ABSTRACT
BACKGROUND
Factors contributing to medication nonadherence among patients with chronic obstructive pulmonary disease (COPD) are poorly understood.
OBJECTIVES
To identify patient characteristics that are predictive of adherence to inhaled medications for COPD and, for patients on multiple inhalers, to assess whether adherence to one medication class was associated with adherence to other medication classes.
DESIGN
Cohort study using data from Veteran Affairs (VA) electronic databases.
PARTICIPANTS
This study included 2,730 patients who underwent pulmonary function testing between 2003 and 2007 at VA facilities in the Northwestern United States, and who met criteria for COPD.
MAIN MEASURES
We used pharmacy records to estimate adherence to inhaled corticosteroids (ICS), ipratropium bromide (IP), and long-acting beta-agonists (LABA) over two consecutive six month periods. We defined patients as adherent if they had refilled medications to have 80 % of drug available over the time period. We also collected information on their demographics, behavioral habits, COPD severity, and comorbidities.
KEY RESULTS
Adherence to medications was poor, with 19.8 % adherent to ICS, 30.6 % adherent to LABA, and 25.6 % adherent to IP. Predictors of adherence to inhaled therapies were highly variable and dependent on the medication being examined. In adjusted analysis, being adherent to a medication at baseline was the strongest predictor of future adherence to that same medication [(Odds ratio, 95 % confidence interval) ICS: 4.79 (3.22–7.12); LABA: 6.60 (3.92–11.11); IP: 14.13 (10.00–19.97)], but did not reliably predict adherence to other classes of medication.
CONCLUSIONS
Among patients with COPD, past adherence to one class of inhaled medication strongly predicted future adherence to the same class of medication, but only weakly predicted adherence to other classes of medication.
KEY WORDS: medication adherence, pulmonary diseases, health behavior, veterans
INTRODUCTION
Multiple randomized controlled trials have demonstrated the efficacy of pharmacological treatment of chronic obstructive pulmonary disease (COPD) at improving symptoms and quality of life, while reducing the frequency of exacerbations.1–4 Medications cannot be effective when they are not taken. Medication adherence represents a complex set of health behaviors that is not clearly understood. Patients who demonstrate better adherence to an assigned treatment, including placebo treatment in the context of efficacy trials, have been shown to experience better outcomes.5 Although there is a paucity of information about adherence to pharmaceutical therapy for COPD, existing information suggests that medication nonadherence is common.6
In contrast to other chronic conditions such as hypertension and diabetes, there is limited information about predictors of nonadherence to inhaled medication therapies among patients with COPD.7–11 Moreover, the studies of adherence to COPD medications have largely focused on short-acting bronchodilators. Given the wealth of recently published data demonstrating the efficacy of long-acting inhaled therapies for COPD, understanding characteristics of associated medication adherence represents an important opportunity to identify areas where interventions may be designed to improve adherence to therapies with documented benefit. We sought to assess predictors of adherence to inhaled therapies for COPD and, for patients on multiple inhaled medications, to assess whether adherence to one class of medication predicts adherence to other classes of medication.
METHODS
Settings and Subjects
We performed a cohort study of Veterans who had pulmonary function testing. Our study population was identified from the 14,541 patients from three sites within the Veterans Integrated Service Network 20 (VISN 20) medical facilities, who had pulmonary function tests (PFTs) performed between January 2003 and December 2007. Pulmonary function testing was obtained as part of routine clinical care. We enrolled into our cohort those patients who: (1) had spirometric evidence of COPD, as defined by the global initiative for chronic obstructive lung disease (GOLD) criteria (a post-bronchodilator FEV1/FVC ratio less than 0.7), and (2) had a ReComp score for at least one of the study medications during both the baseline and the outcome periods.12 Enrollment did not require a clinician’s diagnosis of COPD. The enrollment date for a patient was the date of their first PFT within the specified time period that met criteria for obstruction. Of note, the patient could have had PFTs prior to January 2003, and thus the enrollment date does not necessarily reflect a new diagnosis of COPD. The protocol was approved by the institutional review boards at the University of Washington and the Veterans Affairs (VA) Puget Sound Health Care System.
