provided in Supplemental Materials available online.
Results
Effect of Krit1 haploinsufficiency on the development of inflammatory arthritis
We postulated that in Krit1+/− mice, the decrease in junction complex stability could affect their ability to maintain vascular barrier function in response to inflammatory stimuli. Therefore we assessed inflammatory responses in these mice using the K/BxN model of inflammatory arthritis. This is a well-characterized model of rheumatoid arthritis and is serum-transferable to normal recipients, allowing for the study of inflammatory arthritis in other mouse lines without the need for backcrossing 21. This model is marked by a predominantly neutrophil infiltrate and edema, which resolve over time 22.
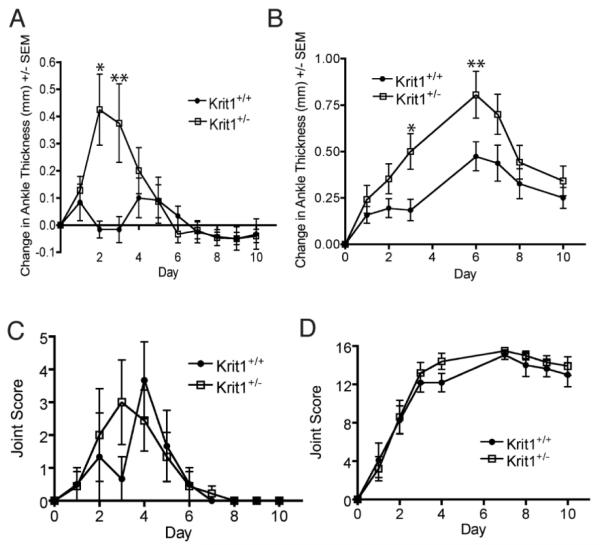
To measure the sensitivity of Krit1+/− mice to inflammatory stimuli, we performed two sequential injections of 50μl of K/BxN serum, which induced a sub-maximal inflammatory response as shown by the number of involved joints (Figure 1C). This dosing regimen was sufficient to induce swelling and arthritis in Krit1+/− mice, however this response was absent in wildtype animals (Figure 1A), indicating Krit1+/− mice are more sensitive to the induction of inflammation. A higher single dose of 150μl also induced a severe polyarthritis in both Krit1+/− and wildtype littermates (Figure 1B). At this dose, both groups developed arthritis to the same degree over the course of the experiment (Figure 1D). However, even at the higher serum dose, the Krit1+/− mice exhibited a significant increase in ankle thickness versus wildtype controls by 4 days post-serum injection, which continued until 7-days post injection at which time the inflammation began to resolve (Figure 1B). Both groups reached their maximum change in ankle size at Day 7, suggesting that the severity, and not the progression of the inflammation was affected.
Figure 1. Reduced Krit1 expression enhances edema formation.
A & C) Response to two 50μl doses of K/BxN serum. Change in ankle thickness is given relative to Day 0, *p<0.001, **p<0.01 vs. wildtype. Joint scoring, maximum score=16. B & D) Response to single 150μl dose of K/BxN serum, Change in ankle thickness, *p<0.05, **p<0.01 vs. wildtype. Joint scoring, maximum score=16.
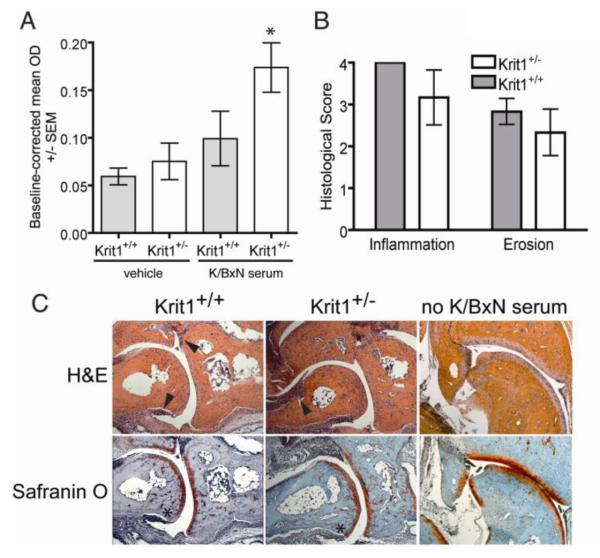
To examine whether the increase in ankle thickness was due to an increase in microvascular leak in response to K/BxN serum, we injected Evans Blue dye 15 minutes prior to injection of serum, then quantified dye accumulation in the paws and feet (representative images shown in Supplemental Figure I) following formamide extraction. As expected, microvascular dye flux was increased in response to K/BxN serum in Krit1+/− animals compared to wildtype controls (Figure 2A). In addition, we examined the influx of inflammatory cells into the ankle joint of arthritic animals by immunohistochemistry. Ankle sections taken at the peak of cellular infiltration (Day 9) from a separate cohort of mice treated in an identical manner were stained with hematoxylin and eosin to assess leukocyte infiltration and bone erosion (i.e. inflammation) and with Safranin O to evaluate peptidoglycan loss and cartilage destruction. All sections were scored by an observer blinded to section identity using an established scale 23. While a slight trend towards decreased inflammatory and erosion scores in Krit1+/− mice was observed, this was not statistically significant (Figure 2B). Representative images are shown in Figure 2C; un-injected tissue is shown for comparison. Arrowheads indicate areas of leukocyte infiltration; asterisks denote cartilage degradation. Thus inflammatory activation by K/BxN serum triggered a larger increase in protein flux across the vessel wall in Krit1+/− mice, but did not affect the accumulation of leukocytes in the inflamed joint.
