Abstract
Weight gain typically accompanies smoking cessation, and women smokers concerned about postcessation weight gain are prone to substantial gain. Little is known about the ways in which cessation affects dietary composition. Understanding postcessation changes in dietary composition may inform the design of smoking cessation interventions to address postcessation weight gain. Participants were women smokers concerned about postcessation weight gain enrolled in a randomized trial and assigned to either bupropion or placebo, and either standard cessation intervention or standard intervention plus components to address weight concerns. Women completed three, 24-hour food recall interviews at baseline, and at 1 and 6 months following a targeted quit date. At 6 months, 22% of women were abstinent and had gained 3.6 (±2.7) kg, compared to 0.91 (±2.0) kg for women who continued to smoke, p = 0.42. Abstinent women reported significantly higher energy intake and consumed a smaller percentage of fat across assessment points than did those who continued to smoke. Intervention was not associated with differential weight gain, or change in percent of calories from protein, fat or carbohydrates. This study is the first documentation of energy and macronutrient intake during smoking cessation treatment using a validated 24-hour dietary recall methodology. Although cessation was associated with overall increases in energy intake among women, neither bupropion nor weight concerns treatment affected energy or macronutrient intake. Future research to understand the relation between cessation and dietary intake needs to replicate and extend these findings to elucidate how, if at all, smoking cessation affects dietary intake.
Keywords: Smoking cessation, Dietary intake, Weight concerns, Macronutrients
Introduction
It is well established that smokers gain weight upon successful cessation, with weight gains of approximately 5.4 kg (12 lb) during the first six months after quitting (Hudmon, Gritz, Clayton, & Nisenbaum, 1999; Klesges et al., 1997). Although weight gain tends to stabilize during the second six months of the first year postcessation (McBride, French, Pirie, & Jeffery, 1996), there is some evidence of continued weight gain years after quitting (Lycett et al., 2011; O’Hara et al., 1998). Specific subgroups of smokers, notably women smokers who endorse specific concerns about postcessation weight gain, tend to gain even larger amounts after quitting than do typical smokers. For example, weight-concerned women who quit and remained quit for one year gained an average 7.7 kg (17 lb) after quitting (Perkins et al., 2001). Thus, several studies have evaluated interventions to address postcessation weight gain and concerns about weight following cessation.
Bupropion, an efficacious treatment for smoking cessation (Ahluwalia, Harris, Catley, Okuyemi, & Mayo, 2002; Aubin et al., 2004; Fiore, 2000; Hurt et al., 1997; Jorenby et al., 1999), has been associated with less postcessation weight gain than placebo, regardless of pretreatment weight concerns (Ahluwalia et al., 2002; Hays et al., 2001; Hurt et al., 1997; Parsons, Shraim, Inglis, Aveyard, & Hajek, 2009). Among women expressly concerned about postcessation weight gain, cognitive behavioral treatment (CBT) designed to decrease smoking-related weight concerns also has been reported to improve rates of continuous smoking abstinence one year posttreatment relative to a standard cessation program (Perkins et al., 2001). Like bupropion, CBT for weight concerns attenuated weight gain among women who remained abstinent (Perkins et al., 2001). However, the weight gain attenuating effects of bupropion do not persist consistently after withdrawal of medication (Parsons et al., 2009), and support for postcessation weight-attenuation following CBT for weight concerns has been inconsistent (Levine et al., 2010). Moreover, the ways in which either intervention affects postcessation energy intake, if at all, are not well understood. Although it has been suggested that changes in macronutrient intake, that is energy intake from proteins, carbohydrates and fat, affect weight gain after cessation (Hughes & Hatsukami, 1997), little is known about changes in intake from carbohydrates, fats and proteins, during cessation treatment.
Of particular relevance to the present investigation, an initial evaluation of the efficacy of CBT designed to decrease smoking-related weight concerns by our research group indicated that CBT improved rates of smoking abstinence one year posttreatment relative to a standard cessation program (Perkins et al., 2001). More recently, we examined the combination of CBT for cessation-related weight concerns and bupropion, based on the rationale that a weight concerns intervention combined with bupropion would increase cessation rates further. We documented that this combination was efficacious for smoking cessation but neither postcessation weight gain or smoking-related weight concerns differed by intervention group (Levine et al., 2010).
