Gallant-Behm et al. identify a novel mechanism of transcriptional repression by ΔNp63α, a p53 family member that functions as an oncogene in squamous cell carcinomas. They find that although ΔNp63α and p53 bind almost identical DNA sequence motifs, they regulate nonoverlapping gene sets, and that ΔNp63α oncogenicity is mediated independently of p53. Also identified is a new p63 target, SAMD9L, an anti-proliferative gene.
Keywords: transcriptional repression, squamous cell carcinoma, oncogene, SAMD9L, SRCAP, corepressor
Abstract
ΔNp63α is a member of the p53 family of transcription factors that functions as an oncogene in squamous cell carcinomas (SCCs). Because ΔNp63α and p53 bind virtually identical DNA sequence motifs, it has been proposed that ΔNp63α functions as a dominant-negative inhibitor of p53 to promote proliferation and block apoptosis. However, most SCCs concurrently overexpress ΔNp63α and inactivate p53, suggesting the autonomous action of these oncogenic events. Here we report the discovery of a novel mechanism of transcriptional repression by ΔNp63α that reconciles these observations. We found that although both proteins bind the same genomic sites, they regulate largely nonoverlapping gene sets. Upon activation, p53 binds all enhancers regardless of ΔNp63α status but fails to transactivate genes repressed by ΔNp63α. We found that ΔNp63α associates with the SRCAP chromatin regulatory complex involved in H2A/H2A.Z exchange and mediates H2A.Z deposition at its target loci. Interestingly, knockdown of SRCAP subunits or H2A.Z leads to specific induction of ΔNp63α-repressed genes. We identified SAMD9L as a key anti-proliferative gene repressed by ΔNp63α and H2A.Z whose depletion suffices to reverse the arrest phenotype caused by ΔNp63α knockdown. Collectively, these results illuminate a molecular pathway contributing to the autonomous oncogenic effects of ΔNp63α.
ΔNp63α is a member of the p53 family of transcription factors that is essential for epithelial maintenance and epidermal morphogenesis (Yang et al. 1998; Mills et al. 1999). ΔNp63α is predominantly expressed in epithelial stem cells and undifferentiated basal keratinocytes, where it acts as a critical proliferative factor (Senoo et al. 2007). ΔNp63α functions as a potent oncogene in squamous cell carcinomas (SCCs) of diverse origins, where it drives proliferation and blocks apoptosis (Parsa et al. 1999; Rocco et al. 2006). In fact, ΔNp63α expression is both a diagnostic marker for specific cancer subtypes and an indicator of poor prognosis (Graziano and De Laurenzi 2011). Despite its undisputed relevance in epithelial biology and cancer, the mechanism of action of ΔNp63α remains poorly characterized.
ΔNp63α contains a DNA-binding domain that is highly conserved with the other p53 family members and binds to DNA response elements nearly identical to those bound by p53, as determined by both in vitro selection of preferred DNA sequences and in vivo genome-wide chromatin-binding studies (Ortt and Sinha 2006; Perez et al. 2007; Kouwenhoven et al. 2010). However, ΔNp63α lacks the canonical N-terminal transcriptional activation domain found in p53, TAp63, and TAp73 due to alternative promoter usage within the TP63 locus. Accordingly, ΔNp63α was found to function as a transcriptional repressor of several genes within the p53 network (Westfall et al. 2003; DeYoung et al. 2006; Rocco et al. 2006; Mundt et al. 2010). One of the prevailing hypotheses in the literature is that ΔNp63α represses p53 target genes by simply acting as a dominant negative to prevent p53 occupancy at the shared DNA response elements (Yang et al. 1998). According to this view, overexpression of ΔNp63α in SCCs would broadly shut down the p53 transcriptional program by inactivating this important tumor suppressor at the level of chromatin binding. However, this model is challenged by epidemiological studies showing that most SCCs exhibit a “gain of function” in the form of overexpression of ΔNp63α concurrently with “loss-of-function” mutations in p53, indicating that the two oncogenic events are nonredundant during SCC progression (Neilsen et al. 2011). This could be explained by the existence of p53-independent oncogenic functions of ΔNp63α in SCC cells and/or a failure of endogenous ΔNp63α to act as a true dominant negative of p53 in this cellular context. Deciphering the precise mechanism of action of ΔNp63α will greatly facilitate the design of therapeutic strategies targeting this oncogene in SCCs.
Here we report the results of our research on the regulatory interactions between endogenous ΔNp63α and p53 in rare SCC cells expressing wild-type versions of both regulators. Using an isogenic cell system, we found that SCC cells are addicted to ΔNp63α regardless of p53 status, thus confirming the overall autonomous action of these transcription factors. Intriguingly, our mechanistic studies revealed that although ΔNp63α and p53 share a large number of DNA response elements in the genome, they regulate largely nonoverlapping subsets of genes. Surprisingly, activated p53 effectively binds to ΔNp63α-repressed loci but fails to transactivate them. Conversely, ΔNp63α binds to p53 target genes but fails to repress them. Identification of ΔNp63α-interacting proteins by mass spectrometry revealed an association with subunits of the SRCAP complex previously implicated in H2A.Z deposition. ChIP analysis revealed that ΔNp63α mediates recruitment of SRCAP subunits to chromatin as well as H2A.Z deposition. Furthermore, knockdown of SRCAP subunits or H2A.Z leads to specific derepression of ΔNp63α targets. Finally, we identified SAMD9L as a key anti-proliferative gene repressed by ΔNp63α, SRCAP subunits, and H2A.Z that is required for the cell proliferation arrest observed upon ΔNp63α depletion. Thus, our results identify a novel molecular mechanism mediating ΔNp63α oncogenicity in a p53-autonomous fashion.
Results
ΔNp63α drives proliferation of SCC cells independently of p53 status
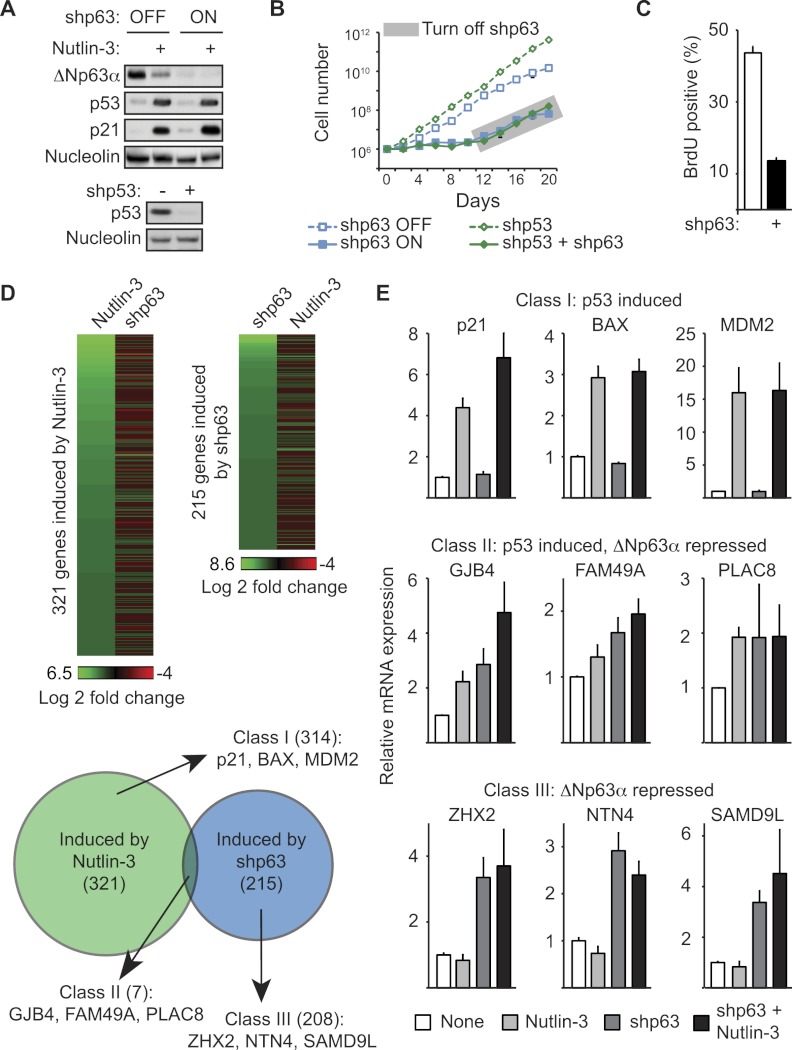
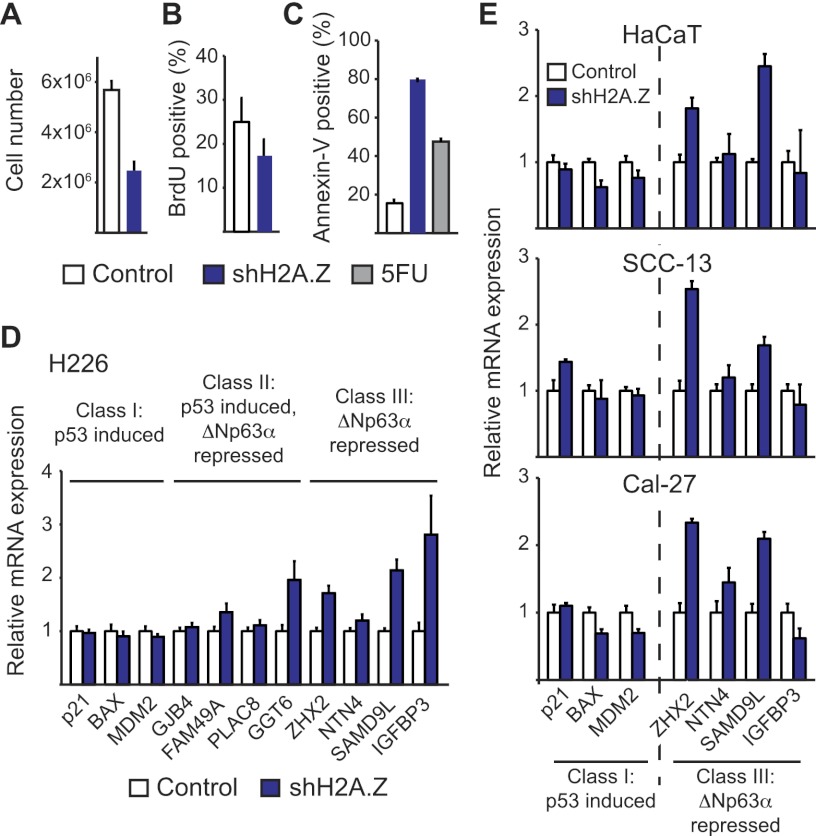
SCCs frequently accrue both loss-of-function mutations of the tumor suppressor p53 and overexpression of the oncogene ΔNp63α, suggesting that these two oncogenic events are not functionally redundant (Nekulova et al. 2011). In order to investigate the possible functional interplay between ΔNp63α and p53, we screened a panel of SCC cell lines in search of those expressing wild-type versions of both genes. Several lines of evidence indicate that H226 cells (lung SCC) express both ΔNp63α and functional p53. First, Western blot analysis of protein extracts from H226 cells run alongside extracts from insect cells expressing various myc-tagged human p63 isoforms confirmed that H226 cells express only ΔNp63α and not other p63 variants (Supplemental Fig. 1A). Second, shRNAs directed against ΔNp63α-specific mRNA isoforms effectively deplete the Western blot signal from H226 extracts (Fig. 1A). Third, treatment of H226 cells with Nutlin-3, a specific inhibitor of the MDM2–p53 interaction, leads to effective p53 accumulation and induction of p21 (CDKN1A) and many other well-characterized p53 target genes and results in cell cycle arrest (Fig. 1A,D,E; Supplemental Figs. 1B, 2). Of note, p53 activation by Nutlin-3 leads to a modest but reproducible decrease in ΔNp63α expression (Fig. 1A).