Figure 2. Reduced Krit1 expression increases edema formation but not leukocyte infiltration in response to K/BxN serum.
A) OD values from formamide extracted paws and feet normalized to serum dye OD, *p<0.05 vs. wildtype + K/BxN serum. B) Mean histological scoring of mice at Day 9 following administration of 150μl of K/BxN serum, +/− SD. C) Representative brightfield images (50x magnification) of inflammation in the talo-navicular joints of animals used for histological scoring. H&E (hematoxylin and eosin) shows general tissue morphology and presence of leukocyte infiltration (arrowheads); Safranin O staining demonstrates local erosion of bone (light blue/purple) and cartilage (red) (asterisks).
Reduced KRIT1 expression increases baseline microvessel permeability
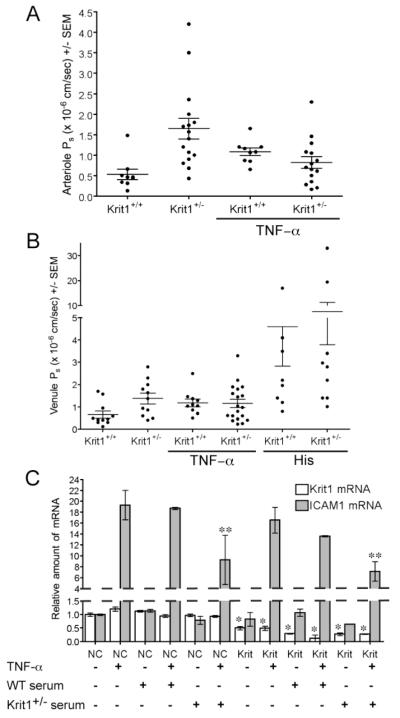
Our studies using the passive K/BxN arthritis model indicated that reduced expression of KRIT1 increases the flux of protein rich fluid into the tissue during inflammation, supporting our hypothesis that Krit1+/− mice exhibit defective fluid homeostasis. To examine whether these changes correlated with lower vascular barrier function in vivo, we directly measured the permeability (Ps) using intravital microscopy, under conditions in which changes in hydrostatic forces are minimal 16. Krit1+/− arterioles exhibited a mean Ps of 1.646 × 10−6 cm/sec, significantly higher than that observed in wildtype littermates (0.531 × 10−6 cm/sec, Table 1). As shown in Figure 3A, this difference is clearly due to both an overall upward shift in value, but also an increase in the variation of Ps values, including an increase in the proportion of highly leaky vessels. The effect of reduced KRIT1 expression in venules is less extreme (mean Ps of 1.383 vs. 0.664 × 10−6 cm/sec, Figure 3B, Table 1), but also clearly demonstrates an upward shift in permeability values. Thus reduced Krit1 expression is sufficient to reduce barrier function in vivo, confirming our earlier work in isolated cells. Notably, this increase was seen in the absence of any inflammatory stimulus, suggesting that KRIT1 haploinsufficiency predisposes these animals to edema formation.