Although our previous study did not find that bupropion, CBT or their combination affected post-cessation weight gain in weight-concerned women, understanding changes in postcessation dietary intake may help in design of treatments to prevent postcessation weight gain in weight concerned women. Previous work has suggested that changes in macronutrient intake affect weight gain after cessation (Hughes & Hatsukami, 1997). However, much of the extant research on energy intake after smoking cessation has been conducted cross-sectionally, comparing smokers to nonsmokers (de Castro & Taylor, 2008), or using self-report questionnaire-based measures of dietary intake (Allen, Hatsukami, Brintnell, & Bade, 2005; de Castro & Taylor, 2008; Vander Weg, Klesges, Eck Clemens, Meyers, & Pascale, 2001). Because individuals tend to under-report intake on questionnaires, the use of food recall interviews, which use a five-step multiple pass method reflects changes in intake over time more accurately than questionnaires (Conway, Ingwersen, Vinyard, & Moshfegh, 2003), and are particularly amenable to use in a clinical trial. Indeed, although underreporting has been documented in all measures of dietary intake, evidence suggests that automated multiple pass methods are more valid measures of nutrient intake than food frequency questionnaires among motivated, normal weight women (Blanton, Moshfegh, Baer & Kretsch, 2006).
In summary, little is known about how or whether macronutrient composition changes during smoking cessation treatment. Moreover, the use of a detailed, interview-based methodology to determine dietary intake pre- and postcessation may provide a more complete understanding of changes in women’s dietary intake patterns after quitting, which in turn may inform the design of intervention to address weight gain postcessation. Accordingly, we examined changes in macronutrient intake during our previously reported study of the separate and combined utility of CBT and bupropion for smoking cessation in weight concerned women. (Levine et al., 2010), and examined the effects of smoking cessation on energy and macronutrient intake. We hypothesized that overall energy intake would increase with abstinence, and that bupropion and CBT treatment would be associated with smaller changes in overall intake after cessation.
Method
Participants
Participants were women who participated in a randomized, double-blind, placebo-controlled trial of weight-concerned women smokers (Levine et al., 2010) and completed food-recall assessments. Women were randomly assigned to either a smoking cessation intervention designed to address weight concerns or a standard smoking cessation group (SS), and either bupropion (B) or placebo (P). All women smoked a minimum of 10 cigarettes per day and endorsed concern about postcessation weight gain on one of two questions designed to assess degree of postcessation weight concerns. Participants in the current study (N = 337) also completed telephone nutrition assessments at baseline, and at 1 and 6 months after a targeted quit date (TQD). On average, women were 42.0 (±10.1) years old, smoked 20.7 (±8.4) cigarettes per day, had smoked for 24.1 (±10.2) years, and had a BMI of 27.3 (±5.5) pretreatment. Most were white (86.1%), married (74.4%), and had some college education (85%).
Procedure
As detailed previously (Levine et al., 2010), women received weekly group cessation counseling (either CBT or a standard smoking cessation treatment) over a three month period and medication (bupropion or placebo) was provided for 6 months. Women were given a TQD in the second week of the program and scheduled to complete food recall interviews prior to quitting, and at approximately 1 and 6 months after the TQD. Demographic information was collected prior to randomization. Assessments of smoking and weight were completed at baseline and prior to each counseling group session.
Assessment of Weight
Women were weighed in street clothing without shoes prior to each session. Height was measured at baseline using a mounted stadiometer and BMI calculated as weight (kg)/height (m)2. Precessation weight was computed as the average of weights at the three treatment sessions prior to the TQD.
Smoking Cessation Outcome
At each visit, expired-air carbon monoxide was collected and women were asked about use of tobacco products since their last visit, using a timeline follow-back methodology. Relapse was defined as the self-report of smoking for seven consecutive days at any point after the TQD or any biochemical indication of smoking. Women who did not quit or who dropped out of treatment were considered to have relapsed as of the day following the last visit on which abstinence was verified. In cases where CO did not confirm abstinence or were not available, women were coded as relapsed. Point prevalent abstinence was defined as the self-report of no smoking during the seven days prior to the assessment and a CO reading of 8 or less. Prolonged abstinence (PA) was used as the main assessment of abstinence for this report and was defined as repeatedly meeting criteria for point prevalence.
