Abstract
Background
Despite uncertainty about their effectiveness in chronic dialysis patients, statin use has increased in recent years. Little is known about the demographic, clinical, and geographic factors associated with statin exposure in end-stage renal disease (ESRD) patients.
Objective
To analyze the demographic, clinical, and geographic factors associated with use of statins among chronic dialysis patients.
Design
Cross-sectional analysis.
Setting
Prevalent dialysis patients across the U.S.
Participants
55,573 chronic dialysis patients who were dually eligible for Medicaid and Medicare services during the last four months of 2005.
Methods
Using Medicaid prescription drug claims and United States Renal Data System core data, we examined demographics, comorbid conditions, and state of residence using hierarchical logistic regression models to determine their associations with statin use.
Intervention
Prescription for a statin.
Outcome Measures
Factors associated with a prescription for a statin.
Results
Statin exposure was significantly associated with older age, female sex, Caucasian (versus African-American) race, body mass index, use of self-care dialysis, diabetes, and comorbidity burden. Moreover, there was substantial state-by-state variation in statin use, with a greater than 2.3-fold difference in adjusted odds ratios between the highest- and lowest-prescribing states.
Conclusions
Among publicly insured chronic dialysis patients, there were marked differences between states in the use of HMG-CoA reductase inhibitors above and beyond patient characteristics. This suggests substantial clinical uncertainty about the utility of these medications. Understanding how such regional variations impact patient care in this high-risk population is an important focus for future work.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-012-2112-7) contains supplementary material, which is available to authorized users.
KEY WORDS: HMG-CoA reductase inhibitors, statins, dialysis, end stage renal disease, Medicare, insurance
INTRODUCTION
Substantial geographic variation exists across the U.S. in many domains of healthcare delivery. For example, there are regional differences in Medicare spending,1,2 quality of drug prescribing,3 quality of clinical management during hospitalization,4 use of surgical procedures,5 and adherence to medication guidelines for use of drugs like HMG-CoA reductase inhibitors.6 Geographic variation in care is also marked in the kidney disease population, in whom substantial geographic variation in care has been demonstrated for pre-dialysis access to a nephrologist,7 vascular access creation,7 and selection of peritoneal dialysis as a modality of renal replacement therapy.8,9 We have recently demonstrated that substantial regional variation also exists in use of cardioprotective antihypertensive medications in the chronic dialysis population, even after adjustment for other factors.10
A medication class of great interest to both nephrologists and the general medical community is the HMG-CoA reductase inhibitors, or “statins”. The role of these drugs in the prevention of cardiovascular events in end stage renal disease (ESRD) patients on dialysis is uncertain. Despite the completion of three randomized clinical trials (RCTs) in dialysis patients,11–13 the efficacy of statins in reducing mortality or cardiovascular events has not been definitively established. This was unexpected, given both earlier observational studies that had suggested a survival benefit of statins in this population14,15 as well as a strong underlying clinical rationale to treat these patients, given their high rates of cardiovascular disease (CVD).16
Despite this uncertainty, several studies have, nevertheless, reported a dramatic rise in statin use in dialysis patients since the late 1990s17,18 (a trend mirroring that of the general population19), suggesting the presence of a belief among practitioners that the benefits observed in non-dialysis patients may extend to dialysis patients. However, the factors associated with statin use in dialysis patients have never been rigorously investigated, prompting the present study. We were especially interested in the role that geography might play in differential rates of medication prescribing. The existence of substantial geographically-based variation in the delivery of health care, after accounting for identifiable clinical factors, would suggest the existence of clinical uncertainty over medication use, warranting further investigation.20 In addition, because dually eligible persons rely upon the two largest medical public assistance programs, understanding practice patterns in these patients has important policy implications for vulnerable patients’ access to medications. Therefore, we used a linked national data source, encompassing dually eligible (Medicare & Medicaid) dialysis patients, to examine geographic patterns of exposure to statins in a period prior to the reporting of the results from recent RCTs.
PATIENTS AND METHODS
Data Sources for Analysis
We conducted a cross-sectional analysis of statin prescription drug claims for prevalent, dually eligible (Medicare–Medicaid) chronic dialysis patients during a four-month period, September through December 2005.10 Chronic dialysis patients were defined, in standard fashion as per the United States Renal Data System (USRDS), as those individuals dialyzing for at least 90 days and who therefore had reached dialysis-requiring end stage renal disease with virtually no prospects of spontaneous renal recovery.
Data were obtained from the USRDS and the Centers for Medicare & Medicaid Services (CMS) Medicaid files. From the USRDS, we received standard patient records that include demographics, comorbidities, functional status, dialysis modality, and the time of dialysis commencement. From CMS, we obtained Medicaid Analytic eXtract Personal Summary Files as well as the final action prescription drug claims files. The USRDS performed a deterministic match of these Medicaid beneficiaries against the core USRDS files to identify dually eligible individuals on chronic dialysis. The MAX final action prescription drug claims were used to determine medication exposure.10
Study Cohort and Rationale for Analytic Approach
We identified unique individuals over the age of 18 years who were on chronic dialysis and who were enrolled in Medicaid and Medicare programs simultaneously and continuously during the 4-month period of September through December 2005. We eliminated individuals who initiated dialysis on or after September 1, 2005 or who died or received a kidney transplant on or before December 31, 2005 to ensure a complete window of observation. Patients enrolled in Medicaid managed care plans were excluded since medication data were not available. (Of note, persons on chronic dialysis were not generally eligible for Medicare managed care plans prior to 2006.) We specifically excluded individuals residing in Arizona and Tennessee because all Medicaid patients in these states are enrolled in managed care plans, and in Delaware and Kentucky because there were <25 eligible dialysis patients meeting our eligibility criteria. Finally, we eliminated individuals who did not fill at least one Medicaid prescription during this time.