Table 1. Mean Ps values in microvessels of wildtype and Kritl+/− mice.
| Genotype | Microvessel type |
Treatment | Mean Ps (×10−6 cm/sec) +/− SEM | p value* | n= |
|---|---|---|---|---|---|
| Krit1 +/− | Arterioles | none | 1.646 +/− 0.25 | <0.01 | 16 |
| Venules | none | 1.383 +/− 0.24 | <0.01 | 11 | |
| Arterioles | TNF-α | 0.820 +/− 0.15 | n.s† | 15 | |
| Venules | TNF-α | 1.158 +/− 0.19 | n.s† | 19 | |
| Venules | His | 7.510 +/− 1.72 | <0.01 | 9 | |
| Krit1 +/+ | Arterioles | none | 0.531 +/− 0.13 | - | 9 |
| Venules | none | 0.664 +/− 0.16 | - | 11 | |
| Arterioles | TNF-α | 1.189+/− 0.09 | <0.05 | 9 | |
| Venules | TNF-α | 1.189 +/− 0.17 | <0.05 | 10 | |
| Venules | His | 4.607 +/− 1.77 | <0.001 | 9 |
p values are calculated versus wildtype for unstimulated vessels, and versus unstimulated vessels of same genotype for TNF-α and histamine treated animals.
n.s.-not significant.
Figure 3. Reduced Krit1 expression directly alters microvessel permeability.
A) Permeability of albumin in arterioles of Krit1+/− and Krit1+/+ mice. Data shown is mean Ps +/− SEM, Overall p<0.0001 by ANOVA. B) Permeability of albumin in venules of Krit1+/− and Krit1+/+ mice. Overall p<0.001 by ANOVA. C) Relative ICAM-1 and Krit1 mRNA expression in siRNA-transfected mouse MVEC treated with either TNF–α, or serum isolated from Krit1+/+ (WT) or Krit1+/− mice, or both. NC- negative control siRNA transfected cells, Krit1- anti-Krit1 siRNA transfected cells, n=3.
Reduced KRIT1 expression blocks increased permeability in response to TNF–α, but not histamine
We then asked whether the increase in edema formation in the K/BxN model was the result of an increase in Ps in response to the inflammatory stimulus. This model relies on cytokine-dependent processes to trigger edema formation and leukocyte migration. Specifically, those mediated by increased expression of TNF–α and/or IL-1β 19, 24, common inflammatory signals released by leukocytes during inflammatory activation 25. Therefore, we assessed permeability changes in response to TNF–α and IL-1β stimulation.
Injection of IL-1β 1-4 hours prior to imaging failed to induce significant changes in Ps in control or Krit1+/− animals, while triggering a massive leukocyte response in both strains (data not shown). However, injection of TNF–α 4 hours prior to imaging increased both arteriolar and venular Ps ~2-fold in wildtype littermates (Figure 3A and B), in agreement with previous studies 15 which established that this dose of TNF-α induces a maximum increase in permeability. Intriguingly, TNF–α stimulation was unable to increase Ps in Krit1+/− venules (Figure 3B), and surprisingly decreased Ps in Krit1+/− arterioles, though this difference was not statistically significant. We considered whether the lack of permeability increase in response to TNF–α was due to altered responses to TNF–α in Krit1+/− animals. TNF–α levels were similar between groups (Supplemental Figure IIA), as was the serum levels of other inflammatory cytokines or chemokines (Supplemental Figure IIB and IIC). Thus the difference in responsiveness is unlikely to be explained by altered TNF–α or cytokine expression.
Furthermore, reduced KRIT1 expression in endothelial cells does not appear to affect the activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway by TNF–α, considered a major regulatory link between cytokine stimulation and permeability regulation. Krit1 mRNA expression decreased by >60% following anti-Krit1 siRNA transfection of human dermal microvascular endothelial cells (MVEC, Supplemental Figure IIIB) 8. Knockdown of Krit1 did not affect NFκB-dependent luciferase expression in response to TNF–α treatment (Supplemental Figure IIIA), nor did it alter the NFκB-dependent induction of ICAM-1 mRNA by TNF–α 26(Figure 3C). To examine whether the endothelial response was affected by environmental factors, we also cultured siRNA-transfected mouse dermal microvascular endothelial cells (mMVEC) in media containing 10% Krit1+/+ (WT) or Krit1+/− serum. We observed a decrease in TNF-α induced ICAM expression in both negative control and Krit1 siRNA-transfected cells in the presence of Krit1+/− serum, decreasing from an ~18-fold increase to an ~8-fold increase. However, the physiological relevance of this change is uncertain, given that an 8-fold increase in expression is still a significant up-regulation. Thus the absence of a TNF–α–induced increase in permeability in Krit1+/− mice is not likely due to an inability of KRIT1-deficient endothelium to respond to TNF–α stimulation. Finally, the lack of a permeability change in the Krit1+/− mice in response to TNF–α is not due to the maximal loss of barrier function, as treatment of cremaster muscle preps with histamine, and acute mediator of the inflammatory response, increased Ps in both Krit1+/+ and Krit1+/− venules (Figure 3B).