Nutrition Data
Nutrition assessments were completed by telephone prior to the TQD and at 1 and 6 months after the TQD. Three 24-hour dietary recalls were conducted at each assessment point using the interactive, computerized software program, the Nutrition Data System for Research (NDSR), developed by the University of Minnesota Nutrition Coordinating Center. Food recalls are reliable assessments of group energy and macronutrient intake (Conway, Ingwersen, & Moshfegh, 2004) and assess dietary intake within 10% of actual intake under controlled conditions (Conway et al., 2003). In addition, multiple pass food recall interviews can be completed accurately by telephone (Tran, Johnson, Soultanakis, & Matthews, 2000).
Interviewers were trained to use a computerized dietary recall system, which uses specific probes to help the respondent remember all of the foods eaten and details on how foods were prepared. Interviewers used a multiple pass approach in which women were first prompted to generate a quick list of foods consumed. Next, the list was reviewed using prompts to help individuals recall easily forgotten foods (e.g., butter on toast) and for all occasions of food consumption (e.g., coffee breaks). Third, individuals were asked to describe foods and beverages by brand name, ingredients and preparation, portion size, and quantity eaten. Finally, the list was reviewed to ensure completeness. Food recall interviews were conducted on Tuesdays and Wednesdays, and women were asked to recall one weekend and two weekdays (Monday and Tuesday).
Data Analysis
Descriptive statistics and quantile plots for nutrition data were generated to screen for abnormal values and to check normality assumptions. There were five cases in which women reported energy intake of less than 400 kcal/day, which is substantially below the expected intake. Consistent with previous reports using data from 24-hour dietary recalls, these outliers were excluded from analysis (Hebert et al., 2002; Samuel-Hodge, Fernandez, Henriquez-Roldan, Johnston, & Keyserling, 2004). Thus, the total energy intake ranged from 434 to 5,015 kcal with a mean of 1,558, ± 570 kcal.
We applied mixed-effects models to nutrition data collected from the whole sample using all time points, with terms including abstinence status, treatment group, time and the abstinence status by time interaction. A secondary analysis was carried out including women who met criteria for PA at 6 months. In this analysis, treatment group and time were evaluated. The interaction between treatment group and time was not included for energy or macronutrient intakes as there was no main effect of treatment on these variables.
Results
Changes in dietary intake by smoking status
At 6 months, 22.0% (74/337) of participants were abstinent. Women who were abstinent gained an average of 3.6 (±2.7) kg compared to a 0.91 (±2.0) kg gain among women who were still smoking, p = 0.42. As expected, women reported significantly increased energy intake over time across all treatment groups, F (2,791) = 3.64, p = 0.03 (d = .12) and with cessation, F (1,791) = 5.37, p = 0.02 (d = .16) with no interaction between time and cessation, F (2,791) = 0.01, p = 0.99. As illustrated in Figure 1, energy intake increased over time in both women who did and did not quit (140 ± 98 kcal and 127 ± 62 kcal in abstinent and smoking women, respectively). In addition, although differences in energy intake were not significant at any individual assessment point, women who were abstinent at 6 months reported higher energy intake at each assessment than did those who were not abstinent.
Figure 1.