Descriptive Variables
Demographic and clinical variables were drawn from the CMS 2728 dialysis intake form, completed at the time of dialysis initiation. Demographic variables included age, sex, and race by ethnicity (four mutually exclusive groups comprising non-Hispanic Caucasians, non-Hispanic African-Americans, Hispanics, and Others), and employment. Risk behaviors included smoking and substance abuse (alcohol or illicit drugs), and functional status markers were ability to ambulate and transfer. Cause of ESRD, for which the CMS 2728 form requires a single best answer to be selected, was categorized as diabetes, hypertension, glomerulonephritis, or other. Major clinical comorbidities were diabetes (types I and II combined), hypertension, congestive heart failure, coronary artery disease, cerebrovascular disease, peripheral vascular disease, and cardiac dysrhythmia. Since the CMS 2728 form is structured such that diseases like diabetes or hypertension may be considered as both a cause of ESRD and/or a comorbidity, diabetes and hypertension were considered present in an individual if they were listed as either the cause of ESRD or as a comorbidity.21 The sole laboratory value analyzed was hemoglobin, which was dichotomized at 11 g/dL. Dialysis modality was categorized as in-center hemodialysis or self-care dialysis (home HD or peritoneal dialysis (PD)). We also included, as a summary measure of comorbidity burden, the Liu comorbidity index,22 which includes both cardiovascular and non-cardiovascular (e.g., chronic obstructive pulmonary disease, liver disease, and cancer) conditions, as well as cause of ESRD.
Medication Exposure
We matched drug name and therapeutic class information in the Medicaid drug claims at the national drug code (NDC) level using Multum Lexicon (Cerner Corporation, www.cerner.com). Statins were divided into monotherapy and combination-agent (e.g., a statin plus a calcium channel blocker) groups. We looked across a 4-month period of exposure since some state Medicaid programs allow for 100-day supplies of maintenance medications. Consistent with our cross-sectional approach, and for the purposes of determining the relative prescribing frequencies of the individual drugs, we limited the analysis to any-versus-no prescription for each person.
Statistical Analyses
Person-Level Analyses
We generated descriptive statistics (means and standard deviations for continuous variables and frequencies for categorical variables) to illustrate how statin users differed from non-users. Bivariate analyses comparing each of the explanatory variables by use versus non-use were performed using Pearson’s chi-squared test or Student’s t-test, as appropriate. To identify independent factors associated with statin use, we generated a multi-level logistic regression model using generalized linear mixed modeling (GLMM)23 with medication status being regressed simultaneously on all a priori selected explanatory variables. Cause of ESRD was not included among these a priori selected variables, though we selected the Liu comorbidity burden (a summary measure of overall disease burden) that includes cause of ESRD for this model. Additionally, we explored potential interactions based upon a priori discussions of variables likely to have important clinical implications. The model included a random effect for state. To assess the fit, we generated an unconditional logistic regression model that instead treated state as a fixed effect, and conducted the Hosmer–Lemeshow goodness-of-fit test24 on this fixed effects model. The parameter estimates between the two models indicated similar predicted probabilities, and by extension similar observed versus expected quantities; therefore we used the Hosmer–Lemeshow test result from the fixed effects model as a proxy for the fit of our model with the random effect for state.
Due to the large sample size, statistical significance was inferred only when P < 0.01. All statistical analyses were done with SAS 9.2 (SAS Institute, Inc., www.sas.com).
State-by-State Medication Exposure
In addition to the person-level analysis, we conducted a state-by-state comparison for treatment with statins. For each state, we determined whether the observed proportion of persons treated with a statin, called “observed” (O), was above or below the “expected” value (E) based on our GLMM, which adjusted for individual-level characteristics. We utilized the random coefficients for state from the GLMM to facilitate state-level observed versus expected (O/E) comparisons. Specifically, we derived the estimates of the random coefficients for each state as these parameters modify each state’s log-odds of medication treatment (and hence its proportion treated) from the overall cross-state (fixed) model effects. Taking the anti-log of these estimates generated state-specific observed versus expected (O/E) odds ratios (ORs). Using the estimated standard errors of the predictions, we estimated confidence intervals for these state-specific O/E odds ratios.25
Compliance and Research Participant Protection
The research protocol was approved by the institutional review board at the University of Kansas Medical Center (KUMC), and the project was undertaken according to the principles of the Declarations of Helsinki. Data use agreements (DUA) between KUMC and the USRDS and CMS permitted the data linking across the USRDS, Medicare and Medicaid files.
Results
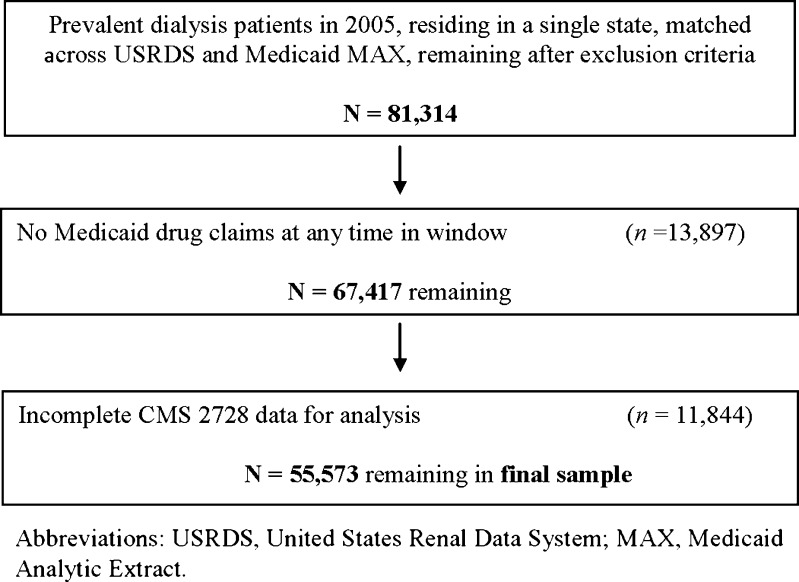
There were 81,314 individuals who met the initial inclusion criteria (Fig. 1). After limiting the cohort to persons who demonstrated active Medicaid use (in the form of filling at least one prescription), 67,417 persons remained. Of those, 55,573 had complete CMS 2728 data and were therefore suitable for analysis. The overall sample is characterized in the first column of Table 1. The mean age of the sample was 60.1 years and included more females (54.4 %) than males and more African-Americans (44.3 %) than Caucasians (29.2 %), Hispanics (18.8 %), or individuals of other races/ethnicities (7.7 %). The vast majority had hypertension (85.5 %) and over half (57.2 %) had diabetes. Nearly 95 % were using in-center HD.