Contribution of the Krit1+/− leukocyte population to microvessel permeability
We then examined the possibility that the role of KRIT1 in vascular permeability regulation may not be confined to its function in the endothelium. We had previously reported that KRIT1 protein is expressed in the hematopoietic lineage 11, and indeed appears to be expressed in most cell types. Furthermore, KRIT1 is an effector of the GTPase Rap1, a known modulator of leukocyte adhesion 27. Given that the interplay between leukocyte and endothelial behavior is complex, it is difficult to predict the exact outcome of altered signaling between or within these two cell populations. Therefore, to examine the potential contribution of Krit1+/− leukocytes to edema formation and microvessel permeability, we generated reciprocal bone marrow chimeras.
We used congenic B6.SJL-Ptprca Pepcb/BoyJ mice (referred to herein as CD45.1 B6) as the wildtype donor/recipient strain. These mice express the differential B cell antigen Ly5.1 (CD45.1), which allows differentiation between donor and recipient cell populations by fluorescence-activated cell sorting (FACS) analysis. Krit1+/− mice are on a C57BL/6N background, and express the Ptprcb allele (CD45.2). Bone marrow cells isolated from either Krit1+/− or CD45.1 B6 donors were injected into irradiated Krit1+/− or CD45.1 B6 hosts. The four groups of mice, which included Krit1+/− bone marrow transferred into Krit1+/− recipients (Krit1+/− chimeras), CD45.1 B6 bone marrow into CD45.1 B6 recipients (CD45.1 B6 chimeras), Krit1+/− bone marrow into CD45.1 B6 recipients (Krit1+/−BM/B6host chimeras) and CD45.1 B6 bone marrow into Krit1+/− recipients (B6BM/Krit1+/−host chimeras) were allowed to reconstitute for 8 weeks, at which time peripheral blood cells were stained for CD45.1, CD45.2, and B220. In all four groups of mice, the percent reconstitution was greater than 95% (Supplemental Figure IV and Supplemental Table I).
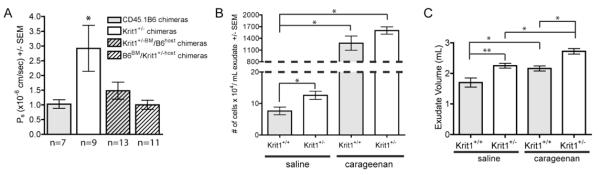
We then measured Ps in the venules of recipient animals by intravital microscopy. Baseline permeability of the chimeric animals was increased compared to non-irradiated control animals (Figure 4A versus Figure 3A). However, similar to our previous observations using non-irradiated Krit1+/− and Krit1+/+ mice, Krit1+/− chimeras exhibited a 3-fold increase in Ps compared to the CD45.1 B6 chimeras. The presence of Krit1+/− leukocytes in the Krit1+/−BM/B6host chimeras slightly increased mean Ps over control levels (1.1 +/− 0.29 in Krit1+/−BM/B6host chimeras versus 1.026 +/− 0.1433 in CD45.1 B6 chimeras), but did not rise to the levels observed in the Krit1+/− chimeras. Interestingly, mean Ps levels in the B6BM/Krit1+/−host chimeras were not significantly different than the mean Ps levels in the control CD45.1 B6 chimeras, indicating that wild-type hematopoietic cells were sufficient to reverse the increased permeability observed in the Krit1+/− chimeras. Together, these data suggest, unexpectedly, that the observed increase in permeability in Krit1+/− mice is due to a requirement for Krit1 in both the hematopoietic and endothelial populations.
Figure 4. Reduced Krit1 expression in bone marrow derived cells is required for increased permeability; reduced Krit1 expression increases fluid extravasation in the murine air pouch model.
A) Permeability of albumin in cremaster microvessels following adoptive transfer, * p<0.01 vs. CD45.1 B6 chimera. Number of vessels analyzed is indicated below columns. B) Number of cells recovered per milliliter exudate, *p<0.01. C) Recovered exudate volumes from saline and carageenan treated mice, *p<0.05, **p<0.001.