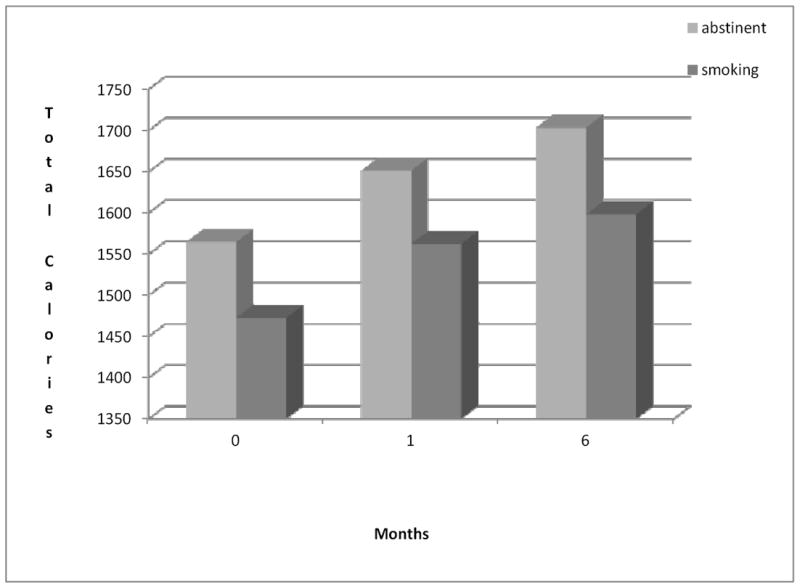
Total Calories by Smoking Status over Time.
Women who were abstinent at 6 months also consumed a smaller percentage of intake from fat than did those who were not abstinent, F (1, 791) = 4.64, p = 0.03 (d = .15), but there was no significant interaction between abstinence status and time for fat intake (p = 0.73). Women who were abstinent at 6 months ate 34.2%, 34.9 and 34.5% of their calories from fat at baseline, 1- and 6 months postcessation, compared to 36.2%, 35.7 and 36.4%, respectively among women who did not maintain abstinence. Thus, women who remained abstinent did not differentially change the proportion of calories consumed from fat relative to those who did not achieve sustained abstinence. Further there were no differences in the percent of energy intake from protein or carbohydrates over time among abstinent women (p > 0.15).
Changes in dietary intake by treatment group
Although weight gain among abstinent women did not differ across the treatment groups, we were interested in examining changes in macronutrient intake by treatment group to identify potential explanations for previous weight attenuating effects of bupropion and CBT for weight concerns. Among women who remained abstinent through 6 months, intervention condition was not related to changes in dietary intake. There were no differences in energy intake over time among women who received bupropion, CBT, or the combination [F (3,198) = 2.07, p = 0.11]. Moreover, the percent of calories consumed from protein, fat or carbohydrates among abstinent women did not differ among the intervention conditions (p’s > 0.09).
Conclusions
Weight concerned women who successfully sustained smoking cessation for six months reported greater overall energy intake and consumed a smaller proportion of energy intake from fat than did women who did not sustain smoking abstinence. Although effect sizes were small, women in the current study who successfully maintained abstinence over a six months period reported higher energy intake, a finding consistent with the well-documented changes in body weight that occur after smoking cessation. Notably, however, all women participating in an intervention for smoking cessation, regardless of successful cessation, reported increased dietary intake over time. Thus, the increased energy intake over time in both successful and unsuccessful abstainers may represent increased intake related to efforts to stop smoking. This finding is consistent with the notion that individuals need to accept that efforts to quit smoking may be accompanied by weight gain (Perkins, 2001; Perkins, Levine, Marcus, & Shiffman, 1997).
In addition, women who successfully maintained smoking abstinence through six months reported eating less dietary fat than did those who did not quit. However, the absolute differences in fat intake were small, and the clinical significance of these differences are not clear as all women reported a proportion of fat intake on the upper end of that recommended for American adults (USDA, 2010). Moreover, although abstinent women reported less fat intake at each assessment than did women who did not quit, abstinent women did not differentially increase consumption of fats, proteins or carbohydrates over time after smoking cessation. This finding suggests that cessation-related weight gain may not be preferentially associated with increased intake of fats and carbohydrates, as has been suggested previously (Hughes & Hatsukami, 1997). Although available data has indicated that smokers who quit and remain quit report increased consumption of sweets (French, Hennrikus, & Jeffery, 1996), calories from sugar (Rodin, 1987) and calories from carbohydrates (Spring, Wurtman, Gleason, Wurtman, & Kessler, 1991), we did not confirm these findings. Finally, the present data also suggest that neither bupropion nor CBT for weight concerns, both of which have been associated with weight gain attenuation in previous research (Perkins et al., 2001; Ahluwalia, Harris, Catley, Okuyemi, & Mayo, 2002; Hays et al., 2001; Hurt et al., 1997; Parsons et al., 2009), are associated with changes in intake of overall calories or with differential changes in women’s macronutrient intake after smoking cessation.