Figure 1.
ΔNp63α drives SCC cell proliferation independently of p53. (A) Western blot of H226 cell extracts following 48 h of ΔNp63α knockdown and/or 12 h of p53 activation with 10 μM Nutlin-3 (top panels) or following stable p53 knockdown (bottom panels). Nucleolin served as a loading control. (B) Cell proliferation assay performed by direct cell counting. Cells carrying stably integrated shRNAs against ΔNp63α and p53 were pretreated with doxycycline for 5 d prior to seeding to induce ΔNp63α knockdown. Cells (1 × 106) were seeded at day 0, and doxycycline was removed from the medium at day 10 to allow re-expression of ΔNp63α. (C) Flow cytometry analysis of BrdU incorporation following 5 d of ΔNp63α knockdown. (D) Heat map and Venn diagrams of microarray results following 48 h of ΔNp63α knockdown or 12 h of p53 activation by 10 μM Nutlin-3. Significant induction upon ΔNp63α knockdown or p53 activation is defined as a >1.5-fold change and P < 0.05 relative to control samples. Genes with an increased expression following p53 activation are referred to hereafter as “class I.” Genes that are up-regulated following ΔNp63α knockdown are “class III.” Genes with an increased expression following both treatments are “class II.” (E) Validation of microarray results by qRT–PCR analysis of selected class I, II, and III target genes following 12 h of 10 μM Nutlin-3 treatment, 48 h of ΔNp63α knockdown, or combination treatment.
To test for a functional interaction, or lack thereof, between ΔNp63α and p53 in H226 cells, we established isogenic cell lines stably expressing shRNAs targeting each mRNA (Fig. 1A). We used a tet-inducible shRNA against ΔNp63α (referred to herein as shp63), which allowed us to toggle cells between ΔNp63α-competent and ΔNp63α-deficient states by simply adding doxycycline to the media. We then tested this inducible shp63 alone or in combination with a stably integrated, constitutively expressed shRNA against p53 (shp53). Upon ΔNp63α knockdown, primary keratinocytes and various SCC cell lines have been shown to undergo decreased proliferation, increased apoptosis, and/or senescence (Rocco et al. 2006; Truong et al. 2006; Rivetti di Val Cervo et al. 2012). Upon ΔNp63α knockdown, H226 cells undergo effective proliferation arrest with little sign of apoptosis, as evidenced by a complete stalling of cell numbers, a decrease in BrdU incorporation, and a drop in S-phase cells without a significant increase in Annexin V staining (Fig. 1B,C; Supplemental Fig. 1C–E). Importantly, this proliferation arrest is completely reversible even after 10 d of ΔNp63α knockdown, as evidenced by an increase in cell numbers immediately upon doxycycline removal (gray box in Fig. 1B), indicating that H226 cells do not undergo senescence during ΔNp63α knockdown. Surprisingly, concomitant knockdown of p53 does not rescue the proliferation arrest caused by ΔNp63α depletion (Fig. 1B). However, p53 knockdown alone does increase the proliferation rate of H226 cells, confirming the anti-proliferative effects of p53 in this cell line (Fig. 1B; Supplemental Fig. 1C).
Taken together, these results demonstrate that ΔNp63α drives proliferation of SCC cells independently of p53 status.
ΔNp63α and p53 regulate largely nonoverlapping gene expression programs in SCC cells
In order to better understand the autonomous phenotypic effects of ΔNp63α and p53, we investigated the impact of p53 activation versus ΔNp63α depletion on global gene expression in H226 cells using microarray analysis. Toward this end, we compared cells treated with Nutlin-3 or doxycycline to induce p53 activation or ΔNp63α depletion, respectively. Interestingly, the gene set induced by Nutlin-3 treatment showed negligible overlap with the gene set derepressed upon ΔNp63α knockdown (Fig. 1D). Nutlin-3 treatment led to significant induction (>1.5-fold, P < 0.05) of 321 genes, including many well-characterized p53 target genes such as p21, BAX, and MDM2 (Supplemental Table 1). From now on, we refer to this group as class I genes. Using the same cutoff, we found that ΔNp63α knockdown leads to up-regulation of 215 genes (referred to as class III genes), only seven of which are also up-regulated by Nutlin-3 (class II genes). Importantly, quantitative RT–PCR (qRT–PCR) analysis of several genes within each class validates the microarray results (Fig. 1E; Supplemental Fig. 2). Furthermore, qRT–PCR revealed that class III genes remain insensitive to Nutlin-3-activated p53 even after ΔNp63α knockdown. Among class I genes, only p21 showed a modest increase in expression in the combination treatment.
These results demonstrate that ΔNp63α has negligible impact on the p53 transcriptional program in H226 cells, indicating that ΔNp63α does not function as a broadly acting dominant-negative inhibitor of p53 in this setting. Interestingly, although ΔNp63α was shown to repress canonical p53 target genes such as p21, 14-3-3σ (SFN), GADD45A, PUMA (BBC3), and NOXA (PMAIP1) in some other cell types (Westfall et al. 2003; Rocco et al. 2006), none of these genes were induced in H226 cells upon ΔNp63α knockdown (Fig. 1E; Supplemental Fig. 2). Thus, we conclude that ΔNp63α represses a distinct class of genes not activated by p53 in H226 cells. As nearly all of the class II and III genes have not previously been described as being ΔNp63α target genes, we chose to investigate whether these genes were also transcriptionally repressed by ΔNp63α in squamous cells of different origins and p53 status. qRT–PCR analysis of HaCaT (immortalized keratinocytes), SCC-13 (head and neck SCC [HNSCC] and tongue), and Cal-27 (HNSCC and oral) cells (all p53 mutant) demonstrates that several class II and III genes, but not class I genes, are similarly up-regulated following ΔNp63α knockdown, indicating that they are bona fide ΔNp63α target genes (Supplemental Fig. 3). Furthermore, the differential effects of p53 on class I versus class III genes is still observed when using 5-fluorouracil (5FU), a p53-activating agent that acts by a mechanism different from Nutlin-3 (Supplemental Fig. 4). Considering the fact that ΔNp63α and p53 were found to recognize virtually identical DNA sites both in vitro and in vivo, the lack of coregulation by the two family members in H226 cells prompted us to pose several mechanistic questions. Do p53 and ΔNp63α bind the same genomic sites in H226 cells? Can the lack of regulatory overlap be explained by yet-unappreciated differences in the chromatin-binding properties of ΔNp63α and p53?