Figure 1.
Construction of the analytic sample.
Table 1.
Descriptive Characteristics of the Total Eligible Cohort and of the Statin Users
| Characteristics | All | Statin users | Statin non-users | P-value* |
|---|---|---|---|---|
| Number of cases | 55,573 (100.0) | 18,979 (34.2) | 36,594 (65.8) | |
| Age, yr | 60.1 ± 15.2 | 63.1 ± 13.1 | 58.5 ± 16.0 | <0.0001 |
| Sex, n (%) | <0.0001 | |||
| Women | 30,259 (54.4) | 11,233 (37.1) | 19,026 (62.9) | |
| Men | 25,314 (45.6) | 7746 (30.6) | 17,568 (69.4) | |
| Race/Ethnicity, n (%) | <0.0001 | |||
| African-American | 24644 (44.3) | 7196 (29.2) | 17,448 (70.8) | |
| Caucasian | 16,200 (29.2) | 6373 (39.3) | 9827 (60.7) | |
| Hispanic | 10,449 (18.8) | 3724 (35.6) | 6725 (64.4) | |
| Other | 4280 (7.7) | 1686 (39.4) | 2594 (60.6) | |
| BMI category, n (%) | <0.0001 | |||
| <20 kg/m2 | 4907 (8.8) | 1186 (24.2) | 3721 (75.8) | |
| 20–24.9 kg/m2 | 15,970 (28.7) | 4892 (30.6) | 11,078 (69.4) | |
| 25–29.9 kg/m2 | 15,030 (27.0) | 5306 (35.3) | 9724 (64.7) | |
| 30+ kg/m2 | 19,666 (35.4) | 7595 (38.6) | 12,071 (61.4) | |
| Smoker, n (%) | <0.0001 | |||
| Yes | 3502 (6.3) | 1035 (29.6) | 2467 (70.4) | |
| No | 52,071 (93.7) | 17,994 (34.5) | 34,127 (65.5) | |
| Substance abuser, n (%) | <0.0001 | |||
| Yes | 1830 (3.3) | 319 (17.4) | 1511 (82.6) | |
| No | 53,743 (96.7) | 18,660 (34.7) | 35,083 (62.3) | |
| Unemployed, n (%) | <0.0001 | |||
| Yes | 3226 (5.8) | 18,125 (34.6) | 34,222 (65.4) | |
| No | 52347 (94.2) | 854 (26.5) | 2372 (73.5) | |
| Unable to ambulate, n (%) | 0.22 | |||
| Yes | 1897 (3.4) | 673 (35.5) | 1224 (64.5) | |
| No | 53,676 (96.6) | 18,306 (34.1) | 35,370 (65.9) | |
| Unable to transfer, n (%) | 0.94 | |||
| Yes | 609 (1.1) | 207 (34.0) | 402 (66.0) | |
| No | 54,964 (98.9) | 18,772 (34.2) | 36,192 (65.9) | |
| Dialysis type, n (%) | <0.0001 | |||
| In-center HD | 52,632 (94.7) | 17,857 (33.9) | 34,775 (66.1) | |
| Self-care | 2941 (5.3) | 1122 (38.2) | 1819 (61.9) | |
| Hemoglobin, n (%) | <0.0001 | |||
| <11.0 g/dL | 42,659 (76.8) | 14,071 (33.0) | 28,588 (67.0) | |
| ≥11.0 g/dL | 12,914 (23.2) | 4908 (38.0) | 8006 (62.0) | |
| Comorbidities | ||||
| HTN, n (%) | <0.0001 | |||
| Yes | 47,492 (85.5) | 16,447 (34.6) | 31,045 (65.4) | |
| No | 8081 (14.5) | 2532 (31.3) | 5549 (68.7) | |
| DM, n (%) | <0.0001 | |||
| Yes | 31,785 (57.2) | 13,552 (42.6) | 18,233 (57.4) | |
| No | 23,788 (42.8) | 5427 (22.8) | 18,361 (77.2) | |
| CHF, n (%) | <0.0001 | |||
| Yes | 15,257 (27.5) | 6051 (39.7) | 9206 (60.3) | |
| No | 40,316 (72.5) | 12,928 (32.1) | 27, 388 (67.9) | |
| CAD, n (%) | <0.0001 | |||
| Yes | 10,640 (19.1) | 5156 (48.5) | 5484 (51.5) | |
| No | 44,933 (80.9) | 13,823 (30.8) | 31,110 (69.2) | |
| PVD, n (%) | <0.0001 | |||
| Yes | 5795 (10.4) | 2492 (43.0) | 3303 (57.0) | |
| No | 49778 (89.6) | 16,487 (33.1) | 33,291 (66.9) | |
| CVA, n (%) | <0.0001 | |||
| Yes | 4429 (8.0) | 1901 (42.9) | 2528 (57.1) | |
| No | 51,144 (92.0) | 17,078 (33.4) | 34,066 (66.6) | |
| Arrhythmia, n (%) | <0.0001 | |||
| Yes | 1880 (3.4) | 746 (39.7) | 1134 (60.3) | |
| No | 53,693 (96.6) | 18,233 (34.0) | 35, 460 (66.0) | |
| Liu Comorbidity Score | 6.7 ± 4.0 | 7.5 ± 3.9 | 6.3 ± 4.0 | <0.0001 |
| Cause of ESRD, n (%) | <0.0001 | |||
| Diabetes | 26,835 (48.3) | 11,651 (43.4) | 15,184 (56.6) | |
| Hypertension | 15,302 (27.5) | 4180 (27.3) | 11,122 (72.7) | |
| Glomerulonephritis | 5862 (10.5) | 1284 (21.9) | 4578 (78.1) | |
| Other | 7574 (13.6) | 1864 (24.6) | 5710 (75.4) | |