Medication Adherence
Our primary outcome measure was medication adherence in the six month period after enrollment into the cohort. We measured adherence to three different classes of medication: inhaled corticosteroids (ICS), ipratropium bromide (IP), and long-acting beta-agonists (LABA). We used the ReComp algorithm to estimate medication adherence based on information included in VA computerized pharmacy records. The ReComp measure is similar to a medication possession ratio and it accounts for overstocking and medication gaps; it has been shown to correlate better with physiologic outcomes than previous measures of adherence, and has been used in prior studies.13–15 To account for stock of medications received prior to the period of interest (in this case the six months prior to and following enrollment), ReComp incorporates a run-in period assessing medications dispensed in the six months prior to the period of interest. A ReComp value of 0 indicates that no medication was available to the patient, and a value of 1 indicates that medication was available for all days in the period of interest. ReComp scores greater than 1 were truncated to 1.0. A ReComp score was only calculated for a medication if the patient had been dispensed that medication. Thus, a patient who had received the medication but never refilled it during the period of interest would have a ReComp score of 0, while a patient who was not prescribed a class of medication would not have a ReComp score for that medication. We defined adherence to a medication as having a ReComp score greater than or equal to 0.80, similar to previous studies.5 Outcome adherence reflects adherence in the six month period following the date of enrollment, and baseline adherence represents the six month period prior to enrollment. Our measure of outcome adherence was not a measure of primary adherence, as patients had been prescribed these medications prior to the date of enrollment.
Our primary exposure variable was medication adherence in the six month period before enrollment into the cohort. We categorized medication adherence during the exposure time period into either poor (ReComp score 0 to <0.20), moderate (≥0.20 to <0.80), good (≥0.80 to <1.0), or excellent adherence (1.0).
Covariates
Baseline sociodemographic characteristics were obtained from the VA computerized medical record system. These included age, gender, race, smoking status (defined as current smoker or nonsmoker based on VA health factors), and number of missed clinic visits in the twelve months prior to study enrollment.
Comorbid illnesses were assessed using ICD-9 diagnoses included in the patient’s VA electronic medical record in the twelve months prior to their study enrollment date. We also collected information about additional medication classes that had been dispensed through VA pharmacies in the twelve months prior to enrollment.
Several variables were included as markers of COPD disease severity. We collected data on pulmonary function testing by utilizing PFT data included in the VA electronic medical record. We obtained information regarding the frequency of both inpatient and outpatient COPD exacerbations within the year prior to enrollment; an inpatient exacerbation was defined as having a primary inpatient diagnosis of COPD (ICD-9 codes 491.%, 492.%, 493.2 %, or 496.%), while an outpatient exacerbation was defined as having an outpatient diagnosis of COPD, accompanied by a prescription of either prednisone or an antibiotic within two days of the visit. Frequent exacerbators were patients with both an FEV1 <30 percent-predicted and ≥2 COPD exacerbations (inpatient or outpatient) within the past year. We defined patients as having a baseline diagnosis of COPD if they had either an inpatient or outpatient diagnosis of COPD in the year before enrollment.
Data Analysis
Bivariate analyses were performed using appropriate parametric or non-parametric tests. Logistic regression modeling was conducted to assess independent predictors of medication adherence. To be included in the analyses, a subject was required to have a ReComp score for the medication of interest during both the outcome and the baseline periods. Our primary exposure was medication adherence in the six months prior to study enrollment. To assess the effect of potential confounding factors and identify important predictors of adherence, we added covariates in blocks to the models with the primary exposure variable (medication adherence in the six months prior to enrollment), starting with sociodemographic characteristics, markers of COPD severity, health behaviors, and comorbid illnesses. Our final models included the following covariates: age, gender, race, number of missed clinic visits, FEV1 measured as percent-predicted, occurrence of ≥1 outpatient COPD exacerbations, asthma, lung cancer, and total number of medication classes. A two sided p-value of <0.05 was used to determine statistical significance. Analyses were performed using STATA version 11 and SAS version 9.2.
RESULTS
We identified 2,730 subjects who met our enrollment criteria. As demonstrated in Table 1, which shows baseline patient characteristics during the period before enrollment, members of the cohort were predominantly older (mean age 66.1 ± 10.8 years), white (74.9 %) and male (97.1 %) Veterans. By GOLD criteria, 60.7 % had severe to very severe COPD (the mean FEV1 was 43.4 ± 20.8 percent-predicted). Prior to enrollment, 83.1 % carried a diagnosis of COPD . Ipratropium (IP) was the most commonly dispensed medication, followed by ICS, and then LABA. Baseline adherence to medication was generally poor, with only 25.6 % adherent to IP, 19.8 % adherent to ICS and 30.6 % adherent to LABA.
