Abstract
Several serine protease enzymes are known to be involved in both normal desquamation and the inflammatory processes of the skin. Alteration in the activity of these proteases should also affect corneocyte maturity and size as well as stratum corneum thickness. The aim of the present work was to characterise the baseline changes in corneocyte size, corneocyte maturity, selected protease activity (specifically, Kallikreins-5 and 7, tryptase), protein content and trans-epidermal water loss (TEWL) as a function of anatomic site. The anatomic sites investigated were: cheek, abdomen, wrist and mid-ventral forearm. TEWL values were highest for the cheek (p < 0.05). The TEWL values were also significantly higher (p < 0.05) for cheek and wrist compared with other sites. Protein content was significantly lower for wrist (p < 0.05) compared with other sites. Corneocyte maturity and surface area were significantly (p < 0.05) lower for cheek and wrist compared with other sites. An excellent correlation (r2 = 0.99) was obtained for maturity and surface area measurements. Kallikrein-5 and tryptase activity were significantly higher for the cheek compared with other sites but Kallikrein-7 values were uniform across sites. The findings have significant implications for skin permeability to drugs and other substances such as environmental toxins depending on the anatomic site of delivery or exposure.
KEY WORDS: kallikrein, maturity, protease, skin, transepidermal water loss
INTRODUCTION
The major barrier function of the skin resides primarily in the epidermis and is localised in the stratum corneum (SC) typically 10–20 μm thick (1). Inter- and intra-variability in human skin barrier as a function of anatomic site has previously been reported and reviewed by a number of authors (2–5). The methods used to determine such variation have included techniques to assess corneocyte size (2,6), epidermal turnover (7,8), biophysical measurements such as transepidermal water loss (TEWL; 6,9) or spectroscopic measurements (10).
Recently, we have developed and validated a range of techniques to probe the biophysical and molecular properties of the SC (11). Changes in corneocyte surface area, corneocyte maturity, selected protease activities and TEWL were characterised for the volar forearm. Corneocyte maturity and surface area decreased with increasing number of tape strippings, i.e. depth into the skin. More mature corneocytes were typically larger than less mature corneocytes. The protease activities of both the desquamatory and inflammatory enzymes together with the protein content were highest in the outer layers of the stratum corneum and decreased with depth. As expected, TEWL increased as more stratum corneum layers were removed.
In the present work, we have used the methods previously reported to investigate SC characteristics at a number of anatomic sites. In addition to the mid-ventral forearm, the other sites investigated included the cheek, wrist and abdomen. An understanding of barrier function at different body sites will have implications for the permeation of drugs and xenobiotics at these locations; it should also provide insight into optimal locations for application of devices such as transdermal patches or sprays.
MATERIALS AND METHODS
Materials
Standard D-Squame(R) tape (2.2 cm in diameter, 3.8 cm2) was obtained from CuDerm Corporation (Dallas, TX, USA). Sodium lauryl sulphate, ethylene diaminetetra-acetic acid, Triton X-100, acetic acid and Nile Red were obtained from Sigma-Aldrich, UK. High-performance liquid chromatography (HPLC) analytical grade water and methanol were obtained from Fisher Scientific, UK. DL-dithiothreitol and Tris-hydrochloride buffer (pH 8.0) were obtained from Fluka Analytical (UK). Dimethyl sulphoxide was obtained from VWR International Ltd, UK and phosphate-buffered saline tablets were obtained from Oxoid UK TEWL was measured using an Aquaflux AF103 (Biox Systems, London, UK) and protein absorbance was measured at 850 nm using a SquameScan A850 infrared densitometer (Heiland Electronic, Wetzlar, Germany). The primary monoclonal antibody, anti-human involucrin (clone SY5), was purchased from Cambridge Scientific, Monosan, UK and the rabbit polyclonal antibody to mouse fluorescent IgG–H&L (whole molecule) fluorescein isothiocyanate antibody was obtained from Abcam, Cambridge, UK. Aminomethyl coumarin (AMC) and all fluorogenic peptide substrates (tryptase “Tos-Gly-Pro-Lys-AMC”, kallikrein-5 (KLK5) “Boc-Phe-Ser-Arg-AMC”, kallikrein-7 (KLK7) “MeOSuc-Arg-Pro-Tyr-AMC”) were generous gifts from DSM Nutritional Products Ltd., Switzerland.