yr years; BMI body mass index; HD hemodialysis; HTN hypertension; DM diabetes; CHF congestive heart failure; CAD coronary artery disease; PVD peripheral vascular disease; CVA cerebrovascular accident; ESRD end stage renal disease
*P-value is for the comparison between statin users and non-users
Patient Characteristics Associated with Use of Statins
A total of 18,979 (34.2 %) of the individuals received a statin. Table 1 shows, by row, the proportion of persons within a specific group who were and were not statin-treated, and provides the results of bivariate analyses. For example, 37.1 % of women, versus 30.6 % of men, were treated with statins (P < 0.0001).
In addition to sex, use differed significantly in the bivariate analysis by race, with African-Americans having the lowest use rates of any group. Use increased as BMI category increased. Use was significantly higher in non-smokers and in persons who did not abuse substances, but functional status was not associated with statin use. Persons on self-care dialysis had higher use of statins, and use was positively associated with each comorbidity. Cause of ESRD was associated with statin use, with persons having ESRD due to diabetes having higher absolute use rate than other individuals.
Prescribed Agents
Atorvastatin, at 49.1 %, and simvastatin, at 32.7 %, were the most commonly-prescribed agents (Table 2). Statins in the form of combination agents represented only 4.3 % of the total, with the most commonly-prescribed combination agent being simvastatin-ezetimibe (3.0 %).
Table 2.
Distribution of the Specific Statin Agents and their Classes Used by Dually Eligible Dialysis Patients
| Class | Specific Agent | Percent of total |
|---|---|---|
| Monotherapy | Atorvastatin | 49.1 |
| Simvastatin | 32.7 | |
| Pravastatin | 6.2 | |
| Lovastatin | 4.1 | |
| Rosuvastatin | 2.1 | |
| Fluvastatin | 1.5 | |
| Combination Therapy | Simvastatin-ezetimibe | 3.0 |
| Atorvastatin-amlodipine | 1.0 | |
| Lovastatin-niacin | 0.2 | |
| Pravastatin-aspirin | 0.1 |
Patient Characteristics Associated with Statin Use
Using multivariable analysis (Table 3), we estimated the adjusted odds ratios (AORs) for factors associated with statin use. Since we observed an interaction between diabetes and age, diabetics and non-diabetics were considered separately with respect to age. Among non-diabetics, use was significantly higher in the 50–80 and ≥80 year-old age groups, compared to individuals <50 years old. In contrast, statin use among diabetics ≥80 years of age was not different from the rate of use in the in the youngest group. Statin use was higher among persons in higher BMI categories and among persons on self-care dialysis. It was negatively associated with male gender, African-American race, substance abuse, inability to ambulate, and anemia at baseline. Concerning comorbidities, hypertension, coronary artery disease, and a history of a CVA were all associated with statin use. Specific examination of diabetes (not shown in the table) demonstrated that diabetes was consistently associated with statin use across age groups: in diabetic individuals (compared to non-diabetics) aged <50 years, the AOR for use was 2.82 (99 % CIs, 2.52–3.16); in diabetic individuals aged 50–80 years, it was 1.78 (1.66–1.90); and in diabetics >80, it was 1.55 (1.32–1.82). Overall, individuals with greater comorbidity burdens, as calculated with the Liu comorbidity score, had significantly more statin use.
Table 3.