p53 effectively binds to genes repressed by ΔNp63α but fails to transactivate them
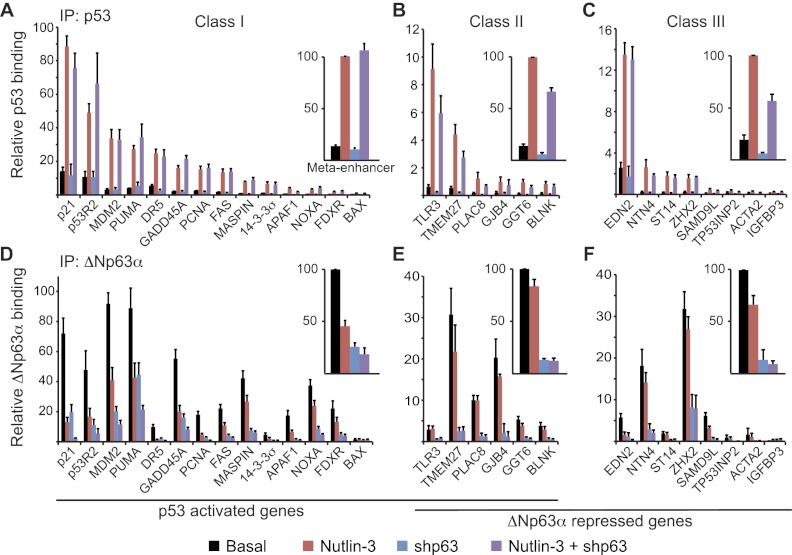
In order to investigate the molecular basis of gene-specific regulation by ΔNp63α and p53, we performed a series of chromatin immunoprecipitation (ChIP) assays to test their binding to the enhancers of class I, II, and III genes in H226 cells. Detailed maps of each loci tested depicting the location of the ΔNp63α/p53-binding sites are shown in Supplemental Figure 5. We used qPCR to analyze ChIP-enriched DNA under four experimental conditions: basal (high ΔNp63α expression and low p53 expression), Nutlin-3 (activated p53), shp63 (low expression of both ΔNp63α and p53), and Nutlin-3 + shp63 (activated p53 and low ΔNp63α expression). First, we analyzed p53 binding to chromatin under these conditions. Expectedly, we observed significant p53 binding to all enhancers in class I genes upon Nutlin-3 treatment, albeit with different degrees of occupancy (Fig. 2A; Supplemental Fig. 6A). Among these genomic sites, the p21 enhancer displayed the highest p53 occupancy, while the BAX enhancer showed the lowest, yet all of these sites presented significant enrichment over control immunoprecipitations, and all sites demonstrated a significant enrichment of p53 binding following Nutlin-3 treatment as compared with basal conditions. Interestingly, p53 binding was also easily detected at class II and class III genes, with increased occupancy upon Nutlin-3 treatment (Fig. 2B,C; Supplemental Fig. 6B,C). As a group, class II and III genes showed lower p53 ChIP signals than class I genes (note the different scales on the Y-axis in Fig. 2B,C; Supplemental Fig. 6B,C). However, many class II and class III genes showed higher p53 occupancy than most class I genes. For example, EDN2 (class III) shows a level of p53 binding comparable with or higher than that observed at canonical p53 target genes such as FAS and 14-3-3σ (class I). Of note, p53 activation with 5FU produces the same overall pattern of p53 binding as that observed with Nutlin-3 (Supplemental Fig. 7). Importantly, basal and activated levels of p53 binding to all three gene classes are not increased in cells depleted of ΔNp63α (cf. red and purple bars in Fig. 2A–C). This observation argues against the notion that ΔNp63α competes with p53 for binding to common genomic sites. If anything, p53 binding to some class II and III genes seems to require ΔNp63α expression (cf. red and purple bars in class II and III Meta-enhancers in Fig. 2A–C).
Figure 2.
ΔNp63α does not prevent p53 access to its genomic sites. ChIP assays were performed with whole-cell extracts from control cells (black bars) following 12 h of 10 μM Nutlin-3 treatment (red bars), 48 h of ΔNp63α knockdown (blue bars), or the combination of Nutlin-3 treatment and ΔNp63α knockdown (purple bars). Antibodies specific for p53 (A–C) and ΔNp63α (D–F) were used. ChIP-enriched DNA was quantified by real-time PCR for the p53/p63 response elements of each indicated gene. See Supplemental Figure 5 for gene maps and amplicon locations. Meta-enhancer values were calculated as the average PCR signal for each treatment group relative to Nutlin-3-treated (p53 IP) or basal (ΔNp63α IP) values for all response elements tested within a gene class.
Overall, these results demonstrate that p53 effectively binds to genomic sites in all three gene classes but somehow fails to transactivate class III genes.
ΔNp63α binds to genes activated by p53 and is lost from chromatin upon p53 activation
ChIP analysis of ΔNp63α revealed that, prior to p53 activation by Nutlin-3, ΔNp63α effectively binds to the genomic sites in all three gene classes (Fig. 2D–F; Supplemental Fig. 6D–F). Interestingly, the levels of ΔNp63α occupancy across the response elements does not fully correlate with the levels of p53 binding. For example, p53 shows greater than fivefold occupancy at the p53R2 enhancer than the MASPIN enhancer, yet ΔNp63α binding is similar at these two sites. These differences in relative occupancy between p53 and ΔNp63α are also observed at class II and III genes. In a similar fashion to p53, the class I genes as a group also show stronger ΔNp63α occupancy (cf. Y scales in Fig. 2D–F). However, this trend has many exceptions, with some genes in classes II and III (e.g., TMEM27 and ZHX2) showing greater ΔNp63α occupancy than most genes in class I. Expectedly, ΔNp63α ChIP signals decrease considerably at all sites in cells expressing shp63 (cf. black vs. blue bars in Fig. 2D–F). Interestingly, ΔNp63α occupancy also drops upon Nutlin-3 treatment, most significantly at class I genes (cf. black vs. red bars in Fig. 2D–F; Supplemental Fig. 6D–F). This effect could be explained by the decrease in ΔNp63α expression upon Nutlin-3, as seen by Western blot (Fig. 1A) and/or displacement from chromatin by incoming p53. Of note, the loss of ΔNp63α binding upon p53 activation is also observed when using 5FU instead of Nutlin-3 (Supplemental Fig. 7). Regardless of mechanism, it is clear that ΔNp63α does not prevent p53 from accessing its genomic sites.
Taken together, these results show that p53 and ΔNp63α display a significant overlap in their genomic binding sites yet do not coregulate the hundreds of genes contained in classes I and III. Therefore, their regulatory capacity cannot be solely explained by their chromatin-binding properties.
ΔNp63α associates with subunits of the SRCAP chromatin regulatory complex involved in histone exchange
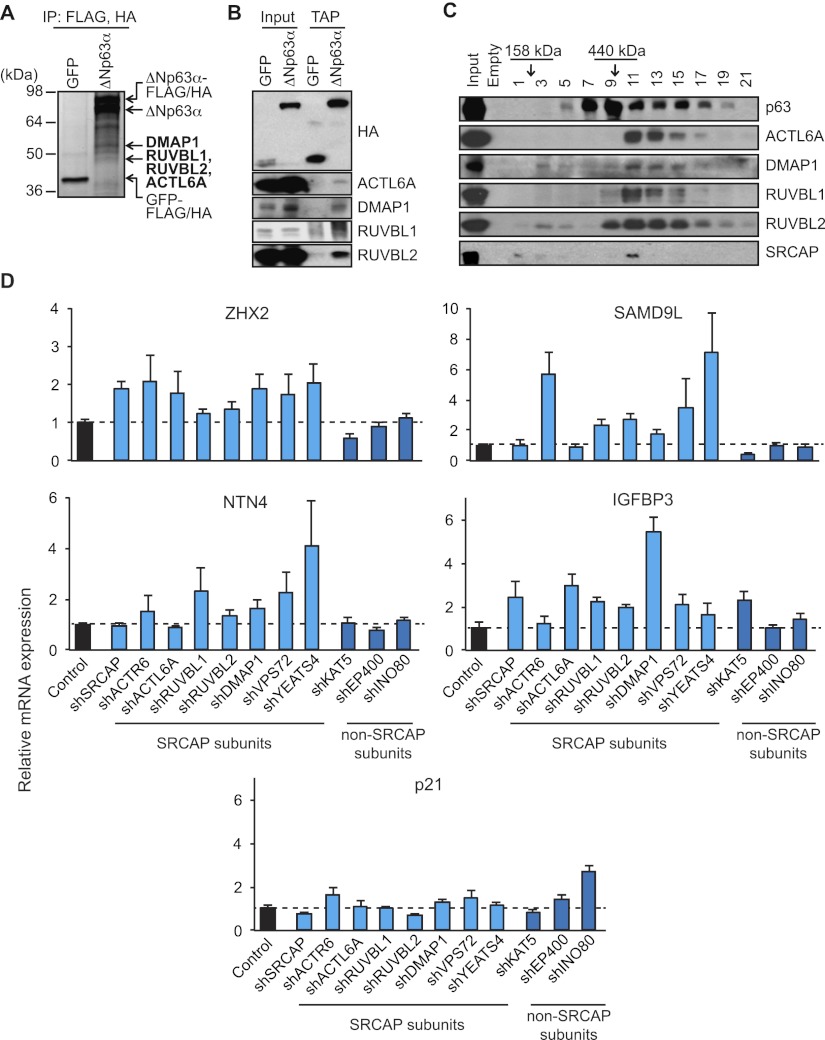
The structure of ΔNp63α contains several protein–protein interaction domains, including a proline-rich domain and a sterile α motif. These domains may enable ΔNp63α to recruit both positive and negative transcription regulatory factors to its target gene loci. In an unbiased approach to identify ΔNp63α-interacting proteins, we performed a tandem affinity purification (TAP) of C-terminal Flag/HA-tagged ΔNp63α or control GFP proteins followed by SDS-PAGE and mass spectrometry, as previously described (Ramsey et al. 2011). Interestingly, we found that ΔNp63α interacts with four proteins previously identified as components of diverse chromatin regulatory complexes: DMAP1 (found in the TIP60 and SRCAP complexes) as well as RUVBL1, RUVBL2, and ACTL6A (found in the TIP60, SRCAP, BAF, and INO80 complexes) (Fig. 3A; Supplemental Fig. 8A; Jin et al. 2005; Huen et al. 2010; Billon and Côté 2012). All of these interactions were confirmed by TAP followed by Western blot (Fig. 3B). Furthermore, fractionation experiments using a 10%–40% glycerol density gradient show that a significant portion of ΔNp63α copurifies in a large-molecular-weight complex with DMAP1, RUVBL1, RUVBL2, and ACTL6A (Fig. 3C). Although the SRCAP subunit was not detected by our mass spectrometry protocol, Western blot analysis of the glycerol gradient shows that this subunit copurifies in the same fraction as the rest of the complex (see fraction 11 in Fig. 3C).