Reduced KRIT1 expression does not significantly promote leukocyte migration or adhesion
It is well established that leukocyte activation and adhesion plays an important role in the regulation of vascular permeability. Thus given the apparent contribution of hematopoietic cells to increased permeability in Krit1+/− mice, it appeared obvious to examine whether the increase in permeability was accompanied by a change in leukocyte behavior. Consequently, we utilized the air pouch inflammation model to study the effect of reduced expression of KRIT1 on leukocyte chemotaxis. We injected a previously created subcutaneous air space with either saline or carageenan, the latter of which induces fluid (exudate) accumulation and the infiltration of polymorphonuclear leukocytes (PMN) within 24 hours. There was no difference in leukocyte infiltration following carageenan treatment in Krit1+/− mice versus wildtype controls (Figure 4B), though we did observe a Krit1+/− dependent increase in exudate volume in carageenan treated mice (Figure 4C). Somewhat surprisingly, we observed a small but significant increase in the number of cells found in the air pouch of saline-treated Krit1+/− mice (Figure 4B), concomitant with a significant increase in exudate volume (Figure 4C). The cells found in the exudate of carageenan treated mice and saline controls were primarily PMNs (>95%, data not shown), with a few monocytes present, suggesting some low level of inflammation is present even in saline controls. Thus reduced expression of Krit1 may increase leukocyte extravasation under sub-optimal inflammatory conditions, but has little to no effect on the leukocyte response to inflammatory stimuli.
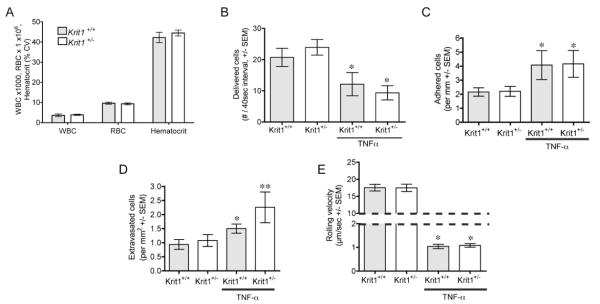
As leukocyte migration is predicated on the adhesion of these cells to the vessel wall, we then directly examined leukocyte adhesion in microvessels, both in the absence of inflammation and following injection of TNF–α. Total white blood cell counts were equivalent and within the normal range in both strains (Figure 5A), and although the differential cell counts showed modest changes in some cell types, these were not significant and were not seen in a more detailed analysis of several hematopoetic markers (Supplemental Figure V). Subsequently, there was no difference between untreated Krit1+/− mice and wildtype littermate controls, in either the delivered (Figure 5B), or adhered (Figure 5C) leukocytes. Measurements were within the expected range for unstimulated vessels 20. While Krit1+/− mice exhibited a slight increase in leukocyte transmigration, this was not statistically significant (Figure 5D). TNF–α induced a significant decrease in leukocyte rolling velocity (Figure 5E), and an increase in adhesion (Figure 5C) and the number of transmigrated cells (Figure 5D) in both groups of mice compared to untreated animals. The TNF–α response appeared as expected 15 in wildtype animals, and no significant increase in velocity, adhesion or extravasation in response to TNF–α was seen in Krit1+/− mice. Thus we conclude that under unstimulated conditions, Krit1+/− leukocytes do not exhibit increased activation, as measured by increased interaction with the vessel wall. Furthermore, these data suggest that the lack of permeability increase in response to TNF–α in Krit1+/− mice is not due to an inability of the leukocyte population to activate adhesion signaling in response to TNF–α stimulation.
Figure 5. Reduced Krit1 expression does not affect leukocyte adhesion.
A) Total cell counts from peripheral blood; WBC, white blood cells, RBC, red blood cells, +/− SEM. B) Delivered flux -visible fraction of leukocytes near the wall/40 sec- in unstimulated and TNF–α treated mice, *p<0.01 vs. untreated. C) Number of adhered leukocytes per mm vessel wall, *p<0.05 vs. untreated. D) Number of leukocytes within 40μm of the vessel wall, per mm2, *p<0.05 vs. untreated Krit1+/+, **p<0.05 vs. untreated Krit1+/−. E) Rolling velocity of wall-associated leukocytes, *p<0.001 vs. untreated.