Factors Associated with Use of Statins
| AOR | 99 % CI’s | |
|---|---|---|
| Age | ||
| Among non-diabetics* | ||
| Age <50 yr | 1.00 | − |
| Age 50–80 yr | 2.18 | 1.98–2.40 |
| Age ≥80 yr | 1.89 | 1.63–2.19 |
| Among diabetics* | ||
| Age <50 yr | 1.00 | − |
| Age 50–80 yr | 1.37 | 1.26–1.50 |
| Age ≥80 yr | 1.04 | 0.91–1.19 |
| Male sex | 0.83 | 0.79–0.87 |
| Race/Ethnicity | ||
| Caucasian | 1.00 | – |
| African-American | 0.77 | 0.72–0.82 |
| Hispanic | 0.93 | 0.86–1.01 |
| Other | 1.13 | 1.02–1.25 |
| BMI category | ||
| <20 kg/m2 | 0.80 | 0.72–0.89 |
| 20–24.9 kg/m2 | 1.00 | − |
| 25–29.9 kg/m2 | 1.13 | 1.06–1.21 |
| 30+ kg/m2 | 1.24 | 1.16–1.32 |
| Smoker | 1.00 | 0.90–1.12 |
| Substance abuser | 0.66 | 0.55–0.78 |
| Employed | 0.92 | 0.82–1.03 |
| Inability to ambulate | 0.81 | 0.69–0.94 |
| Inability to transfer | 0.90 | 0.69–1.17 |
| Use of self-care dialysis | 1.30 | 1.17–1.45 |
| Hemoglobin <11.0 | 0.84 | 0.79–0.89 |
| Comorbidities | ||
| HTN | 1.12 | 1.04–1.20 |
| CHF | 0.95 | 0.90–1.01 |
| CAD | 1.59 | 1.49–1.70 |
| PVD | 1.00 | 0.92–1.08 |
| CVA | 1.16 | 1.06–1.27 |
| Arrhythmia | 0.90 | 0.79–1.03 |
| Liu Comorbidity Score† | 1.05 | 1.01–1.09 |
AOR adjusted odds ratios; CI confidence intervals; yr, years old; BMI body mass index; HTN hypertension; CHF congestive heart failure; CAD coronary artery disease; PVD peripheral vascular disease; CVA cerebrovascular accident
*Because diabetes interacts with age, association of age with statin use must be stratified according to the presence of diabetes. As a result, diabetes is not listed separately as a comorbidity
†Per 5 unit increase in the Liu Comorbidity Score
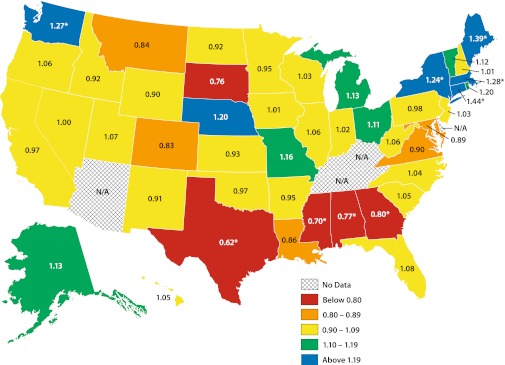
Geographic Variation
In the state-by-state geographic comparisons (Fig. 2), we categorized states according to their adjusted rates of statin utilization. While the majority of states analyzed (n = 46, plus the District of Columbia) had confidence intervals spanning unity, 9 states had O/E odds ratios significantly different from 1.0. The shadings demonstrate this wide state-by-state variation across the extremes, with a 2.3-fold variation between the highest- (Connecticut, O/E odds ratio = 1.44) and lowest- (Texas, O/E odds ratio = 0.62) using states. The former result therefore indicates that the odds of use were 44 % higher in Connecticut than was expected, after adjustment for the individual-level factors of each patient in that state. Other states with higher-than-expected use were Maine, Massachusetts, Washington, and New York. Other states with lower-than-expected use were Mississippi, Alabama, and Georgia. In Appendix Table 1 (available online), O/E odds ratios for individual states are shown.
Figure 2.
Observed versus expected odds ratios for use of statins, by state.
Discussion
In this study, we examined patterns of statin use in a large cohort of dually eligible chronic dialysis patients. Numerous demographic and clinical factors were associated with statin use, many of which would be expected. However, a striking association is attributable to state of residence. While the majority of states (roughly 80 %) had O/E odds ratios clustering around 1.0, there was still a 2.3-fold difference in rates between the highest- and lowest-prescribing states.
Our findings contribute to the literature documenting geographic variation of care in the general and dialysis populations. While our work does not permit us to draw definitive conclusion about why such differences might exist, the large geographic variations in care we report may well reflect a lack of consensus regarding optimal management in the time period examined. Before the advent of recent RCTs, observational evidence from at least two retrospective cohort studies14,15 suggested significant, independent effects of statins on cardiac and non-cardiac mortality. Although such studies could not provide definitive evidence of efficacy, some physicians may have believed that dialysis patients would benefit from statins in a fashion similar to non-dialysis patients, while others may not have. In the absence of consensus, other factors impacting use, such as local physician or healthcare “culture” or local and regional patterns in training may have predominated. Since more recent randomized controlled trials have provided conflicting evidence on the benefits of statins,11–13 it will be important to revaluate statin use in the coming years to assess what, if any, affect the recent RCTs have on prescribing patterns.
It is important to note than some of the variation may be driven by Medicaid policies. Each state operates its own Medicaid program with a degree of flexibility, albeit within federal guidelines, so policy details such as requirements for drug preauthorizations or caps on total monthly prescriptions vary by state. During 2005, 12 states had prior authorization requirements for statins, and fourteen states (some overlapping) had monthly prescription caps or limits to the number of prescriptions beneficiaries could have covered by Medicaid.26 Prior authorization policies did not appear to greatly influence utilization in this analysis: Alabama was the only state with prior authorization required for statins that had lower-than-expected use, while Maine and Massachusetts, both with prior authorization restrictions, had higher-than-expected use. Prescription limits may have had a stronger impact, since three of the four states that demonstrated lower-than expected use (Georgia, Mississippi, and Texas) employed monthly caps while four of the five states showing higher-than expected use do not employ caps (New York being the exception). In the absence of further evidence, we are hesitant to unduly ascribe difference in the O/E odds ratios to policy differences for two reasons: first, many states with prior authorization restrictions and/or caps did not differ in their expected-to-observed ratio, and second, our analysis of patterns of raw claims showed that caps were not strictly enforced (as is the case in our own state, Kansas).