Table 1.
Patient Characteristics During the Baseline Period
| Total N = 2730 | ICS N = 1541 | LABA N = 966 | IP N = 2155 | |
|---|---|---|---|---|
| Demographic/Behavioral | ||||
| Age in years (m, sd) | 66.1 (10.8) | 65.8 (11.3) | 66.0 (10.9) | 66.4 (10.5) |
| Male (%) | 97.1 | 97.1 | 97.1 | 97.4 |
| Race (%) | ||||
| White | 74.9 | 74.7 | 74.7 | 75.4 |
| Other | 5.3 | 6.7 | 6.9 | 4.6 |
| Unknown | 19.8 | 18.6 | 18.4 | 20.1 |
| Smoker within past year (%) | 41.1 | 35.7 | 35.3 | 43.9 |
| Missed clinic visits (m, sd) | 0.5 (1.0) | 0.5 (1.1) | 0.6 (1.1) | 0.5 (1.0) |
| COPD Severity | ||||
| FEV1 in percent-predicted (m, sd) | 43.4 (20.8) | 43.6 (19.9) | 41.2 (19.1) | 41.5 (20.7) |
| FEV1 in liters (m, sd) | 1.7 (0.7) | 1.7 (0.7) | 1.6 (0.7) | 1.7 (0.7) |
| ≥1 inpatient COPD exacerbation (%) | 4.7 | 5.7 | 5.8 | 5.2 |
| ≥1 outpatient COPD exacerbation (%) | 23.0 | 26.7 | 29.6 | 25.1 |
| Frequent exacerbators (%) | 4.3 | 5.6 | 6.6 | 4.7 |
| % with clinical COPD diagnosis | 83.1 | 82.1 | 85.2 | 86.9 |
| Comorbidities (%) | ||||
| Asthma | 23.8 | 33.7 | 32.9 | 17.5 |
| Acute Coronary Syndrome | 2.5 | 2.4 | 2.6 | 2.3 |
| Congestive Heart Failure | 12.6 | 12.5 | 13.3 | 13.0 |
| Depression | 20.6 | 19.6 | 18.3 | 20.6 |
| Diabetes | 17.9 | 19.0 | 20.3 | 16.8 |
| Edema | 6.2 | 6.2 | 5.6 | 6.4 |
| Hypertension | 55.8 | 54.5 | 53.8 | 56.5 |
| Lung Cancer | 3.1 | 2.7 | 2.8 | 3.3 |
| Neuromuscular disease | 0.3 | 0.3 | 0.2 | 0.3 |
| Sleep Apnea | 5.9 | 6.2 | 6.4 | 5.6 |
| Schizophrenia | 2.2 | 2.3 | 1.9 | 2.3 |
| Medication classes (m, sd) | 11.9 (6.4) | 12.6 (6.5) | 12.7 (6.5) | 12.2 (6.5) |
| Medication adherence | ||||
| % adherent to ICS | – | 19.8 | – | – |
| % adherent to LABA | – | – | 30.6 | – |
| % adherent to IP | – | – | – | 25.6 |
*m = mean; sd = standard deviation
In bivariate analyses, as seen in Table 2, there was significant heterogeneity in the baseline patient characteristics that were associated with our outcome, adherence to the three classes of medication in the six months following enrollment. Gender, race, and smoking status were not associated with medication adherence. Overall, medication adherence was not generally associated with the presence of particular comorbid conditions, nor with the number of total drug classes that patients had received. Older age and fewer missed clinic visits were associated with LABA adherence only. A previous diagnosis of lung cancer or asthma was associated with decreased adherence to LABA, while a diagnosis of acute coronary syndrome or depression was associated with decreased adherence to IP.
Table 2.