Figure 3.
Subunits of the SRCAP complex associate with ΔNp63α and repress its target genes. (A) Silver-stained gel following TAP of C-terminal Flag/HA-tagged ΔNp63α or control GFP proteins in JHU029 SCC cells. Novel ΔNp63α-interacting proteins identified by mass spectrometry analysis are indicated in bold. (B) TAP/Western blot confirmation of specific binding of ΔNp63α to ACTL6A, DMAP1, RUVBL1, and RUVBL2. (C) Cofractionation of ΔNp63α, ACTL6A, DMAP1, RUVBL1, RUVBL2, and SRCAP on a 10%–40% glycerol density gradient. Fraction numbers and molecular weight standards are indicated. (D) qRT–PCR analysis of gene expression in H226 cells stably expressing shRNAs against SRCAP subunits SRCAP, ACTR6, ACTL6A, RUVBL1, RUVBL2, DMAP1, VPS72, and YEATS4 and non-SRCAP subunits KAT5, EP400, and INO80.
In order to test for the impact of these novel ΔNp63α interactors on the expression of ΔNp63α target genes, we established stable knockdowns for subunits of the SRCAP complex as well as subunits of other overlapping protein complexes (Fig. 3D; Supplemental Figs. 8B, 9). Interestingly, depletion of the SRCAP subunits SRCAP, ACTR6, ACTL6A, RUVBL1, DMAP1, VPS72, and YEATS4 results in the selective derepression of specific class III genes, including ZHX2, NTN4, SAMD9L, and IGFBP3, with no significant effects on the class I gene p21 (Fig. 3D; Supplemental Fig. 8B). In contrast, knockdown of the non-SRCAP subunits KAT5 (TIP60), EP400 (p400), and INO80 does not produce the same overall pattern of gene-specific derepression. While the precise effect of the six SRCAP subunits tested varies across class III genes, overall, our results implicate subunits of the known SRCAP complex in mediating transcriptional repression by ΔNp63α.
Depletion of ΔNp63α leads to loss of H2A.Z and RNA polymerase II (RNAPII) activation
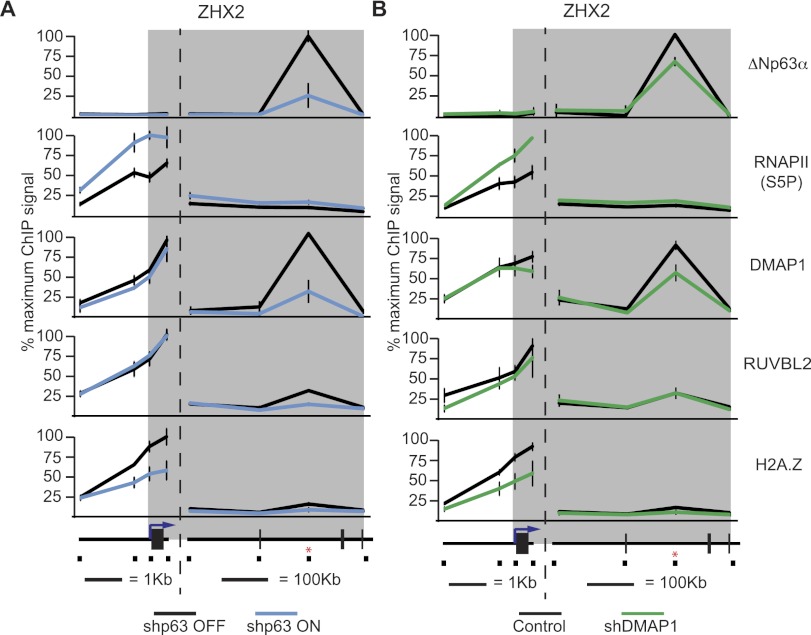
Both the SRCAP complex and a smaller subcomplex composed of RUVBL1, RUVBL2, DMAP1, and ACTL6A have been shown to exchange histone H2A with the histone variant H2A.Z (Jin et al. 2005; Choi et al. 2009; Huen et al. 2010; Billon and Côté 2012). Interestingly, H2A.Z has been previously identified as a transcriptional repressor in some settings (Gévry et al. 2007; Marques et al. 2010). These observations led us to hypothesize that ΔNp63α could use this complex as a corepressor, perhaps via H2A.Z deposition. To investigate this possibility, we performed ChIP analysis of the class III gene ZHX2, which was derepressed to varying degrees upon the loss of any of ΔNp63α, SRCAP, ACTR6, ACTL6A, DMAP1, RUVBL1, RUVBL2, VPS72, and YEATS4 (Figs. 1E, 3D). ZHX2 is a zinc finger homeobox transcription factor that has recently been shown to induce cell cycle arrest via repression of S-phase cyclins in hepatocellular carcinoma cells (Yue et al. 2012). ZHX2 is also commonly down-regulated in lung SCCs and HNSCCs as well as large cell lung carcinoma and invasive breast carcinoma (Supplemental Fig. 10) and thus may be a relevant target of ΔNp63α-mediated repression during oncogenesis. ChIP assays show that under basal conditions, strong ΔNp63α binding is detected at the intronic ZHX2 enhancer site but not elsewhere across the gene locus (Fig. 4A; Supplemental Fig. 11A). Expectedly, ΔNp63α signals drop significantly in cells expressing shp63. ChIP analysis of the actively initiating form of RNAPII [Ser5-phosphorylated (S5P)-RNAPII] shows that ΔNp63α knockdown increases RNAPII activity at the ZHX2 promoter. Analysis of DMAP1 and RUVBL2 occupancy indicates that both factors are found at the ZHX2 promoter and enhancer sites (Fig. 4A; Supplemental Fig. 11A). Importantly, ΔNp63α depletion leads to a significant decrease in the binding of these factors exclusively at the enhancer site. Analysis of H2A.Z throughout the ZHX2 locus showed significant occupancy at the promoter region and to a lesser extent at the intragenic enhancer, and ΔNp63α knockdown leads to a significant drop in H2A.Z signals at both sites (Fig. 4A; Supplemental Fig. 11A). Thus, ΔNp63α promotes H2A.Z deposition, possibly through a mechanism that involves gene looping from the enhancer to the promoter. Given that H2A.Z has been found to colocalize with CTCF to bridge and loop DNA (Millau and Gaudreau 2011), it is not necessarily surprising that the ΔNp63α-specific deposition of H2A.Z may be mediated by the action of these subunits at the distal enhancer site. Of note, ΔNp63α knockdown also causes decreased occupancy of DMAP1 and RUVBL2 at the enhancers of the class III genes NTN4, SAMD9L, and IGFBP3 as well as reduced H2A.Z signals at the promoters of NTN4 and SAMD9L (Supplemental Fig. 12). Overall, these results suggest that ΔNp63α-mediated recruitment of SRCAP subunits and H2A.Z deposition could mediate transcriptional repression at ΔNp63α target genes. As a first test of this hypothesis, we examined the impact of DMAP1 knockdown. Depletion of DMAP1 led to concomitant loss of H2A.Z and increase in S5P-RNAPII at the ZHX2 promoter (Fig. 4B; Supplemental Fig. 11B). Furthermore, ΔNp63α binding was modestly but reproducibly reduced upon DMAP1 depletion, suggesting a self-enforcing mechanism of recruitment between ΔNp63α and its corepressor (Fig. 4B; Supplemental Fig. 11B).
Figure 4.
ΔNp63α depletion leads to RNAPII activation concurrently with loss of SRCAP subunits and H2A.Z at the ZHX2 locus. ChIP assays of the ZHX2 locus were performed using cell extracts from H226 cells. (A) Extracts from cells before (black lines) and 48 h after (blue lines) ΔNp63α knockdown were used. (B) Extracts from control cells (black lines) and DMAP1 constitutive knockdown cells (green lines) were used. Antibodies specific for p63, Ser5-phosphorylated (S5P) RNAPII, DMAP1, RUVBL2, and H2A.Z were used. ChIP-enriched DNA was quantified by real-time PCR. The position of each PCR amplicon is indicated on a linear scale model of the locus. The position of the p53/p63 response element is indicated by a red asterisk. The gray band represents the transcribed region of the locus.
H2A.Z functions as a specific repressor of ΔNp63α target genes
Next, we investigated the impact of H2A.Z knockdown on cellular behavior and expression of p53 and ΔNp63α target genes. H2A.Z knockdown in H226 cells produces a strong decrease in cell viability, as evidenced by a reduction in total cell numbers and BrdU incorporation, and, more prominently, an increase in the number of apoptotic cells (Fig. 5A–C; Supplemental Fig. 13). Strikingly, qRT–PCR analysis revealed that H2A.Z depletion produces a specific increase in the expression of class III genes such as ZHX2, NTN4, SAMD9L, and IGFBP3 (Fig. 5D). In contrast, the class I genes p21, BAX, and MDM2 were unaffected by H2A.Z knockdown. Among class II genes, FAM49A and GGT6 also showed increased expression, while GJB4 and PLAC8 were unaffected. Thus, H2A.Z knockdown largely mimics the effects of ΔNp63α knockdown across the three gene classes. To define the generality of these effects, we tested the effects of H2A.Z knockdown in other cell types overexpressing ΔNp63α and lacking functional p53. In fact, H2A.Z depletion in HaCaT, SCC-13, and Cal-27 cells leads to a specific increase in the expression of the class III genes, most notably ZHX2 and SAMD9L, without significant effects on class I genes (Fig. 5E). Intriguingly, IGFBP3 was not sensitive to H2A.Z depletion in these cell lines.