Like all changes in body weight, postcessation weight gain is a function of an imbalance between energy intake and expenditure. The present data pertain only to the intake side of this energy balance equation. Although changes in energy expenditure also may contribute to postcessation weight gain, the preponderance of evidence suggests that change in body weight after smoking cessation relate more to increases in intake than to sustained change in expenditure. For example, although there is an acute metabolic effect of nicotine (Perkins, 1993), short-term smoking cessation has been associated with increased weight and energy intake, but not with changes in resting energy expenditure (Ferrara, Kumar, Micklas, McCrone, & Goldberg, 2001; Vander Weg et al., 2001). Women who sustained abstinence did not change the proportion of any macronutrient group over time suggesting that women do not have a preference for high fat, high sugar snacks while quitting. Although episodes of snacking, per se, are not captured by 24-hour food recalls, typical snack foods are often high in carbohydrates and fat, rather than protein. Thus, the absence of differential changes in macronutrient content following cessation suggests that women consumed more food overall rather than higher fat, higher calorie foods specifically.
There were many strengths to the current study. Notably, this study was the first to use the automated multiple pass method to determine dietary intake among women quitting smoking over time, and included collection of high quality smoking status and dieatry data as part of a larger clinical trial for weight-concerned women smokers. Nevertheless, although dietary recalls are reliable indicators of change in energy and macronutrient intake (Blanton, Moshfegh, Baer, & Kretsch, 2006; Conway et al., 2004; Conway et al., 2003) and are often used in weight loss trials (Dolecek, Stamler, Caggiula, Tillotson, & Buzzard, 1997; Thomson et al., 2003), there are problems with all self-report methods of nutritional assessment. Limitations of this specific method of assessing intake include errors in recalling past eating events, missing food recall interviews, and interviewer error in recording and entering foods data. In addition, the fact that only women concerned about postcessation weight gain were studied limits the generalizability of these data. For example, the previously observed effects of bupropion on weight gain during smoking cessation (Parsons et al., 2009) were not observed in this subgroup of women smokers with specific concerns about weight gain.
In summary, the present study extends the literature on smoking-related weight gain and weight concerns among women. However, additional prospective research is needed to examine the potential implications of the current findings. On the one hand, interventions may need to promote the acceptance of moderately increased energy intake and weight gain with cessation (Perkins, Levine, Marcus, & Shiffman, 1997) and strive to mitigate excessive cessation-related weight gain (Perkins et al., 2001). Alternatively, the current findings suggest that weight management interventions emphasizing the need for control of overall levels of energy intake offered prior to or initiated concurrently with a quit attempt (e.g., Spring et al., 2004), may not impede smoking cessation efforts. Finally, given the importance of understanding the role of energy intake and weight gain during smoking cessation attempts, studies designed to evaluate whether a weight control or weight gain acceptance intervention may have differential utility for particular sub-groups of smokers may be worthwhile.
Acknowledgments
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (R01 DA04174 (PI: Marcus). Dr. Levine’s effort was partially supported by K01DA15396 (PI: Levine) and R01 DA04770 (PI: Levine).
Footnotes
Portions of this article were presented at the 30th annual meeting of the Society for Behavioral Medicine (Montreal, Quebec; April, 2009).
GlaxoSmithKline provided bupropion SR 150 mg and matching placebo for the parent study free of charge.
Contributor Information
Michele D. Levine, Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center
Yu Cheng, Departments of Statistics and Psychiatry, University of Pittsburgh, Pittsburgh PA.