Associations Between Outcome Adherence and Baseline Patient Characteristics
| Outcome ICS Adherence | Outcome LABA Adherence | Outcome IP Adherence | ||||
|---|---|---|---|---|---|---|
| Adherent N = 525 | Nonadherent N = 1016 | Adherent N = 487 | Nonadherent N = 479 | Adherent N = 863 | Nonadherent N = 1292 | |
| Demographic/Behavioral | ||||||
| Age in years (m, sd) | 66.1 (11.2) | 65.7 (11.3) | 67.3 (10.6)* | 64.7 (11.0)* | 66.2 (10.3) | 66.6 (10.6) |
| Male (%) | 97.7 | 96.8 | 97.7 | 96.5 | 97.8 | 97.1 |
| Race (%) | ||||||
| White | 71.8 | 74.8 | 75.8 | 73.5 | 75.7 | 75.2 |
| Other | 7.2 | 6.4 | 5.8 | 8.1 | 4.2 | 4.8 |
| Unknown | 21.0 | 18.8 | 18.5 | 18.4 | 20.2 | 20.1 |
| Smoker within past year (%) | 34.7 | 36.2 | 34.9 | 35.7 | 45.1 | 43.1 |
| Missed clinic visits (m, sd) | 0.5 (1.1) | 0.6 (1.1) | 0.5 (1.0)* | 0.8 (1.3)* | 0.6 (1.1) | 0.5 (1.0) |
| COPD severity | ||||||
| FEV1 in percent-predicted (m, sd) | 42.3 (19.2) | 44.2 (20.3) | 39.2 (18.4)* | 43.3 (19.8)* | 37.7 (20.0)* | 44.0 (20.8)* |
| FEV1 in liters (m, sd) | 1.6 (0.7)* | 1.7 (0.7)* | 1.5 (0.6)* | 1.7 (0.7)* | 1.6 (0.7)* | 1.8 (0.7)* |
| ≥1 inpt. COPD exac. (%) | 5.7 | 5.6 | 6.0 | 5.6 | 5.7 | 4.8 |
| ≥1 outpt. COPD exac. (%) | 26.3 | 26.9 | 30.0 | 29.2 | 29.6* | 22.1* |
| Frequent exacerbators (%) | 4.6 | 6.1 | 7.4 | 5.9 | 6.2* | 3.8* |
| % clinical COPD diagnosis | 85.0* | 80.6* | 87.9* | 82.5* | 91.5* | 83.8* |
| Comorbidities (%) | ||||||
| Asthma | 35.6 | 32.8 | 29.6* | 36.3* | 16.8 | 17.9 |
| Acute Coronary Syndrome | 2.7 | 2.3 | 2.7 | 2.5 | 1.4* | 2.9* |
| Congestive Heart Failure | 11.4 | 13.1 | 13.4 | 13.2 | 11.7 | 13.9 |
| Depression | 18.5 | 20.2 | 16.2 | 20.5 | 17.8* | 22.4* |
| Diabetes | 16.4 | 20.3 | 18.9 | 21.7 | 16.1 | 17.3 |
| Edema | 5.9 | 6.3 | 5.3 | 5.9 | 5.8 | 6.7 |
| Hypertension | 52.4 | 55.6 | 53.8 | 53.9 | 56.3 | 56.6 |
| Lung Cancer | 1.7 | 3.3 | 1.6* | 4.0* | 2.8 | 3.6 |
| Neuromuscular Disease | 0.0 | 0.5 | 0.0 | 0.4 | 0.4 | 0.2 |
| Sleep Apnea | 6.3 | 6.2 | 6.4 | 6.5 | 4.9 | 6.1 |
| Schizophrenia | 1.7 | 2.6 | 1.4 | 2.3 | 2.2 | 2.4 |
| Medication Classes (m, sd) | 12.2 (6.4) | 12.7 (6.5) | 12.4 (6.3) | 13.0 (6.8) | 12.0 (6.4) | 12.2 (6.6) |
| Medication Adherence | ||||||
| % adherent to ICS at baseline | 34.9* (N = 525) | 12.0* (N = 1016) | 33.8* (N = 367) | 17.3* (N = 341) | 25.7* (N = 470) | 17.8* (N = 585) |
| % adherent to LABA at baseline | 41.0* (N = 300) | 26.5* (N = 408) | 45.6* (N = 487) | 15.5* (N = 479) | 37.8* (N = 299) | 30.2* (N = 358) |
| % adherent to IP at baseline | 40.0* (N = 365) | 26.7* (N = 690) | 38.7* (N = 346) | 29.9* (N = 311) | 44.5* (N = 863) | 12.9* (N = 1292) |
*indicates significant association with p < 0.05
Examining the relationship between adherence and markers of COPD severity, higher adherence to LABA and IP was associated with greater severity of airflow obstruction, as defined by absolute and percent-predicted FEV1; whereas higher adherence to ICS was associated with severity of airflow obstruction only when it was defined in absolute FEV1. Medication adherence was not associated with previous inpatient COPD exacerbations. Higher adherence to IP was associated with prior treatment for a COPD exacerbation in the outpatient setting and with the group of frequent exacerbators. Having a baseline clinical diagnosis of COPD was associated with improved adherence to all classes of medications.