Discussion
We have now clearly demonstrated that KRIT1 plays an integral role in microvessel homeostasis in vivo. Our data indicate that a reduction in KRIT1 expression leads to an increase in basal microvessel permeability in both arterioles and venules (Figures 3A and B), though the responses in these vessel types show subtle differences. We previously demonstrated, in wildtype animals, that flux occurs across the arteriolar wall, though to a lesser extent than in venules. Krit1+/− mice exhibit enhanced arteriolar permeability vs. venular permeability, suggesting that arteriolar endothelial cells may be more sensitive to loss of KRIT1 expression. Continued exploration of the KRIT1 function in specific vessel types is of particular interest, not the least because of the recent suggestion that CCM lesions are restricted to the venous network. Even so, loss of KRIT1 expression plainly has important implications for vascular homeostasis and edema formation during inflammation (Figure 1). Under homeostatic conditions, the increase in protein/fluid flux into the tissue in Krit1+/− mice appears to be compensated for by a concomitant increase in lymphatic drainage, as these mice do not exhibit edema when unchallenged. Following inflammatory stimuli, permeability may increase beyond the ability of the lymphatics to compensate, leading to severe swelling. This is seen most dramatically in the passive K/BxN model, in which reduced KRIT1 expression led to edema at a dose that did not induce a response in wildtype littermates (Figure 1A). However, we also demonstrated an enhanced response to inflammation in the air pouch synovitis model (Figure 4B and C) and following direct stimulation of a microvascular network in vivo (histamine, Figure 3D). The consequence of this higher basal permeability and sensitivity to inflammation in regards to the development of CCM are unclear. However, it is possible that alterations in the inflammatory response could play a role in lesion development or severity. It has been suggested that the presence of inflammatory cells in CCM lesions is associated with risk of hemorrhage and significant symptomatic disease 28. Thus inflammation could increase the relative risk of hemorrhage from the lesion, or the persistence of post-bleed symptoms. Current clinical practices make it difficult to assess this premise in the human population, but new mouse models of CCM will enable us to test this hypothesis in future studies.
Our initial hypothesis predicts that the mechanism that underlies the higher baseline permeability and increase in edema formation is likely downstream of the destabilizing effect of reduced KRIT1 expression on endothelial cell-cell junctions. This hypothesis was based in part on the assumption that the essential physiological functions of KRIT1 are confined to the endothelial compartment. Despite the fact that CCM patients exhibit a germline defect in Krit1 expression in all cell types, CCM is considered an endothelial disease, as mouse models indicate that loss of CCM proteins in endothelial cells is sufficient to generate lesion formation 29. Therefore, we were very surprised to find that the increase in baseline permeability observed in Krit1+/− animals was completely blocked when the Krit1+/− mice were reconstituted with wildtype bone marrow (Figure 4A). This result contrasts with our findings in vitro, which indicated that reduced KRIT1 expression was sufficient to increase endothelial leak in the absence of immune cells 8. Such differences highlight the need for in vivo studies, in which complex cellular interactions are present that may significantly affect the measured physiological responses.
Nonetheless, our findings suggest that Krit1+/− hematopoietic cells contribute to the enhanced permeability in Krit1+/− mice, a novel finding that opens many new channels of research. Cellular interactions between the vascular endothelial wall and leukocytes have a well-documented ability to modify vascular permeability, thus we closely examined this interaction in vivo. The interaction between leukocytes and the vessel wall occurs to a similar extent in unstimulated Krit1+/− mice compared to wildtype controls (Figure 5), under conditions in which the permeability change is evident (Figure 3A). Furthermore, adoptive transfer of Krit1+/− hematopoietic cells failed to increase permeability in wildtype animals (Figure 4A), and KRIT1 haploinsufficiency enhanced fluid extravasation during inflammation in both the K/BxN and air pouch models without significantly affecting leukocyte extravasation (Figures 1 and 4). While we did observe increased leukocyte extravasation in unstimulated Krit1+/− mice (Figure 4B), this likely reflects an increased likelihood for extravasation due to a decrease in barrier function, and the magnitude of this increase suggests this is unlikely to have physiological implications. It is perhaps more noteworthy that we now can show that full KRIT1 expression is not required for effective leukocyte adhesion, implying that Krit1 is unlikely to be a relevant Rap1 effector in this cell type. Despite these significant findings, future studies will be required to determine what hematopoietic cell compartment is responsible for the enhanced permeability response in Krit1+/− mice.
It is now apparent that the contribution of reduced KRIT1 expression in the hematopoietic compartment to vascular permeability is independent of leukocyte activation and adhesion, (Figure 5), and not due to global differences in cytokine or chemokine expression (Supplemental Figure IIB and IIC). Leukocytes can produce many permeability-inducing factors such as reactive oxygen species, and vascular endothelial growth factor (VEGF), commonly as part of the leukocyte activation process. Intriguingly, it was recently shown that reduced KRIT1 expression in endothelial cells is linked to increased reactive oxygen species signaling 30. Furthermore, we previously published that reduced KRIT1 expression increases expression of VEGF in endothelial cells through activation of the canonical β-catenin pathway 11. Indeed, we observed higher levels of serum VEGF in Krit1+/− mice as well as increased VEGF mRNA in isolated Krit1+/− bone marrow cells (Supplemental Figure IIB and VI). Thus it is possible that changes in VEGF, reactive oxygen species, or other permeability inducing factors drive the increase in basal vascular permeability seen in Krit1+/− mice, a question we are actively pursuing in ongoing studies.