Figure 5.
H2A.Z represses ΔNp63α target genes. Cell proliferation was assayed by direct cell counting (A) and BrdU incorporation (B) in H226 cells with and without H2A.Z knockdown. See Supplemental Figure 13 for H2A.Z knockdown efficiency. (C) Apoptosis was assayed by Annexin V staining in flow cytometry assays. 5FU was used as a positive control. (D) qRT–PCR analysis of selected class I, class II, and class III genes following 5 d of H2A.Z knockdown in H226 cells. (E) qRT–PCR analysis of selected class I and class III genes following 5 d of H2A.Z knockdown in HaCaT, SCC-13, and Cal-27 cells.
Overall, our observations indicate that ΔNp63α, H2A.Z, and subunits of known H2A.Z exchange complexes function coordinately to repress the transcription of class III genes independently of p53 status. Since ΔNp63α is required for binding of H2A.Z and recruitment of SRCAP subunits to these loci, our results indicate that ΔNp63α uses H2A.Z deposition to repress RNAPII activity at these genomic sites.
SAMD9L is a novel anti-proliferative gene repressed by ΔNp63α and H2A.Z
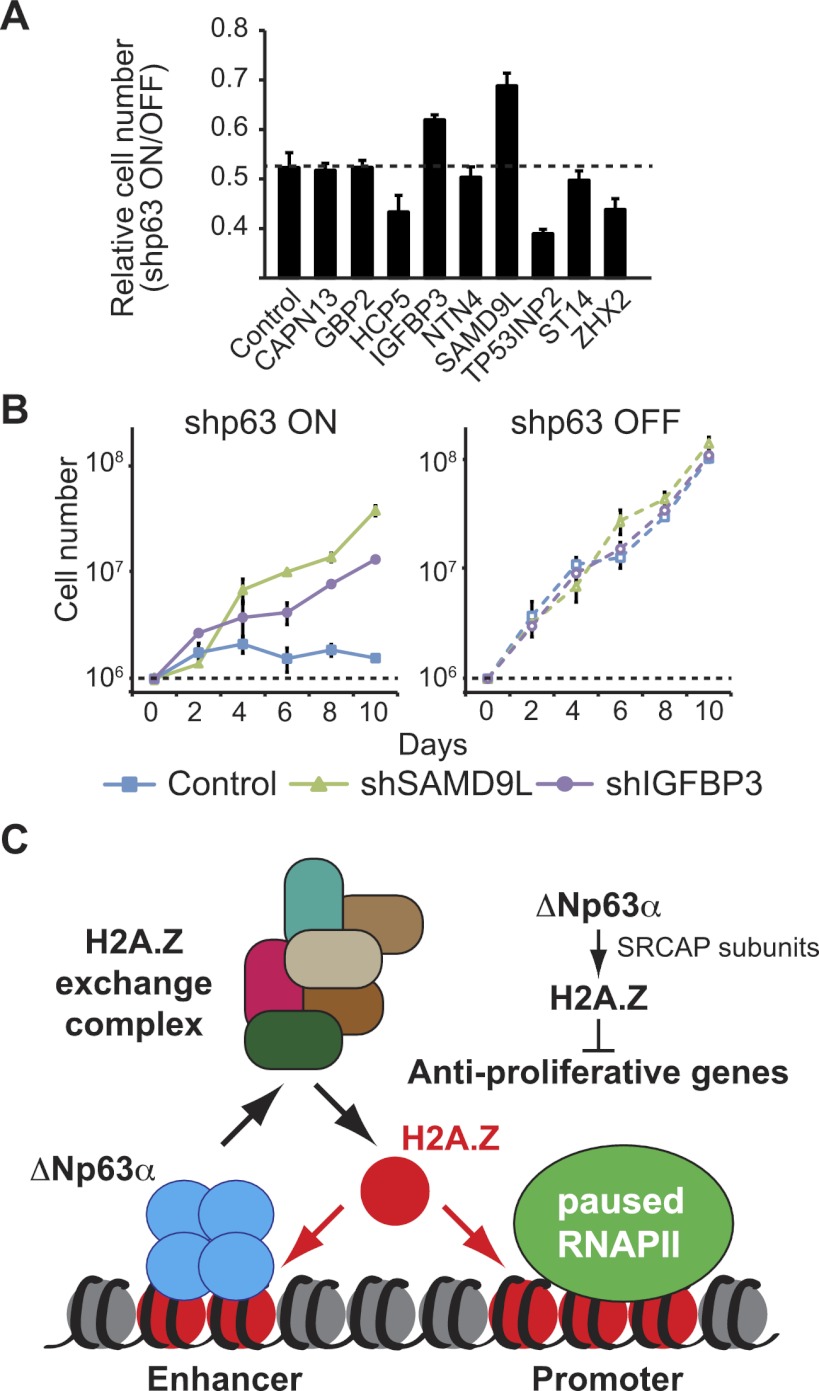
In order to investigate the biological significance of gene repression by the ΔNp63α/H2A.Z axis, we aimed to define the contribution of class III genes to the proliferation arrest caused by ΔNp63α depletion. We hypothesized that the arrest phenotype could be driven by up-regulation of one or more anti-proliferative genes normally repressed by ΔNp63α via H2A.Z. To test this possibility, we established a series of stable knockdowns for validated class III genes in cell lines carrying inducible shp63. We selected genes that have previously been described as anti-proliferative (e.g., IGFBP3, NTN4, TP53INP2, and ST14) and/or whose expression is often down-regulated in cancer tissues (e.g., SAMD9L, CAPN13, GBP2, HCP5, and ZHX2). As previously shown in Figure 1, H226 cell cultures stop dividing when shp63 expression is turned on. Interestingly, among the nine genes tested, only concurrent knockdown of SAMD9L or IGFBP3 leads to an increase in cell numbers (Fig. 6A). Most strikingly, a time-course experiment revealed that depletion of SAMD9L suffices to rescue the proliferation arrest caused by ΔNp63α knockdown (Fig. 6B). A second shRNA targeting SAMD9L reproduces this result, indicating that it is unlikely to be due to off-target effects (Supplemental Fig. 14). Knockdown of IGFBP3, a previously characterized anti-proliferative target of ΔNp63α-mediated repression (Barbieri et al. 2005), using two different shRNAs (Fig. 6B; Supplemental Fig. 14) also allowed cell cultures to continue proliferation upon ΔNp63α depletion. Expectedly, single knockdowns of SAMD9L and IGFBP3 do not affect cell proliferation in ΔNp63α-proficient cells, as these genes are expressed at very low basal levels (Fig. 6B; Supplemental Fig. 14, right panel).
Figure 6.
Depletion of SAMD9L rescues the proliferation arrest caused by ΔNp63α knockdown. (A) Cell proliferation was monitored by sulforhodamine B (SRB) assays following 5 d of ΔNp63α knockdown in H226 cell lines stably expressing shRNAs against the indicated ΔNp63α target genes. Results are presented as the ratio of absorbance (A510) after/before doxycycline treatment to induce p63 knockdown. (B) Time course of cell proliferation performed by direct cell counting. Cells (1 × 106) were seeded at day 0. Cells were pretreated with doxycycline for 5 d prior to seeding to induce ΔNp63α knockdown. Isogenic H226 cell lines with stable SAMD9L knockdown or IGFBP3 knockdown were compared with ΔNp63α knockdown alone (Control). See Supplemental Figure 14 for a second shRNA for each of SAMD9L and IGFBP3. (C) ΔNp63α recruits subunits of the SRCAP complex and mediates H2A.Z incorporation to repress RNAPII at anti-proliferative genes in a mechanism that is autonomous of p53.
Taken together, these results show that gene repression mediated by ΔNp63α and H2A.Z contributes to the hyperproliferative nature of SCC cells and accounts, at least in part, for the oncogenic properties of ΔNp63α.
Discussion
ΔNp63α plays critical roles in normal development and cancer. TP63 knockout mice show profound defects in limb, craniofacial, and epithelial development (Yang et al. 1999). Exon-specific knockout experiments showed that these developmental effects are not driven by the TA isoforms (Suh et al. 2006; Romano et al. 2012). In fact, ΔNp63α is a proliferative factor required for maintenance of the stem cell population in stratified epithelia (Senoo et al. 2007). In humans, loss-of-function germline mutations in the TP63 gene cause a plethora of human ectodermal syndromes (Rinne et al. 2007). In the context of cancer, ΔNp63α is an oncogene that promotes cell survival and proliferation and whose overexpression is widespread among SCCs of multiple origins, including head and neck, lung, and esophagus (Hibi et al. 2000; Yamaguchi et al. 2000). Despite its undisputed biomedical relevance, the molecular mechanisms of ΔNp63α action remain obscure. It is generally accepted that ΔNp63α can function as both a positive and negative regulator of transcription, but it is unclear exactly how these functions contribute to its developmental roles and oncogenic potential. Here we demonstrate that ΔNp63α is a robust transcriptional repressor of anti-proliferative genes in SCC cells, acting through a previously uncharacterized mechanism involving the recruitment of SRCAP subunits and deposition of the histone variant H2A.Z. Our findings can be summarized as follows: (1) ΔNp63α is required for proliferation of SCC cells independently of p53 status. (2) ΔNp63α is a transcriptional repressor of a unique subset of genes that are bound by p53 yet are not transactivated by this tumor suppressor. (3) ΔNp63α does not prevent p53 binding to its enhancer sites, which contrasts with the view of ΔNp63α action as a dominant p53 inhibitor. (4) ΔNp63α associates with and recruits subunits of the SRCAP complex, and knockdown of SRCAP subunits results in up-regulation of ΔNp63α target genes. (5) The histone variant H2A.Z is deposited in a ΔNp63α-dependent manner at ΔNp63α target loci, and H2A.Z knockdown activates these genes. (6) SAMD9L, a gene repressed by ΔNp63α, SRCAP subunits, and H2A.Z, is required to sustain the proliferation defect observed upon ΔNp63α loss. Altogether, these results not only advance our understanding of the molecular mechanism of action of ΔNp63α, but also point to the H2A.Z exchange complexes as important ΔNp63α cofactors and to SAMD9L as a key antagonist of ΔNp63α proliferative effects.