Likewise, in bivariate analyses, adherence to ICS, LABA, and IP during the baseline period was associated with subsequent adherence to all classes of inhaled medications. The magnitude of the effect was strongest, however, for adherence to medications within the same class.
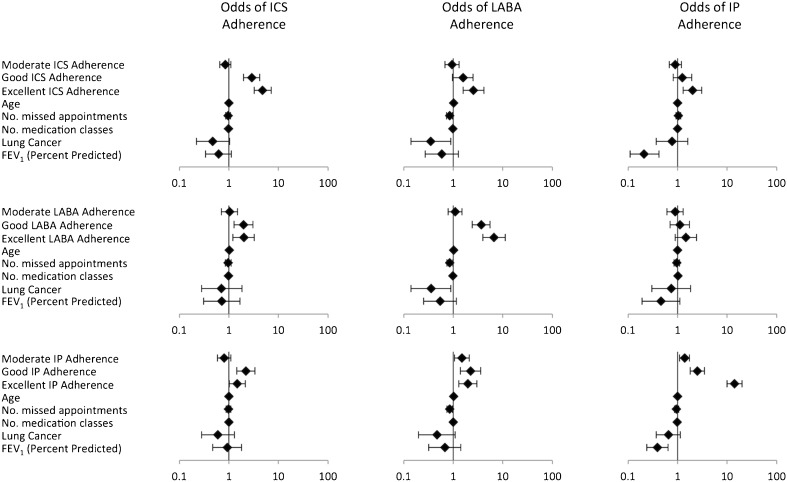
As seen in Figure 1 and Table 3, after adjustment for potentially confounding factors, baseline adherence to an alternate class of inhaled medication was not consistently associated, or only minimally associated, with future adherence to different classes of medication. However, baseline adherence to one class of medication (either ICS, IP, or LABA) was strongly associated with subsequent adherence to the same class of medication. For example, among those with excellent baseline ICS adherence, the odds for future adherence to ICS was 4.79 (95 % CI 3.22–7.12); among those with excellent baseline LABA adherence, the odds for future adherence to LABA was 6.60 (3.92–11.11); and among those with excellent baseline IP adherence, the odds for future adherence to IP was 14.13 (10.00–19.97).
Figure 1.
Odds of medication adherence. Error bars reflect 95 % confidence interval. Reference group for each model is poor baseline adherence. All models were also adjusted for gender, race, occurrence of ≥1 outpatient COPD exacerbations, and asthma, none of which was a significant predictor in any model.
Table 3.
Odds of Medication Adherence Based on Baseline Adherence to Different Classes of Medication
| Baseline Adherence (categorized by ReComp score) | Odds of ICS Adherence (95 % CI) | Odds of LABA Adherence (95 % CI) | Odds of IP Adherence (95 % CI) | |
|---|---|---|---|---|
| Inhaled corticosteroid | Poor (0 to <0.20) | 1.00 | 1.00 | 1.00 |
| Moderate (≥0.20 to <0.80) | 0.85 (0.66–1.10) | 0.98 (0.67–1.41) | 0.88 (0.66–1.18) | |
| Good (≥0.80 to <1.0) | 2.87 (1.96–4.20) | 1.87 (1.09–3.23) | 1.21 (0.77–1.90) | |
| Excellent (1.0) | 4.79 (3.22–7.12) | 3.30 (1.89–5.74) | 1.88 (1.21–2.92) | |
| Long-acting beta-agonist | Poor | 1.00 | 1.00 | 1.00 |
| Moderate | 0.88 (0.60–1.29) | 1.08 (0.79–1.49) | 0.83 (0.56–1.22) | |
| Good | 1.84 (1.17–2.90) | 3.61 (2.39–5.47) | 1.06 (0.67–1.68) | |
| Excellent | 1.71 (1.03–2.84) | 6.60 (3.92–11.11) | 1.43 (0.86–2.38) | |
| Ipratropium bromide | Poor | 1.00 | 1.00 | 1.00 |
| Moderate | 0.85 (0.61–1.19) | 1.49 (0.98–2.27) | 1.38 (1.10–1.73) | |
| Good | 2.30 (1.46–3.61) | 2.24 (1.27–3.94) | 2.52 (1.81–3.50) | |
| Excellent | 1.41 (0.94–2.10) | 1.82 (1.11–3.00) | 14.13 (10.00–19.97) | |
*Reference group for each model is poor baseline adherence (ReComp score <0.20)
†All models were adjusted for age, gender, race, missed clinic visits, FEV1, occurrence of ≥1 outpatient COPD exacerbations, asthma, lung cancer, and number of medication classes
Examining the covariates in each of the individual models demonstrated in Figure 1, we found that none of the additional variables were significantly associated with adherence to ICS. In models predicting adherence to LABA, older age was associated with better adherence to LABA, while missed appointments and a previous diagnosis of lung cancer were associated with poorer adherence to LABA. In models predicting adherence to IP, milder severity of airflow limitation and the total number of drug classes prescribed were both associated with decreased IP adherence. Compared to the strong association between past adherence to a medication class and future adherence to the same medication, the magnitude of effect for all other statistically significant associations was relatively small.