Few, if any, studies have examined factors associated with statin prescribing patterns through use of formal modeling, although Winkelmayer et al. examined statin prescription in the specific setting of post-myocardial infarction care in dialysis patients; none of the factors they analyzed were associated with use, except for age, which had a slight inverse association (odds ratio 0.96, 95 % CIs 0.92–0.99).27 Our findings show an increase in the use of statins among patients with hypertension, diabetes, coronary artery disease, and a history of a CVA, and indeed an overall increased comorbidity burden (as indicated by the Liu comorbidity score22)—reflecting a similar gradation between the risk for CVD and statin use as found non-dialysis populations. Likewise, an association between increasing BMI and statin use was observed, as might be expected. Age, however, followed a biphasic pattern of use, which was highest in those who were 50–80 years old (regardless of diabetic status), declining in individuals> 80 years old. Whether this represents differences in lipid levels with increasing age, concerns about long-term preventive strategies, or other conditions of aging, such as frailty are unknown. We suspect that the greater use in those on self-care dialysis may be a marker of better nutritional or overall health status, or, conceivably, a greater perception by physicians of their ability to adhere to statin therapy. African-Americans were less likely to be treated than their respective counterparts, a finding which echoes those from the general population.28–31 Somewhat counterintuitively, males had less use of statins, a finding at variance with the general population.32,33
The rate of statin use we found, at 34.2 %, is broadly comparable to that found previous studies18,34,35), and recent work utilizing Medicare Part D (at 44 %),36 but is much higher than in older studies using data from the late 1990’s when rates ranged from about 9–17 %,15,37–40 albeit at 16.6 % in U.S. patients. These rate variations likely reflect temporal trends in statin prescribing which reflect trends in the general population.19
It is important to consider several limitations in this study. First, it is the case that the majority of states had O/E odds ratios that were not significantly different from unity. This suggests that the variation in care practices are concentrated across the roughly 20 % or so of states at the two extremes of use, and that efforts to further investigate factors associated with care should concentrate on the states at the extremes. Second, we studied only dually eligible prevalent chronic dialysis patients. By virtue of having Medicaid, these patients were more likely than the general chronic dialysis population to be indigent, female, non-Caucasian, have functional limitations, engage in risk behaviors, and be on in-center hemodialysis.41 Although this somewhat decreases our ability to generalize findings to the US dialysis population as a whole, dually eligible patients are in many ways reflective of growing trends in dialysis patients, such as the increase in females, Hispanics, individuals with functional limitations, and in those on in-center HD. Another important limitation is that, like most observational studies, we do not have actual data on cholesterol levels, so we are unable to determine if patients were indeed hyperlipidemic.
However, to counterbalance these weaknesses and increase confidence in our results, we instituted a variety of analytical safeguards, such as utilizing a modeling approach that takes into account uncertainty in the expected state ratios (thereby accounting for higher uncertainty in states with smaller numbers of patients), excluding patients with any form of managed care (so as to study only “truly observable” patients), examining medications which are widely covered on state formularies and which have only nominal copayments, using a 4-month day prescription window (so as to encompass states which permit more than 100-day supplies), and finally, studying only individuals who filled a prescription (thereby demonstrating actual utilization of Medicaid services). We also used the Liu comorbidity score,22 a method the quantify comorbidity burden specifically in dialysis patients, to bolster our assessment of comorbidities beyond those captured by the CMS 2728 form alone.
In conclusion, we used a novel linked database which included both clinical and medication data for a national cohort of chronic dialysis patients in order to provide a detailed description of the prevalence of HMG-CoA reductase inhibitors in dually eligible chronic dialysis patients. We noted wide variations across US states, suggesting that some states were preferentially using these agents. Our results extend an emerging literature identifying regional differences in the care of chronic dialysis patients. Specialty and procedural care, such as hemodialysis catheter use,42,43 access to kidney transplantation,44 arteriovenous fistula creation,45 and even access to pre-ESRD care46 have all been recently found to vary geographically. Our study extends this realm of inquiry into the provision of readily-available medications, in this case, statins. Further research is needed determine whether changes in payer structure47,48 has affected prescribing patterns in dialysis patients, whether findings of recent RCTs have translated into changes in actual clinical practice, and, most fundamentally, whether use of these medications is “appropriate” in order to guide a more consistent therapeutic approach to treatment in this high-risk population.
Electronic supplementary material
List of observed: expected odds ratios and accompanying 99% confidence intervals for use of any statin, by state (DOC 58 kb)
Acknowledgments