The view that ΔNp63α acts as a dominant-negative inhibitor of p53 was originally proposed based on the observations that ΔNp63α lacks a canonical transactivation domain and that overexpression of ΔNp63α inhibits p53 transactivation in ectopic expression reporter assays (Yang et al. 1998; Parsa et al. 1999). This notion gathered additional support from the fact that p53 and ΔNp63α bind to nearly identical DNA sequences both in vitro and in vivo (Ortt and Sinha 2006; Yang et al. 2006; Perez et al. 2007; Kouwenhoven et al. 2010). In contrast, our research into a possible functional interplay between endogenous p53 and ΔNp63α in cancer cells normally expressing both proteins forced us to discard the dominant-negative hypothesis, leading us instead to an alternative model whereby ΔNp63α represses transcription through a chromatin-based mechanism involving recruitment of a chromatin regulatory complex and H2A.Z deposition.
Our proteomics efforts to uncover novel ΔNp63α cofactors led us to four proteins previously identified as components of diverse chromatin regulatory complexes: DMAP1 (TIP60 and SRCAP complexes) as well as RUVBL1, RUVBL2, and ACTL6A (TIP60, SRCAP, BAF, and INO80 complexes) (Supplemental Fig. 8A). The sophisticated combinatorial assembly of these proteins into various chromatin regulators known to exert both positive and negative roles in transcription prevents a straightforward interpretation of these interaction data. However, knockdown of subunits that are specific to the SRCAP complex leads to differential up-regulation of ΔNp63α target genes in our system, which is not observed for non-SRCAP subunits, indicating that SRCAP or a closely related complex functions as a corepressor complex acting at ΔNp63α target loci. Although the precise effect of each SRCAP subunit varied across ΔNp63α targets, none of them contributed to repression of the p21 gene, which in our system is not repressed by ΔNp63α. Collectively, these observations led us to focus on H2A.Z, whose deposition into nucleosomes can be mediated by either a smaller cofactor complex containing the four core subunits interacting physically with ΔNp63α or a larger complex containing SRCAP itself (Choi et al. 2009; Billon and Côté 2012). Indeed, ChIP analysis of the ZHX2 locus shows that ΔNp63α mediates recruitment of DMAP1 and RUBVL2 and H2A.Z deposition. Unfortunately, our efforts to detect other subunits of the SRCAP complex at ΔNp63α target loci were unsuccessful, mostly due to the lack of ChIP-grade antibodies (data not shown). More importantly, depletion of H2A.Z leads to specific induction of ΔNp63α target genes across multiple cell types, suggesting that this histone variant is a downstream effector of ΔNp63α-mediated repression.
H2A.Z is an evolutionarily conserved variant of the core histone H2A with diverse roles in transcriptional control. Depending on the context, H2A.Z has been described as a positive or negative regulator of transcription. In budding yeast, H2A.Z nucleosomes are observed flanking the transcription start site (TSS), with a nucleosome-free region in between (Raisner et al. 2005). A similar observation was made in human cells, but in this case, H2A.Z was also found at enhancers and insulators (Barski et al. 2007). In yeast, H2A.Z promoter occupancy correlates inversely with transcriptional activity (Guillemette et al. 2005). In humans, the role of H2A.Z is more enigmatic, as H2A.Z incorporation has been associated with both gene activation and repression (Marques et al. 2010; Billon and Côté 2012). In contrast to that observed in yeast, genome-wide studies in human cells show that H2A.Z promoter occupancy correlates positively with gene expression (Barski et al. 2007). Intriguingly, biochemical in vitro assays using mammalian factors showed that H2A.Z-containing nucleosomes are refractory to transcription (Thakar et al. 2010). These seemingly contradictory observations could be reconciled by the precise location of H2A.Z-containing nucleosomes at or near the promoter and the consequent topological effects on transcription factor binding (Marques et al. 2010). Recent genome-wide approaches have demonstrated that while H2A.Z is deposited both immediately upstream of and downstream from the TSSs of active genes, it is uniformly deposited across the promoter of inactive genes (Valdés-Mora et al. 2012). Thus, H2A.Z may regulate transcription positively by reprogramming nucleosome positioning to allow access to a regulatory region or TSS or negatively by preventing access to that regulatory region or TSS (Marques et al. 2010). This hypothesis has been supported by recent H2A.Z ChIP-seq (ChIP combined with deep sequencing) studies performed during mitotic silencing, whereby H2A.Z mediates the upstream shifting of the +1 nucleosome to occupy the TSS and thereby reduces the nucleosome-depleted region, thus silencing gene expression. In this manner, H2A.Z serves to transiently repress transcription during mitosis and poise genes for activation upon H2A.Z removal (Kelly et al. 2010). Future studies will be aimed at deciphering the precise mechanisms by which H2A.Z represses ΔNp63α target genes.
It is important to note that the mechanism of transcriptional repression described here involving H2A.Z does not exclude other mechanisms used by ΔNp63α in other contexts. For example, p63 depletion leads to TAp73-dependent apoptosis in various HNSCC cell lines concurrently with up-regulation of PUMA and NOXA (DeYoung et al. 2006; Rocco et al. 2006). In these cells, ΔNp63α forms hetero-oligomers with TAp73β and prevents binding of TAp73β to the enhancer site in the PUMA locus. Furthermore, our previous work in other HNSCC cell types demonstrated that ΔNp63α physically associates with the histone deacetylases HDAC1 and HDAC2, recruits HDAC1, and promotes histone deacetylation at the PUMA locus (Ramsey et al. 2011). In fact, ΔNp63α-overexpressing cells are significantly more sensitive to HDAC inhibitors, which elicit a form of cell death that can be blocked by BCL2 overexpression (Ramsey et al. 2011). Interestingly, these mechanisms are not conserved in the p53-proficient lung SCC cells used in this study. First of all, H226 cells undergo arrest, not apoptosis, in response to ΔNp63α knockdown. Second, PUMA is not induced upon ΔNp63α knockdown (Supplemental Fig. 2). Third, concomitant knockdown of ΔNp63α and p73 in H226 cells does not rescue the effects of ΔNp63α loss in terms of either cell proliferation arrest or target gene expression (data not shown). Fourth, ΔNp63α depletion has no significant effect on various histone acetylation events analyzed at class I, II, and III genes in H226 cells (data not shown). These context-dependent differences, and particularly the contribution of TAp73 to ΔNp63α-dependent effects in different SCC cells, may in part reflect the higher TAp73 levels observed in SCCs that express mutant p53 (DeYoung et al. 2006). Clearly, ΔNp63α seems to use multiple alternative mechanisms of repression, one of which involves H2A.Z deposition. Importantly, H2A.Z knockdown leads to up-regulation of ΔNp63α target genes in other cell types beyond H226, including HaCaT (immortalized keratinocytes), SCC-13 (tongue HNSCC), and Cal-27 (oral HNSCC), thus proving the conservation of this mechanism. Future studies will further investigate the basis for these cell and context-dependent mechanisms of repression, which may also be due to differential availability of corepressors.
Depending on the context, diverse sets of genes have been identified as targets of ΔNp63α-mediated repression, including canonical p53 target genes such as p21, 14-3-3σ, PUMA, and NOXA (Westfall et al. 2003; Rocco et al. 2006). Interestingly, H2A.Z incorporation has been shown to repress the p53 target gene p21 in colorectal cancer cells (Gévry et al. 2007); however, p21 was not repressed by ΔNp63α, SRCAP subunits, or H2A.Z in the four cell types tested in our study. Instead, our efforts point to a different set of ΔNp63α-repressed genes that contribute to its oncogenic potential. Our study is unique in that we used an unbiased microarray approach in rare SCC cells of lung origin that expresses functional p53 as well as high levels of ΔNp63α. Interestingly, analysis performed through The Cancer Genome Atlas (TCGA; https://tcga-data.nci.nih.gov/tcga) indicates that 196 of the 215 genes up-regulated upon ΔNp63α knockdown in H226 cells have a reduced expression in numerous lung SCC cells as compared with normal tissue (Supplemental Tables 1, 2). Studies of SCC cells expressing endogenous ΔNp63α differ in the degree to which canonical p53 target genes, including p21, MDM2, BAX, NOXA, or PUMA, are repressed by ΔNp63α (Barbieri et al. 2005; Rocco et al. 2006; Gu et al. 2008; Lu et al. 2011). Here our efforts revealed a group of anti-proliferative genes repressed by ΔNp63α across SCC cell types of diverse origin regardless of p53 status, including IGFBP3 and SAMD9L, whose knockdown rescues the proliferation arrest caused by ΔNp63α depletion in H226 cells.