DISCUSSION
We found that adherence to ICS, LABA, and IP was low among those prescribed medication for COPD, and that the strongest predictor of future medication adherence was past adherence to medications within the same drug class. Furthermore, adherence to inhaled medications from different classes and other predictors, including COPD severity, health behaviors, and burden of comorbid illnesses, were not strong predictors of adherence. These results have important implications in the delivery of care to patients with COPD. Inhaled medications represent the cornerstone of therapy for the majority of patients with COPD. Significant emphasis has been recently placed on enhancing medication adherence, because of results from efficacy trials that demonstrate a clear reduction in both symptoms and future COPD exacerbations.1,2 Our results suggest that interventions that are designed to improve medication adherence among patients with COPD will need to focus not only on the general importance of taking these medications, but that these interventions will likely need to be tailored to each individual class of medication. Furthermore, our results suggest that even if a patient is highly adherent to one of their medications, clinicians will still need to inquire about their adherence to other inhaled medications individually.
Why patients may be adherent to one medication and nonadherent to another is not known. We suspect that the higher adherence to IP among patients with more severe airflow obstruction, but not to LABA and ICS, may be related to the perceived immediate benefit of IP on symptom relief. In addition, although patients are typically instructed to use IP at regular intervals, patients may instead be using IP as a rescue medication whereas clear instructions are typically given that neither ICS nor LABA should be used in this manner. We would hypothesize that, because ICS have no immediate effect on symptoms, individuals adherent to ICS may have overall better health behaviors or have health beliefs about the benefits of inhaled medications. This may explain the stronger association between excellent baseline adherence to ICS and subsequent adherence to LABA and IP, compared to the weaker association between baseline adherence to LABA and IP and adherence to other medications.
There are additional potential explanations for the difference in adherence between classes of inhaled medications. Patients may respond better to one class of medication than to the other classes, and for that reason, preferentially use the medication class that provides superior relief. Furthermore, inhalers employ different mechanisms of medication release (metered-dose inhalers, dry powder inhalers, and nebulizers). A patient who does not understand how to use one type of inhaler would likely get little benefit from it and may therefore stop using it. Each medication class has a unique side effect profile; for instance, inhaled corticosteroids have been reported to cause oropharyngeal candidiasis, hoarseness, and dysphonia.16 It is reasonable to expect that a patient who experiences such a side effect to ICS may preferentially adhere to their other inhalers. Inconvenience, in the form of frequent or complex medication dosing regimens, has been identified as an important factor contributing to nonadherence.17 Finally, there is the possibility that patients limit their medication use because of costs, although we would anticipate this effect being attenuated within the VA system where medications are provided free or at low cost.
Our results are consistent with previous studies that documented low adherence rates to inhaled medications among COPD patients.7–9,11,18–23 These studies have produced inconsistent conclusions about the role of sociodemographic factors on adherence to COPD medications.7–9 We found that sociodemographic and behavioral characteristics, markers of COPD severity, and comorbidities were generally poor predictors of medication adherence.
Our study has several strengths. It is the first study to assess the relationship between adherence to different classes of COPD medications simultaneously. This allows us to examine patterns of different medication use within the same individual. Second, our study only enrolled Veterans in our centers who had documented spirometric evidence of COPD, which allowed us to confirm the presence of airflow limitation and to minimize biases that could arise by including patients without confirmed airflow limitation. Finally, VA drug acquisition costs are typically free or require only a small copayment, mitigating some of the effects of access to drugs because of cost.