The authors thank Connie Wang, MD, for technical assistance with manuscript preparation. Funding for this study was supported by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases) grants R01 DK080111-03 (to TIS) and K23 085378-02 (to JBW), by a National Kidney Foundation Young Investigator Award (to JBW) and a Sandra A. Daugherty Foundation Grant (to JBW). The data reported here have been supplied by the USRDS (DUA # 2007-10 and 2009-19 and CMS (DUA #19707). The interpretation and reporting of these data are the responsibility of the authors and in no way should been seen as an official policy or interpretation of the U.S. government.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
References
- 1.Zhang Y, Baicker K, Newhouse JP. Geographic variation in Medicare drug spending. N Engl J Med. 363(5):405–9. [DOI] [PMC free article] [PubMed]
- 2.Donohue JM, Morden NE, Gellad WF, Bynum JP, Zhou W, Hanlon JT, et al. Sources of regional variation in Medicare Part D drug spending. N Engl J Med. 366(6):530–8. [DOI] [PMC free article] [PubMed]
- 3.Zhang Y, Baicker K, Newhouse JP. Geographic variation in the quality of prescribing. N Engl J Med. 363(21):1985–8. [DOI] [PMC free article] [PubMed]
- 4.Havranek EP, Wolfe P, Masoudi FA, Rathore SS, Krumholz HM, Ordin DL. Provider and hospital characteristics associated with geographic variation in the evaluation and management of elderly patients with heart failure. Arch Intern Med. 2004;164(11):1186–91. doi: 10.1001/archinte.164.11.1186. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Greiner MA, DiMartino LD, Schulman KA, Duncan PW, Matchar DB, et al. Geographic variation in carotid revascularization among Medicare beneficiaries, 2003–2006. Arch Intern Med. 170(14):1218–25. [DOI] [PubMed]
- 6.Kumar A, Fonarow GC, Eagle KA, Hirsch AT, Califf RM, Alberts MJ, et al. Regional and practice variation in adherence to guideline recommendations for secondary and primary prevention among outpatients with atherothrombosis or risk factors in the United States: a report from the REACH Registry. Crit Pathw Cardiol. 2009;8(3):104–11. doi: 10.1097/HPC.0b013e3181b8395d. [DOI] [PubMed] [Google Scholar]
- 7.O'Hare AM, Rodriguez RA, Hailpern SM, Larson EB, Kurella Tamura M. Regional variation in health care intensity and treatment practices for end-stage renal disease in older adults. JAMA. 304(2):180–6. [DOI] [PMC free article] [PubMed]
- 8.Wang V, Lee SY, Patel UD, Weiner BJ, Ricketts TC, Weinberger M. Geographic and temporal trends in peritoneal dialysis services in the United States between 1995 and 2003. Am J Kidney Dis. 55(6):1079–87. [DOI] [PMC free article] [PubMed]
- 9.Kutner NG, Zhang R, Huang Y, Wasse H. Patient awareness and initiation of peritoneal dialysis. Arch Intern Med. 171(2):119–24. [DOI] [PubMed]
- 10.Wetmore JB, Mahnken JD, Mukhopadhyay P, Hou Q, Ellerbeck EF, Rigler SK, et al. Geographic variation in cardioprotective antihypertensive medication usage in dialysis patients. Am J Kidney Dis. 58(1):73–83. [DOI] [PMC free article] [PubMed]
- 11.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–48. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 12.Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395–407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 13.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 377(9784):2181–92. [DOI] [PMC free article] [PubMed]
- 14.Seliger SL, Weiss NS, Gillen DL, Kestenbaum B, Ball A, Sherrard DJ, et al. HMG-CoA reductase inhibitors are associated with reduced mortality in ESRD patients. Kidney Int. 2002;61(1):297–304. doi: 10.1046/j.1523-1755.2002.00109.x. [DOI] [PubMed] [Google Scholar]
- 15.Mason NA, Bailie GR, Satayathum S, Bragg-Gresham JL, Akiba T, Akizawa T, et al. HMG-coenzyme a reductase inhibitor use is associated with mortality reduction in hemodialysis patients. Am J Kidney Dis. 2005;45(1):119–26. doi: 10.1053/j.ajkd.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Lopes AA, Bragg-Gresham JL, Ramirez SP, Andreucci VE, Akiba T, Saito A, et al. Prescription of antihypertensive agents to haemodialysis patients: time trends and associations with patient characteristics, country and survival in the DOPPS. Nephrol Dial Transplant. 2009;24(9):2809–16. doi: 10.1093/ndt/gfp212. [DOI] [PubMed] [Google Scholar]
- 17.United States Renal Data System. USRDS 2006 Annual data report: atlas of end-stage renal disease in the United States. In: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2006.
- 18.Chow FY, Polkinghorne KR, Chadban SJ, Atkins RC, Kerr PG. Cardiovascular risk in dialysis patients: a comparison of risk factors and cardioprotective therapy between 1996 and 2001. Nephrology (Carlton) 2003;8(4):177–83. doi: 10.1046/j.1440-1797.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 19.Mann D, Reynolds K, Smith D, Muntner P. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: impact of the 2001 National Cholesterol Education Program guidelines. Ann Pharmacother. 2008;42(9):1208–15. doi: 10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- 20.Wennberg DE. Variation in the delivery of health care: the stakes are high. Ann Intern Med. 1998;128(10):866–8. doi: 10.7326/0003-4819-128-10-199805150-00012. [DOI] [PubMed] [Google Scholar]
- 21.Volkova N, McClellan W, Soucie JM, Schoolwerth A. Racial disparities in the prevalence of cardiovascular disease among incident end-stage renal disease patients. Nephrol Dial Transplant. 2006;21(8):2202–9. doi: 10.1093/ndt/gfl078. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 77(2):141–51. [DOI] [PubMed]
- 23.McCulloch C, Searle S. Generalized, Linear, and Mixed Models. Ch. 8. New York: John Wiley & Sons, Inc.; 2001. [Google Scholar]
- 24.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. New York: John Wiley & Sons, Inc.; 2000. [Google Scholar]
- 25.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. 2. Cary: SAS Institute Inc.; 2006. [Google Scholar]
- 26.National Pharmaceutical Council. Pharmaceutical Benefits Under State Medical Assistance Programs. http://www.npcnow.org/Public/Research_Publications/Publications/pub_res_research/pub_medicaid/Pharmaceutical_Benefits_Under_State_Medical_Assistance_Programs_2005.aspx. accessed December 7, 2010.