IGFBP3 was originally characterized as a p53 target gene whose secretion inhibits mitotic signaling by the insulin-like growth factor IGF-1 (Buckbinder et al. 1995). However, p53 activation does not lead to IGFBP3 induction in H226 cells and other cell types tested (Fig. 1; data not shown), suggesting that its regulation by p53 is not universal. In contrast, IGFBP3 seems to be a common target of ΔNp63α-mediated repression, as first characterized by the Pietenpol laboratory (Barbieri et al. 2005). They showed that ΔNp63α knockdown leads to IGBFP3 induction in keratinocytes and diverse SCC cell lines, that ΔNp63α binds to specific sequences in the IGFBP3 locus to mediate repression, and that expression of ΔNp63α and IGFBP3 is inversely correlated in normal and cancerous tissues (Barbieri et al. 2005). Our studies confirm and extend these findings. Intriguingly, whereas we found that IGFBP3 is commonly induced upon ΔNp63α knockdown in H226, HaCaT, SCC-13, and Cal-27 cells, the effects of H2A.Z knockdown are unique to H226 cells, thus reinforcing the notion of cell type-specific mechanisms of corepression by ΔNp63α.
To the best of our knowledge, we are the first to report SAMD9L as a target of ΔNp63α-mediated repression. Little is known about the biological roles of SAMD9L. Interestingly, SAMD9L and its paralog gene, SAMD9, exist in a short fragment of the human chromosome 7q21 region that is commonly deleted in patients with myeloid leukemia and myelodysplastic syndrome (Asou et al. 2009). SAMD9L is expressed at significantly lower levels in breast carcinomas relative to normal breast epithelia from the same patients (Li et al. 2007). SAMD9L is ubiquitously expressed across normal tissues (Li et al. 2007) and commonly down-regulated in various cancers, including lung SCC as well as large and small cell lung carcinomas (Supplemental Fig. 15). Thus, SAMD9L may be a novel tumor suppressor whose repression is key to the oncogenic effects of ΔNp63α. Our future efforts will focus on the mechanisms of action of SAMD9L and its role in cell proliferation control.
In conclusion, the results presented here demonstrate a novel chromatin-based mechanism by which ΔNp63α promotes cell proliferation through transcriptional repression in SCCs. These findings pave the road for not only more mechanistic studies, but also the design of therapeutic strategies targeting the ΔNp63α/H2A.Z pathway in SCCs of multiple tissue origins.
Materials and methods
Cell culture
H226 cells were grown in RPMI-1640 medium, and HEK293FT, HaCaT, SCC-13, and Cal-27 cells were grown in DMEM medium, all supplemented with 10% (v/v) fetal bovine serum (Sigma-Aldrich) and antibiotic/anti-mycotic mix (Invitrogen) under controlled 5% CO2 and 37°C.
shRNA-mediated knockdown
Isogenic cell lines stably transduced with inducible shRNAs targeting p63 (shp63) or H2A.Z (shH2A.Z) were established using the lentiviral-based shRNA delivery vector pTRIPZ (Open Biosystems). shp53 was expressed using the lentiviral vector pLL3.7. All other shRNAs were generated using the pLKO lentiviral vector (Sigma-Aldrich). For all dual knockdown experiments, shp63 was expressed using the inducible lentiviral vector pLKO-Tet-On (Novartis). All shRNA sequences are listed in Supplemental Table 3. We produced viral particles in HEK293FT cells and used the viral particles to transduce H226 cells, which were subsequently cultured in the presence of 5 μg/mL puromycin (pTRIPZ, pLKO) or 2 μg/mL G418 (pLL3.7, pLKO-Tet-On). Transduced HaCaT, SCC-13, and Cal-27 cells were selected with 0.4 μg/mL, 0.5 μg/mL, and 0.5 μg/mL puromycin, respectively. Expression of shp63 was induced using 1 μg/mL (pTRIPZ) or 2 μg/mL (pLKO-Tet-On) doxycycline, and shH2A.Z was induced using 2 μg/mL doxycycline.
Cell proliferation and flow cytometry analyses
For cell proliferation assays, H226 cells were either counted directly using a Cellometer Auto T4 (Nexcelom Biosciences) or subjected to sulforhodamine B (SRB) assays as described previously (Vichai and Kirtikara 2006). Cell cycle analysis was performed on ethanol-fixed cells using propidium iodide, as described previously (Gomes et al. 2006). For BrdU incorporation analysis, actively growing cells were pulsed with 10 μM BrdU prior to harvest, fixation, anti-BrdU staining, and propidium iodide counterstaining. For apoptosis index assays, cells were trypsinized and washed in PBS and then in Annexin V-binding buffer (10 mM HEPES at pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Cells were then stained with Annexin V-fluorescein conjugate (Invitrogen) and propidium iodide, following the manufacturer's instructions. Flow cytometry analysis was performed on a CyAn flow cytometer (Beckman Coulter) or a C6 flow cytometer (Accuri).
Protein immunoblot analysis
Ten micrograms of total protein extract was loaded onto 8% (p53, p63, or Nucleolin) or 15% (p21, H2A.Z, or actin) SDS-PAGE gels and transferred to PVDF membranes. Blots were probed with the indicated primary antibodies (Supplemental Table 4) and developed with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology) and ECL detection reagents (GE Healthcare). Blots were imaged on the LAS4000 chemiluminescence imager (GE Healthcare).
Microarray gene profiling
H226 cells were grown to 50%–60% confluence and treated with 1–2 μg/mL doxycycline for 48 h to induce shp63 or 10 μM Nutlin-3 (Cayman) for 12 h to activate p53. Total RNA was isolated using TRIzol (Sigma-Aldrich), following the manufacturer's instructions. Samples were labeled and hybridized to Human Gene 1.0 ST arrays (Affymetrix) according to the manufacturer's instructions. Raw data from the *.CEL files were imported into Partek Express (Partek, Inc.) for analysis. Genes were identified as being significantly induced if they displayed a >1.5-fold change and P < 0.05 as compared with control samples. Microarray data have been deposited in the Gene Expression Omnibus (GSE40462).
qRT–PCR
qRT–PCR analyses were performed as described previously (Gomes et al. 2006). cDNA was generated using the qScript cDNA synthesis kit (Quanta Biosciences), and relative mRNA levels were determined relative to 18S rRNA using SYBR Green I chemistry in an ABI 7900HT real-time PCR machine (Applied Biosystems). See Supplemental Table 5 for primer sequences.
ChIP assays
All ChIP analyses were performed as described previously (Gomes et al. 2006). Briefly, H226 cells were grown to 50%–60% confluency, treated with 1 μg/mL doxycycline for 48 h and/or 10 μM Nutlin-3 or 375 mM 5FU for 12 h, and cross-linked with 1% formaldehyde prior to preparation of whole-cell lysates. Cell lysates (1 mg of protein) were subjected to ChIP using the indicated antibodies (Supplemental Table 4), followed by DNA purification. ChIP-enriched DNA was analyzed by qPCR using an absolute quantification method as detailed previously (Gomes et al. 2006) with the indicated primer sets (Supplemental Table 5).
TAP
TAP was performed as previously described (Ramsey et al. 2011). Briefly, cells were stably transfected with pMSCV-ΔNp63α-Flag-HA (C-terminal) or pMSCV-GFP-Flag-HA plasmids, and nuclear extracts were prepared by suspending cells in hypotonic buffer (10 mM Tris-HCl at pH 7.5, 1.5 mM MgCl2, 10 mM KCl) followed by douncing. Nuclear proteins were extracted from pelleted nuclei in 400 mM KCl nuclear buffer (400 mM Tris-HCl at pH 7.5, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol), and then the supernatant was dialyzed against BC-100 buffer (100 mM KCl, 20 mM Tris-HCl at pH 7.5, 0.2 mM EDTA, 20% glycerol). Nuclear extracts were incubated with α-Flag-conjugated beads (M2, Sigma) and then washed with 150 mM, 250 mM, 500 mM, 250 mM, and 150 mM KCl wash buffer (50 mM Tris-HCl at pH 7.5, 5 mM MgCl2, 0.2 mM EDTA, 0.1% NP-40, 10% glycerol). Immune complexes were eluted with 0.5 mg/mL Flag peptide in 150 mM KCl wash buffer and then incubated at 4°C with α-HA-conjugated beads (3F10, Roche). Beads were washed with 150 mM, 200 mM, 250 mM, 200 mM, and 150 mM KCl wash buffer and boiled in Laemmli buffer, and then proteins were visualized using the SilverQuest silver staining kit (Invitrogen). Peptides were identified using microcapillary LC/MS/MS analysis of bands excised from SDS-PAGE gels. Glycerol density gradient fractionation of nuclear extracts was performed on a 10%–40% density gradient as previously described (Binne et al. 2007).
Acknowledgments
We thank the Taplin Mass Spectrometry facility (Harvard Medical School) for performing the mass spectrometry analysis. This work was supported by NIH grants 2R01CA117907-06 (to J.M.E.), R01 DE-015945 (to L.W.E.), and F32CA159521-01 (to C.L.G-B.), and by the ACS/Mass Biotech Council Cancer Research Challenge-AstraZeneca Pharmaceuticals LP Fellowship PF-09-100-01 MGO (M.R.R.). J.M.E. is an HHMI Early Career Scientist. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other supporting agencies.
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.198069.112.