The study has several limitations. First, because the cohort was derived from a VA population, these results may not generalize to non-integrated systems of care or to women. Second, we chose 80 % as a threshold of adherence based on the widespread use of this cut-point in previous studies, including in the COPD literature.5,24–26 However, 80 % adherence has not been shown to be a threshold where COPD medications are truly efficacious. Finally, the information available for our analyses was limited to variables included in our databases. The impact of other variables that may be associated with adherence, such as patient distance from the VA or the specialty of the medication prescriber, could not be assessed in this study.
There are multiple mechanisms for estimating adherence, including patient self-report, pharmacy refill measurement, and electronic drug monitoring; each of these measures presents advantages and disadvantages.27 Our use of pharmacy refill records may be viewed as both a strength and a limitation. This method allowed us to obtain population level data and to avoid recall and social desirability biases. Electronic drug-monitoring has been suggested to be a more accurate measurement of adherence, especially in comparison to patient self-report.28 However, when compared in the same patient population, pharmacy refill records and electronic drug monitoring yield comparable estimates of adherence.29 Moreover, electronic monitoring of patients' medication use in a study such as ours would have been prohibitively expensive. A potential limitation of the pharmacy refill method is that it measures medication acquisition, but not medication use. It is likely reasonable to assume that patients who regularly refill medications over time are using them. An ideal measure of inhaler adherence would take into account not only inhaler use but correct technique of inhaler use (referred to as competence); inhaler devices have been designed which electronically measure both adherence and competence.30 However, such methods of measurement are cumbersome and expensive and thus not feasible in a population level study such as ours.
In conclusion, our results demonstrate that in a large cohort of patients with COPD, adherence to inhaled medication was poor and that the strongest predictor of adherence was being previously adherent to the same medication class. Our finding that adherence to inhaled medications is not generalizable across medication classes, but is instead class-dependent, is relevant to the clinician prescribing these medications. The application of this finding does not require measures of medication adherence by the clinician. A clinician’s confidence about a patient’s adherence to one class of medication should not be used as a proxy for adherence to other medications; rather, our results suggest that clinicians should inquire about adherence to each inhaled therapy for COPD separately. Furthermore, when initiating a patient on a new class of COPD medication, clinicians should be aware that past adherence to other classes of medication cannot be treated as a predictor of adherence to the new medication. Additionally, interventions that are designed to improve adherence to inhaled medication may need to be designed to incorporate variation in adherence between medications. Further study is needed to better understand the individualized reasons underlying this variance.
Acknowledgments
The authors thank all the research volunteers who participated in the study.
This project was funded by the American Lung Association, grant # CI-51755N. Dr. Au was funded by HSR&D, VA Puget Sound Health Care System. The views expressed in this manuscript are those of the authors and do not necessarily represent the opinions of the Department of Veterans Affairs.
Prior Presentations
None.
Conflict of Interest
Dr. Huetsch, Mr. Udris, and Ms. Uman report no conflicts of interest. Dr. Au is a research advisor for Bosch. He also receives grants from NHLBI, AHRQ, the Department of Veterans Affairs, and Gilead Sciences.
REFERENCES
- 1.Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 2.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–54. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 3.Mahler DA, Donohue JF, Barbee RA, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115(4):957–65. doi: 10.1378/chest.115.4.957. [DOI] [PubMed] [Google Scholar]
- 4.Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361(9356):449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 5.Vestbo J, Anderson JA, Calverley PM, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64(11):939–43. doi: 10.1136/thx.2009.113662. [DOI] [PubMed] [Google Scholar]
- 6.Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chronic Obstr Pulmon Dis. 2008;3(3):371–84. doi: 10.2147/copd.s3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolce JJ, Crisp C, Manzella B, Richards JM, Hardin JM, Bailey WC. Medication adherence patterns in chronic obstructive pulmonary disease. Chest. 1991;99(4):837–41. doi: 10.1378/chest.99.4.837. [DOI] [PubMed] [Google Scholar]
- 8.Turner J, Wright E, Mendella L, Anthonisen N. Predictors of patient adherence to long-term home nebulizer therapy for COPD. Chest. 1995;108(2):394–400. doi: 10.1378/chest.108.2.394. [DOI] [PubMed] [Google Scholar]
- 9.Corden ZM, Bosley CM, Rees PJ, Cochrane GM. Home nebulized therapy for patients with COPD: patient compliance with treatment and its relation to quality of life. Chest. 1997;112(5):1278–82. doi: 10.1378/chest.112.5.1278. [DOI] [PubMed] [Google Scholar]
- 10.Earnest MA. Explaining adherence to supplemental oxygen therapy. J Gen Intern Med. 2002;17(10):749–55. doi: 10.1046/j.1525-1497.2002.20218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George J, Kong DCM, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128(5):3198–204. doi: 10.1378/chest.128.5.3198. [DOI] [PubMed] [Google Scholar]
- 12.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. Available at: http://www.goldcopd.org. Accessed April 30, 2012.