Melissa A. Kalarchian, Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center
Kenneth A. Perkins, Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center
Marsha D. Marcus, Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center
References
- Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained release bupropion for smoking cessation in African Americans. Journal of the American Medical Association. 2002;288(4):468–474. doi: 10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- Allen SS, Hatsukami D, Brintnell DM, Bade T. Effect of nicotine replacement therapy on post-cessation weight gain and nutrient intake: a randomized controlled trial of postmenopausal female smokers. Addictive Behaviors. 2005;30(7):1273–1280. doi: 10.1016/j.addbeh.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Aubin HJ, Lebargy F, Berlin I, Bidaut-Mazel C, Chemali-Hudry J, Lagrue G. Efficacy of bupropion and predictors of successful outcome in a sample of French smokers: a randomized placebo-controlled trial. Addiction. 2004;99(9):1206–18. doi: 10.1111/j.1360-0443.2004.00814.x. [DOI] [PubMed] [Google Scholar]
- Blanton CA, Moshfegh AJ, Baer DJ, Kretch MJ. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. Journal of Nutrition. 2006;136(10):2594–2599. doi: 10.1093/jn/136.10.2594. [DOI] [PubMed] [Google Scholar]
- Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validity study. Journal of the American Dietetic Association. 2004;104(4):595–603. doi: 10.1016/j.jada.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and non-obese women. American Journal of Clinical Nutrition. 2003;77(5):1171–1178. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- de Castro JM, Taylor T. Smoking status relationships with the food and fluid intakes of free-living humans. Nutrition. 2008;24(2):109–119. doi: 10.1016/j.nut.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Dolecek TA, Stamler J, Caggiula AW, Tillotson JL, Buzzard IM. Methods of dietary and nutritional assessment and intervention and other methods in the Multiple Risk Factor Intervention Trial. American Journal of Clinical Nutrition. 1997;65(1 Suppl):196S–210S. doi: 10.1093/ajcn/65.1.196S. [DOI] [PubMed] [Google Scholar]
- Ferrara CM, Kumar M, Micklas B, McCrone S, Goldberg AP. Weight gain and adipose tissue metabolism after smoking cessation in women. International Journal of Obesity and Related Metabolic Disorders. 2001;25(9):1322–1326. doi: 10.1038/sj.ijo.0801716. [DOI] [PubMed] [Google Scholar]
- French SA, Hennrikua DJ, Jeffery RW. Smoking status, dietary intake, and physical activity in a sample of working adults. Health Psychology. 1996;15(6):448–454. doi: 10.1037//0278-6133.15.6.448. [DOI] [PubMed] [Google Scholar]
- Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, et al. Sustained-relase bupropion for pharmacological relapse prevention after smoking cessation, a randomized, controlled trial. Annals of Internal Medicine. 2001;135(6):423–433. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- Hebert JR, Ebbeling CB, Matthews CE, Hurley TG, Ma Y, Druker S, et al. Systematic errors in middle-aged women’s estimates of energy intake: comparing three self-report measures to total energy expenditure from doubly labeled water. Annals of Epidemiology. 2002;12(8):577–586. doi: 10.1016/s1047-2797(01)00297-6. [DOI] [PubMed] [Google Scholar]
- Hudmon KS, Gritz ER, Clayton S, Nisenbaum R. Eating orientations, postcessation weight gain, and continued abstinence among female smokers receiving an unsolicited smoking cessation intervention. Health Psychology. 1999;18(1):29–39. doi: 10.1037//0278-6133.18.1.29. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Effects of three doses of transdermal nicotine on post-cessation eating, hunger and weight. Journal of Substance Abuse. 1997;9:151–159. doi: 10.1016/s0899-3289(97)90013-4. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston LC, Dale MA, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. New England Journal of Medicine. 1997;337(17):1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. The New England Journal of Medicine. 1999;340(9):685–91. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Winders SE, Meyers AW, Eck LH, Ward KD, Hultquist CM, et al. How much weight gain occurs following smoking cessation? A comparison of weight gain using both continuous and point prevalence abstinence. Journal of Consulting and Clinical Psychology. 1997;65(2):286–291. doi: 10.1037//0022-006x.65.2.286. [DOI] [PubMed] [Google Scholar]