These data now bring into question the mechanism of KRIT1’s biological effects. It appears clear that the overall physiological effect of KRIT1 haploinsufficiency is unlikely to be confined to its functions in the endothelial cell. Furthermore, we recently demonstrated that the Krit1 deficiency-induced decrease in cell contact stability leads to an increase in β–catenin dependent transcriptional activity in vitro and in vivo 11. Thus the effects of KRIT1 deficiency in vivo, including the ability of the endothelium to respond to normal inflammatory stimuli, may extend beyond the stability of the cell-cell contact. This idea is supported by the inability of Krit1+/− mice to increase permeability in response to TNF–α treatment, yet these animals were able to respond to other permeability inducing factors—including histamine (Figures 3A and B), VEGF31, and LPS 12. This suggests that reduced KRIT1 expression does not merely reduce barrier function in vivo, but may actively modify signaling responses to inflammatory mediators.
In summary, our observations point to an important role for KRIT1 in maintaining normal microvessel homeostasis in vivo. Significantly, the requirement for reduced KRIT1 expression in the hematopoietic cell population for increased permeability may have implications for the pathophysiology of CCM. However, these studies also point to a role for KRIT1 in the regulation of barrier permeability in response to inflammation, and in particular the outcome of individual cytokine and chemokine signaling. Further dissecting these mechanisms will allow for a more detailed understanding of the regulation of microvessel permeability in response to these factors, and may provide important insights into the centrality of endothelial cell-cell contact in vascular permeability, including the relative contribution of the actions of the inflammatory stimuli directly on the structural barrier (i.e. endothelial-dependent effects) versus effects downstream of leukocyte activation and adhesion.
Supplementary Material
Acknowledgements
We thank Dr. Mark H. Ginsberg for critical review of the manuscript and valuable comments.
This work was supported by grants from the NIH HL18208 and HL75186 to I. Sarelius, NIH AI063399 to F. Lund, NIH F32AI080104 to R. Misra, Arthritis Foundation to M. Corr, and the American Heart Association to A. Glading (0930071N).
Non-standard Abbreviations and Acronyms
- CCM
cerebral cavernous malformation
- Krit1
Krev interaction-trapped 1
- TNF–α
tumor necrosis factor-alpha
- BSA
bovine serum albumin
- MVEC
dermal microvascular endothelial cells
Footnotes
The authors have no conflicts to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee WL, Liles WC. Endothelial activation, dysfunction and permeability during severe infections. Curr Opin Hematol. 2011;18:191–196. doi: 10.1097/MOH.0b013e328345a3d1. [DOI] [PubMed] [Google Scholar]
- 2.London NR, Smith MC, Li DY. Emerging mechanisms of vascular stabilization. J Thromb Haemost. 2009;7(Suppl 1):57–60. doi: 10.1111/j.1538-7836.2009.03421.x. [DOI] [PubMed] [Google Scholar]
- 3.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: Molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, Kemler R, Dejana E. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oas RG, Xiao K, Summers S, Wittich KB, Chiasson CM, Martin WD, Grossniklaus HE, Vincent PA, Reynolds AB, Kowalczyk AP. P120-catenin is required for mouse vascular development. Circ Res. 2010;106:941–951. doi: 10.1161/CIRCRESAHA.109.207753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci U S A. 1997;94:6273–6278. doi: 10.1073/pnas.94.12.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glading A, Han J, Stockton RA, Ginsberg MH. Krit-1/ccm1 is a rap1 effector that regulates endothelial cell cell junctions. J Cell Biol. 2007;179:247–254. doi: 10.1083/jcb.200705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labauge P, Denier C, Bergametti F, Tournier-Lasserve E. Genetics of cavernous angiomas. Lancet Neurol. 2007;6:237–244. doi: 10.1016/S1474-4422(07)70053-4. [DOI] [PubMed] [Google Scholar]
- 10.Whitehead K, Plummer N, Adams J, Marchuk D, Li D. Ccm1 is required for arterial morphogenesis: Implications for the etiology of human cavernous malformations. Development. 2004;131:1437–1448. doi: 10.1242/dev.01036. [DOI] [PubMed] [Google Scholar]