References
- Asou H, Matsui H, Ozaki Y, Nagamachi A, Nakamura M, Aki D, Inaba T 2009. Identification of a common microdeletion cluster in 7q21.3 subband among patients with myeloid leukemia and myelodysplastic syndrome. Biochem Biophys Res Commun 383: 245–251 [DOI] [PubMed] [Google Scholar]
- Barbieri CE, Perez CA, Johnson KN, Ely KA, Billheimer D, Pietenpol JA 2005. IGFBP-3 is a direct target of transcriptional regulation by ΔNp63α in squamous epithelium. Cancer Res 65: 2314–2320 [DOI] [PubMed] [Google Scholar]
- Barski A, Cuddapah S, Cui K, Roh T-Y, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K 2007. High-resolution profiling of histone methylations in the human genome. Cell 129: 823–837 [DOI] [PubMed] [Google Scholar]
- Billon P, Côté J 2012. Precise deposition of histone H2A.Z in chromatin for genome expression and maintenance. Biochim Biophys Acta 1819: 290–302 [DOI] [PubMed] [Google Scholar]
- Binne UK, Classon MK, Dick FA, Wei W, Rape M, Kaelin WG Jr, Naar AM, Dyson NJ 2007. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat Cell Biol 9: 225–232 [DOI] [PubMed] [Google Scholar]
- Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, Seizinger BR, Kley N 1995. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature 377: 646–649 [DOI] [PubMed] [Google Scholar]
- Choi J, Heo K, An W 2009. Cooperative action of TIP48 and TIP49 in H2A.Z exchange catalyzed by acetylation of nucleosomal H2A. Nucleic Acids Res 37: 5993–6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung MP, Johannessen CM, Leong CO, Faquin W, Rocco JW, Ellisen LW 2006. Tumor-specific p73 up-regulation mediates p63 dependence in squamous cell carcinoma. Cancer Res 66: 9362–9368 [DOI] [PubMed] [Google Scholar]
- Gévry N, Chan HM, Laflamme L, Livingston DM, Gaudreau L 2007. p21 transcription is regulated by differential localization of histone H2A.Z. Genes Dev 21: 1869–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes NP, Bjerke G, Llorente B, Szostek SA, Emerson BM, Espinosa JM 2006. Gene-specific requirement for P-TEFb activity and RNA polymerase II phosphorylation within the p53 transcriptional program. Genes Dev 20: 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano V, De Laurenzi V 2011. Role of p63 in cancer development. Biochim Biophys Acta 1816: 57–66 [DOI] [PubMed] [Google Scholar]
- Gu X, Coates PJ, Boldrup L, Nylander K 2008. p63 contributes to cell invasion and migration in squamous cell carcinoma of the head and neck. Cancer Lett 263: 26–34 [DOI] [PubMed] [Google Scholar]
- Guillemette B, Bataille AR, Gevry N, Adam M, Blanchette M, Robert F, Gaudreau L 2005. Variant histone H2A.Z is globally localized to the promoters of inactive yeast genes and regulates nucleosome positioning. PLoS Biol 3: e384 doi: 10.1371/journal.pbio.0030384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi K, Trink B, Patturajan M, Westra WH, Caballero OL, Hill DE, Ratovitski EA, Jen J, Sidransky D 2000. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci 97: 5462–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen J, Kakihara Y, Ugwu F, Cheung KL, Ortega J, Houry WA 2010. Rvb1–Rvb2: Essential ATP-dependent helicases for critical complexes. Biochem Cell Biol 88: 29–40 [DOI] [PubMed] [Google Scholar]
- Jin J, Cai Y, Li B, Conaway RC, Workman JL, Conaway JW, Kusch T 2005. In and out: Histone variant exchange in chromatin. Trends Biochem Sci 30: 680–687 [DOI] [PubMed] [Google Scholar]
- Kelly TK, Miranda TB, Liang G, Berman BP, Lin JC, Tanay A, Jones PA 2010. H2A.Z maintenance during mitosis reveals nucleosome shifting on mitotically silenced genes. Mol Cell 39: 901–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouwenhoven EN, van Heeringen SJ, Tena JJ, Oti M, Dutilh BE, Alonso ME, de la Calle-Mustienes E, Smeenk L, Rinne T, Parsaulian L, et al. 2010. Genome-wide profiling of p63 DNA-binding sites identifies an element that regulates gene expression during limb development in the 7q21 SHFM1 locus. PLoS Genet 6: e1001065 doi: 10.1371/journal.pgen.1001065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CF, MacDonald JR, Wei RY, Ray J, Lau K, Kandel C, Koffman R, Bell S, Scherer SW, Alman BA 2007. Human sterile α motif domain 9, a novel gene identified as down-regulated in aggressive fibromatosis, is absent in the mouse. BMC Genomics 8: 92 doi: 10.1186/1471-2164-8-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Yang X, Duggal P, Allen CT, Yan B, Cohen J, Nottingham L, Romano RA, Sinha S, King KE, et al. 2011. TNF-α promotes c-REL/ΔNp63α interaction and TAp73 dissociation from key genes that mediate growth arrest and apoptosis in head and neck cancer. Cancer Res 71: 6867–6877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques M, Laflamme L, Gervais AL, Gaudreau L 2010. Reconciling the positive and negative roles of histone H2A.Z in gene transcription. Epigenetics 5: 267–272 [DOI] [PubMed] [Google Scholar]
- Millau J-F, Gaudreau L 2011. CTCF, cohesin, and histone variants: Connecting the genome. Biochem Cell Biol 89: 505–513 [DOI] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A 1999. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398: 708–713 [DOI] [PubMed] [Google Scholar]
- Mundt HM, Stremmel W, Melino G, Krammer PH, Schilling T, Müller M 2010. Dominant negative (ΔN) p63α induces drug resistance in hepatocellular carcinoma by interference with apoptosis signaling pathways. Biochem Biophys Res Commun 396: 335–341 [DOI] [PubMed] [Google Scholar]
- Neilsen P, Noll J, Suetani R, Schulz R, Al-Ejeh F, Evdokiou A, Lane D, Callen D 2011. Mutant p53 uses p63 as a molecular chaperone to alter gene expression and induce a proinvasive secretome. Oncotarget 2: 1203–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekulova M, Holcakova J, Coates P, Vojtesek B 2011. The role of P63 in cancer, stem cells and cancer stem cells. Cell Mol Biol Lett 16: 296–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortt K, Sinha S 2006. Derivation of the consensus DNA-binding sequence for p63 reveals unique requirements that are distinct from p53. FEBS Lett 580: 4544–4550 [DOI] [PubMed] [Google Scholar]
- Parsa R, Yang A, McKeon F, Green H 1999. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J Invest Dermatol 113: 1099–1105 [DOI] [PubMed] [Google Scholar]
- Perez CA, Ott J, Mays DJ, Pietenpol JA 2007. p63 consensus DNA-binding site: Identification, analysis and application into a p63MH algorithm. Oncogene 26: 7363–7370 [DOI] [PubMed] [Google Scholar]
- Raisner RM, Hartley PD, Meneghini MD, Bao MZ, Liu CL, Schreiber SL, Rando OJ, Madhani HD 2005. Histone variant H2A.Z marks the 5′ ends of both active and inactive genes in euchromatin. Cell 123: 233–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey MR, He L, Forster N, Ory B, Ellisen LW 2011. Physical association of HDAC1 and HDAC2 with p63 mediates transcriptional repression and tumor maintenance in squamous cell carcinoma. Cancer Res 71: 4373–4379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne T, Brunner HG, van Bokhoven H 2007. p63-associated disorders. Cell Cycle 6: 262–268 [DOI] [PubMed] [Google Scholar]
- Rivetti di Val Cervo P, Lena AM, Nicoloso M, Rossi S, Mancini M, Zhou H, Saintigny G, Dellambra E, Odorisio T, Mahé C, et al. 2012. p63-microRNA feedback in keratinocyte senescence. Proc Natl Acad Sci 109: 1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco JW, Leong CO, Kuperwasser N, DeYoung MP, Ellisen LW 2006. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell 9: 45–56 [DOI] [PubMed] [Google Scholar]
- Romano RA, Smalley K, Magraw C, Serna VA, Kurita T, Raghavan S, Sinha S 2012. ΔNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development 139: 772–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F 2007. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell 129: 523–536 [DOI] [PubMed] [Google Scholar]
- Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F 2006. p63 protects the female germ line during meiotic arrest. Nature 444: 624–628 [DOI] [PubMed] [Google Scholar]
- Thakar A, Gupta P, McAllister WT, Zlatanova J 2010. Histone variant H2A.Z inhibits transcription in reconstituted nucleosomes. Biochemistry 49: 4018–4026 [DOI] [PubMed] [Google Scholar]
- Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA 2006. P63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev 20: 3185–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés-Mora F, Song JZ, Statham AL, Strbenac D, Robinson MD, Nair SS, Patterson KI, Tremethick DJ, Stirzaker C, Clark SJ 2012. Acetylation of H2A.Z is a key epigenetic modification associated with gene deregulation and epigenetic remodeling in cancer. Genome Res 22: 307–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichai V, Kirtikara K 2006. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 1: 1112–1116 [DOI] [PubMed] [Google Scholar]
- Westfall MD, Mays DJ, Sniezek JC, Pietenpol JA 2003. The ΔNp63α phosphoprotein binds the p21 and 14-3-3 σ promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol Cell Biol 23: 2264–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Wu L, Caballero OL, Hibi K, Trink B, Resto V, Cairns P, Okami K, Koch WM, Sidransky D, et al. 2000. Frequent gain of the p40/p51/p63 gene locus in primary head and neck squamous cell carcinoma. Int J Cancer 86: 684–689 [DOI] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F 1998. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 2: 305–316 [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, et al. 1999. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398: 714–718 [DOI] [PubMed] [Google Scholar]
- Yang A, Zhu Z, Kapranov P, McKeon F, Church GM, Gingeras TR, Struhl K 2006. Relationships between p63 binding, DNA sequence, transcription activity, and biological function in human cells. Mol Cell 24: 593–602 [DOI] [PubMed] [Google Scholar]
- Yue X, Zhang Z, Liang X, Gao L, Zhang X, Zhao D, Liu X, Ma H, Guo M, Spear BT et al. 2012. Zinc fingers and homeoboxes 2 inhibits hepatocellular carcinoma cell proliferation and represses expression of Cyclins A and E. Gastroenterology 142: 1559–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]