- Levine MD, Perkins KA, Kalarchian MA, Cheng Y, Houck PR, Slane JD, Marcus MD. Bupropion and cognitive behavioral therapy for weight-concerned women smokers. Archives of Internal Medicine. 2010;170(6):543–550. doi: 10.1001/archinternmed.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett D, Munafo M, Johnstone E, Murphy M, Aveyard P. Associations between weight change over 8 years and baseline body mass index in a cohort of continuing and quitting smokers. Addiction. 2011;106(1):188–96. doi: 10.1111/j.1360-0443.2010.03136.x. [DOI] [PubMed] [Google Scholar]
- McBride CM, French SA, Pirie PL, Jeffery RW. Changes over time in weight concerns among women smokers engage in the cessation process. Annals of Behavioral Medicine. 1996;18(4):273–279. doi: 10.1007/BF02895289. [DOI] [PubMed] [Google Scholar]
- O’Hara P, Connet JE, Lee WW, Nides M, Murray R, Wise R. Early and late weight gain following smoking cessation in the Lung Health Study. American Journal of Epidemiology. 1998;148(9):821–30. doi: 10.1093/oxfordjournals.aje.a009706. [DOI] [PubMed] [Google Scholar]
- Parsons AC, Shraim M, Inglis J, Aveyard P, Hajek P. Interventions for preventing weight gain after smoking cessation. Cochrane Database of Systematic Reviews. 2009;(1) doi: 10.1002/14651858.CD006219.pub2. CD006219. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Weight gain following smoking cessation. Journal of Consulting and Clinical Psychology. 1993;61(5):768–777. doi: 10.1037//0022-006x.61.5.768. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Levine MD, Marcus MD, Shiffman S. Addressing women’s concerns about weight gain due to smoking cessation. Journal of Substance Abuse Treatment. 1997;14:173–182. doi: 10.1016/s0740-5472(96)00158-4. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Marcus MD, Levine MD, D’Amico D, Miller A, Broge M, et al. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. Journal of Consulting and Clinical Psychology. 2001;69:604–613. [PubMed] [Google Scholar]
- Rodin J. Weight change following smoking cessation: the role of food intake and exercise. Addictive Behavior. 1987;12(4):303–317. doi: 10.1016/0306-4603(87)90045-1. [DOI] [PubMed] [Google Scholar]
- Samuel-Hodge CD, Fernandez LM, Henriquez-Roldan CF, Johnston LF, Keyserling TC. A comparison of self-reported energy intake with total energy expenditure estimated by accelerometer and basal metabolic rate in African-American women with type 2 diabetes. Diabetes care. 2004;27(3):663–669. doi: 10.2337/diacare.27.3.663. [DOI] [PubMed] [Google Scholar]
- Spring B, Wurtman J, Gleason R, Wurtman R, Kessler K. Weight gain and withdrawal symptoms after smoking cessation: a preventative intervention using d-fenfluramine. Health Psychology. 1991;10(3):216–233. doi: 10.1037//0278-6133.10.3.216. [DOI] [PubMed] [Google Scholar]
- Spring B, Pagoto S, Pingitore R, Doran N, Schneider K, Hedeker D. Randomized controlled trial for behavioral smoking and weight control treatment: Effect of concurrent versus sequential intervention. Journal of Consulting and Clinical Psychology. 2004;72(5):785–796. doi: 10.1037/0022-006X.72.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson CA, Giuliano A, Rock CL, Ritenbaugh CK, Flatt SW, Faeeber S, et al. Measuring dietary change in a diet intervention trial: comparing food frequency questionnaire and dietary recalls. American Journal of Epidemiology. 2003;157:754–762. doi: 10.1093/aje/kwg025. [DOI] [PubMed] [Google Scholar]
- Tran KM, Johnson RK, Soultanakis RP, Matthews DE. In-person vs telephone-administered multiple-pass 24-hour recalls in women: validation with doubly labeled water. Journal of the American Dietetic Association. 2000;100(7):777–783. doi: 10.1016/S0002-8223(00)00227-3. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. Washington, D.C.: U.S. Government Printing Office; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Weg MW, Klesges RC, Eck Clemens LH, Meyers AW, Pascale RW. The relationship between ethnicity, gender, and short-term changes in energy balance following smoking cessation. International Journal of Behavioral Medicine. 2001;8(2):163–177. [Google Scholar]