- 11.Glading AJ, Ginsberg MH. Rap1 and its effector krit1/ccm1 regulate beta-catenin signaling. Dis Model Mech. 2010;3:73–83. doi: 10.1242/dmm.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit rho kinase to stabilize vascular integrity. J Exp Med. 2010;207:881–896. doi: 10.1084/jem.20091258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 14.Miles AA, Miles EM. Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of guinea-pigs. J Physiol. 1952;118:228–257. doi: 10.1113/jphysiol.1952.sp004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumagin R, Kuebel JM, Sarelius IH. Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of icam-1. Am J Physiol Cell Physiol. 2011;301:C804–813. doi: 10.1152/ajpcell.00135.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarelius IH, Kuebel JM, Wang J, Huxley VH. Macromolecule permeability of in situ and excised rodent skeletal muscle arterioles and venules. Am J Physiol Heart Circ Physiol. 2006;290:H474–480. doi: 10.1152/ajpheart.00655.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huxley VH, Curry FE, Adamson RH. Quantitative fluorescence microscopy on single capillaries: Alpha-lactalbumin transport. Am J Physiol. 1987;252:H188–197. doi: 10.1152/ajpheart.1987.252.1.H188. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Romano M, Faggioni R, Sironi M, Sacco S, Echtenacher B, Di Santo E, Salmona M, Ghezzi P. Carrageenan-induced acute inflammation in the mouse air pouch synovial model. Role of tumour necrosis factor. Mediators Inflamm. 1997;6:32–38. doi: 10.1080/09629359791901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumagin R, Sarelius IH. Tnf-alpha activation of arterioles and venules alters distribution and levels of icam-1 and affects leukocyte-endothelial cell interactions. Am J Physiol Heart Circ Physiol. 2006;291:H2116–2125. doi: 10.1152/ajpheart.00248.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. A new mouse model of rheumatoid arthritis: Organ-specific disease provoked by systemic autoimmunity. Ryumachi. 1997;37:147. [PubMed] [Google Scholar]
- 22.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167:1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 23.Choe JY, Crain B, Wu SR, Corr M. Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by toll-like receptor 4 signaling. J Exp Med. 2003;197:537–542. doi: 10.1084/jem.20021850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyburz D, Corr M. The krn mouse model of inflammatory arthritis. Springer Semin Immunopathol. 2003;25:79–90. doi: 10.1007/s00281-003-0131-5. [DOI] [PubMed] [Google Scholar]
- 25.Madge LA, Pober JS. Tnf signaling in vascular endothelial cells. Exp Mol Pathol. 2001;70:317–325. doi: 10.1006/exmp.2001.2368. [DOI] [PubMed] [Google Scholar]
- 26.Ledebur HC, Parks TP. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant nf-kappa b site and p65 homodimers. J Biol Chem. 1995;270:933–943. doi: 10.1074/jbc.270.2.933. [DOI] [PubMed] [Google Scholar]
- 27.Bos J. Linking rap to cell adhesion. Curr Opin Cell Biol. 2005;17:123–128. doi: 10.1016/j.ceb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Shi C, Shenkar R, Du H, Duckworth E, Raja H, Batjer HH, Awad IA. Immune response in human cerebral cavernous malformations. Stroke. 2009;40:1659–1665. doi: 10.1161/STROKEAHA.108.538769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan AC, Drakos SG, Ruiz OE, Smith AC, Gibson CC, Ling J, Passi SF, Stratman AN, Sacharidou A, Revelo MP, Grossmann AH, Diakos NA, Davis GE, Metzstein MM, Whitehead KJ, Li DY. Mutations in 2 distinct genetic pathways result in cerebral cavernous malformations in mice. J Clin Invest. 2011;121:1871–1881. doi: 10.1172/JCI44393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goitre L, Balzac F, Degani S, Degan P, Marchi S, Pinton P, Retta SF. Krit1 regulates the homeostasis of intracellular reactive oxygen species. PLoS One. 2010;5:e11786. doi: 10.1371/journal.pone.0011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitehead KJ, Chan AC, Navankasattusas S, Koh W, London NR, Ling J, Mayo AH, Drakos SG, Marchuk DA, Davis GE, Li DY. The cerebral cavernous malformation signaling pathway promotes vascular integrity via rho gtpases. Nat Med. 2009 doi: 10.1038/nm.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.