- 13.Bryson CL, Au DH, Young B, McDonell MB, Fihn SD. A refill adherence algorithm for multiple short intervals to estimate refill compliance (ReComp) Med Care. 2007;45(6):497–504. doi: 10.1097/MLR.0b013e3180329368. [DOI] [PubMed] [Google Scholar]
- 14.Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Ann Intern Med. 2008;149(11):795–803. doi: 10.7326/0003-4819-149-11-200812020-00004. [DOI] [PubMed] [Google Scholar]
- 15.Thorpe CT, Bryson CL, Maciejewski ML, Bosworth HB. Medication acquisition and self-reported adherence in veterans with hypertension. Med Care. 2009;47(4):474–81. doi: 10.1097/MLR.0b013e31818e7d4d. [DOI] [PubMed] [Google Scholar]
- 16.Yang IA, Fong K, Sim EHA, Black PN, Lasserson TJ. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;CD002991. [DOI] [PubMed]
- 17.Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1(3):189–99. [PMC free article] [PubMed] [Google Scholar]
- 18.Krigsman K, Moen J, Nilsson LG, Ring L. Refill adherence by the elderly for asthma/chronic obstructive pulmonary disease drugs dispensed over a 10-year period. J Clin Pharm Ther. 2007;32(6):603–11. doi: 10.1111/j.1365-2710.2007.00866.x. [DOI] [PubMed] [Google Scholar]
- 19.Krigsman K, Nilsson JL, Ring L. Adherence to multiple drug therapies: refill adherence to concomitant use of diabetes and asthma/COPD medication. Pharmacoepidemiol Drug Saf. 2007;16(10):1120–8. doi: 10.1002/pds.1433. [DOI] [PubMed] [Google Scholar]
- 20.Jung E, Pickard AS, Salmon JW, Bartle B, Lee TA. Medication adherence and persistence in the last year of life in COPD patients. Respir Med. 2009;103(4):525–34. doi: 10.1016/j.rmed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Dompeling E, Grunsven PM, Schayck CP, Folgering H, Molema J, Weel C. Treatment with inhaled steroids in asthma and chronic bronchitis: long-term compliance and inhaler technique. Fam Pract. 1992;9(2):161–6. doi: 10.1093/fampra/9.2.161. [DOI] [PubMed] [Google Scholar]
- 22.Haupt D, Krigsman K, Nilsson JLG. Medication persistence among patients with asthma/COPD drugs. Pharm World Sci. 2008;30(5):509–14. doi: 10.1007/s11096-008-9197-4. [DOI] [PubMed] [Google Scholar]
- 23.Mehuys E, Boussery K, Adriaens E, et al. COPD management in primary care: an observational, community pharmacy-based study. Ann Pharmacother. 2010;44(2):257–66. doi: 10.1345/aph.1M481. [DOI] [PubMed] [Google Scholar]
- 24.Andrade SE, Kahler KH, Frech F, Chan KAC. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–74. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 25.Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25(9):2303–10. doi: 10.1185/03007990903126833. [DOI] [PubMed] [Google Scholar]
- 26.Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43(3):413–22. doi: 10.1345/aph.1L496. [DOI] [PubMed] [Google Scholar]
- 27.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S79–87. doi: 10.1097/01.qai.0000248337.97814.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniels T, Goodacre L, Sutton C, Pollard K, Conway S, Peckham D. Accurate assessment of adherence: self-report and clinician report versus electronic monitoring of nebulizers. Chest. 2011;140(2):425–32. doi: 10.1378/chest.09-3074. [DOI] [PubMed] [Google Scholar]
- 29.Modi AC, Lim CS, Yu N, Geller D, Wagner MH, Quittner AL. A multi-method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros. 2006;5(3):177–85. doi: 10.1016/j.jcf.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Nikander K, Turpeinen M, Pelkonen AS, Bengtsson T, Selroos O, Haahtela T. True adherence with the Turbuhaler in young children with asthma. Arch Dis Child. 2001;96(2):168–73. doi: 10.1136/adc.2010.187724. [DOI] [PubMed] [Google Scholar]