- 27.Winkelmayer WC, Charytan DM, Levin R, Avorn J. Poor short-term survival and low use of cardiovascular medications in elderly dialysis patients after acute myocardial infarction. Am J Kidney Dis. 2006;47(2):301–8. doi: 10.1053/j.ajkd.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Franks P, Tancredi D, Winters P, Fiscella K. Cholesterol treatment with statins: who is left out and who makes it to goal? BMC Health Serv Res. 10:68. [DOI] [PMC free article] [PubMed]
- 29.Mehta JL, Bursac Z, Mehta P, Bansal D, Fink L, Marsh J, et al. Racial disparities in prescriptions for cardioprotective drugs and cardiac outcomes in Veterans Affairs Hospitals. Am J Cardiol. 105(7):1019–23. [DOI] [PubMed]
- 30.Qato DM, Lindau ST, Conti RM, Schumm LP, Alexander GC. Racial and ethnic disparities in cardiovascular medication use among older adults in the United States. Pharmacoepidemiol Drug Saf. 19(8):834–42. [DOI] [PMC free article] [PubMed]
- 31.Robinson JG, Booth B. Statin use and lipid levels in older adults: National Health and Nutrition Examination Survey, 2001 to 2006. J Clin Lipidol. 4(6):483–90. [DOI] [PMC free article] [PubMed]
- 32.Enriquez JR, Pratap P, Zbilut JP, Calvin JE, Volgman AS. Women tolerate drug therapy for coronary artery disease as well as men do, but are treated less frequently with aspirin, beta-blockers, or statins. Gend Med. 2008;5(1):53–61. doi: 10.1016/S1550-8579(08)80008-3. [DOI] [PubMed] [Google Scholar]
- 33.Larkin ME, Backlund JY, Cleary P, Bayless M, Schaefer B, Canady J, et al. Disparity in management of diabetes and coronary heart disease risk factors by sex in DCCT/EDIC. Diabet Med. 27(4):451–8. [DOI] [PubMed]
- 34.Manley HJ, Garvin CG, Drayer DK, Reid GM, Bender WL, Neufeld TK, et al. Medication prescribing patterns in ambulatory haemodialysis patients: comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol Dial Transplant. 2004;19(7):1842–8. doi: 10.1093/ndt/gfh280. [DOI] [PubMed] [Google Scholar]
- 35.Miller LM, Hopman WM, Garland JS, Yeates KE, Pilkey RM. Cardioprotective medication use in hemodialysis patients. Can J Cardiol. 2006;22(9):755–60. doi: 10.1016/S0828-282X(06)70291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frankenfield DL, Weinhandl ED, Powers CA, Howell BL, Herzog CA, St Peter WL. Utilization and costs of cardiovascular disease medications in dialysis patients in Medicare Part D. Am J Kidney Dis. 2012;59(5):670–81. [DOI] [PubMed]
- 37.Trespalacios FC, Taylor AJ, Agodoa LY, Abbott KC. Incident acute coronary syndromes in chronic dialysis patients in the United States. Kidney Int. 2002;62(5):1799–805. doi: 10.1046/j.1523-1755.2002.00638.x. [DOI] [PubMed] [Google Scholar]
- 38.Abbott KC, Trespalacios FC, Agodoa LY, Taylor AJ, Bakris GL. Beta-blocker use in long-term dialysis patients: association with hospitalized heart failure and mortality. Arch Intern Med. 2004;164(22):2465–71. doi: 10.1001/archinte.164.22.2465. [DOI] [PubMed] [Google Scholar]
- 39.Ishani A, Herzog CA, Collins AJ, Foley RN. Cardiac medications and their association with cardiovascular events in incident dialysis patients: cause or effect? Kidney Int. 2004;65(3):1017–25. doi: 10.1111/j.1523-1755.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- 40.Andreucci VE, Fissell RB, Bragg-Gresham JL, Ethier J, Greenwood R, Pauly M, et al. Dialysis Outcomes and Practice Patterns Study (DOPPS) data on medications in hemodialysis patients. Am J Kidney Dis. 2004;44(5 Suppl 2):61–7. doi: 10.1016/S0272-6386(04)01107-2. [DOI] [PubMed] [Google Scholar]
- 41.Wetmore JB, Rigler SK, Mahnken JD, Mukhopadhyay P, Shireman TI. Considering health insurance: how do dialysis initiates with Medicaid coverage differ from persons without Medicaid coverage? Nephrol Dial Transplant. 25(1):198–205. [DOI] [PMC free article] [PubMed]
- 42.Hopson S, Frankenfield D, Rocco M, McClellan W. Variability in reasons for hemodialysis catheter use by race, sex, and geography: findings from the ESRD clinical performance measures project. Am J Kidney Dis. 2008;52(4):753–60. doi: 10.1053/j.ajkd.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Foley RN, Chen SC, Collins AJ. Hemodialysis access at initiation in the United States, 2005 to 2007: still "catheter first". Hemodial Int. 2009;13(4):533–42. doi: 10.1111/j.1542-4758.2009.00396.x. [DOI] [PubMed] [Google Scholar]
- 44.Ashby VB, Kalbfleisch JD, Wolfe RA, Lin MJ, Port FK, Leichtman AB. Geographic variability in access to primary kidney transplantation in the United States, 1996–2005. Am J Transplant. 2007;7(5 Pt 2):1412–23. doi: 10.1111/j.1600-6143.2007.01785.x. [DOI] [PubMed] [Google Scholar]
- 45.O'Hare AM, Dudley RA, Hynes DM, McCulloch CE, Navarro D, Colin P, et al. Impact of surgeon and surgical center characteristics on choice of permanent vascular access. Kidney Int. 2003;64(2):681–9. doi: 10.1046/j.1523-1755.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 46.McClellan WM, Wasse H, McClellan AC, Kipp A, Waller LA, Rocco MV. Treatment center and geographic variability in pre-ESRD care associate with increased mortality. J Am Soc Nephrol. 2009;20(5):1078–85. doi: 10.1681/ASN.2008060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polinski JM, Donohue JM, Kilabuk E, Shrank WH. Medicare Part D's effect on the under- and overuse of medications: a systematic review. J Am Geriatr Soc. 59(10):1922–33. [DOI] [PMC free article] [PubMed]
- 48.Zhang Y, Donohue JM, Lave JR, Gellad WF. The impact of Medicare Part D on medication treatment of hypertension. Health Serv Res. 46(1 Pt 1):185–98. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of observed: expected odds ratios and accompanying 99% confidence intervals for use of any statin, by state (DOC 58 kb)