Volunteer Recruitment
Twenty-two healthy volunteers aged 20–58 years were recruited (13 males, 9 females; 12 Caucasian and 10 Black subjects) in October 2009 and the study was performed in November 2009. The research protocol was approved by the South West London Research Ethics Committee (Reference 10/H0801/69). A participant information leaflet was supplied to all the volunteers prior to the studies and volunteers were also asked to complete a questionnaire detailing their past medical history, family history, general atopic diseases such as eczema, and use of topical formulations, e.g. anti-inflammatory products. None of the volunteers had any history of skin disease and were asked, apart from daily washing, not to apply any moisturiser or cosmetic product to the study treatment sites, beginning 2 weeks prior to the study. The anatomic sites investigated were: cheek, wrist, mid-ventral forearm and abdomen (Fig. 1). A 1 cm2 area was delineated with an indelible marker on the skin within the centre of each anatomic site.
Fig. 1.
Anatomic sites investigated
Four tape strips were consecutively removed from each site. Corneocyte maturity and size were assessed from the first tape. The remaining three tapes were used to investigate protease activity of desquamatory KLK5 and KLK7 as well as inflammatory ‘tryptase’ enzymes. Protein content was measured on all the four tape strips.
Tape Stripping and TEWL Measurements
Volunteers were acclimatised for 15 min prior to all measurements (in ambient conditions of 21 ± 1°C and 45 ± 1% relative humidity). Standard D-Squame® tape was applied to the cheek, wrist, mid-ventral forearm and abdomen at a constant pressure as described previously (11). Four consecutive tape strippings were performed for all the selected areas. TEWL was measured using an Aquaflux® closed-chamber evaporimeter (Biox Systems Ltd, London,UK) as described previously (11,12).
SC Protein Content
Protein content was measured directly from tape strips using infrared densitometry as reported previously (11). Protein absorption was determined at 850 nm using an infrared densitometer SquameScan 850A using the following equation: (13)
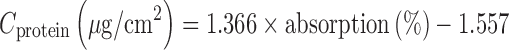 |
1 |
The amount of protein was quantified subsequently using the process described by Voegli et al. (14).
Corneocyte Maturity and Surface Area
Corneocyte maturity was measured using a modification of the method developed by Hirao et al. (15) and reported previously by Mohammed et al. (11). Fluorescence was monitored with a fluorescence microscope equipped with a Fuji S2 Pro 6.2 megapixel camera (Fuji, Japan). The object was magnified 10×, giving 0.800 pixels per micrometer with a resolution of 1,440 × 960 pixels. Images of cells stained with both Nile Red and anti-involucrin were taken separately. ImageJ image analysis software (NIH-Image, USA) was used to analyse separately the red pixels obtained from Nile Red stained cells and the green pixels from the immuno-stained cells (the ratio of red/green pixels providing an estimate of corneocyte maturity). The images obtained from Nile Red stained corneocytes were used to measure corneocyte surface area, also using ImageJ.
SC Protease Activity
SC samples were obtained by tape stripping as described previously. Protease activity was measured using a fluorometric substrate method described by Voegeli et al. (14) and reported previously by Mohammed et al. (10,11). Immediately after measuring protein content, tape strips were placed in 1.5 ml Eppendorf tubes. Released AMC was quantified by HPLC using a Symmetry C18 column, 5 μm, 4.6 × 150 mm (Waters, Milford, MA, USA). The HPLC system consisted of a HP series 1050 quaternary pump, HP series 1050 autosampler, HP series 1050 degasser, Waters 470 Fluorescence Detector. The data were analysed with PRIME software (Ver.4.2.0, HPLC Technology Co. Ltd, UK). Emission and excitation wavelengths were 442 and 354 nm, respectively. The mobile phases used were 50% water, 50% methanol and 0.1% TFA; pH 2.0 to analyse KLK5 and tryptase activity; and 40% water, 60% methanol and 0.1% TFA, pH 2.0 to analyse KLK7. The flow rate was 1 ml/min and the injection volume was 20 μl. The retention time for KLK5 and tryptase was 2.5 min and 3.6 min for KLK7. The released AMC was then calculated from a standard AMC calibration curve. The limit of detection was 1 ng/mL.
Statistical Analysis
SPSS version 18 and Microsoft® Excel Office 2007 were used to analyse the data. Normality was assessed using the Kolmogorov–Smirnov statistical test. Parametric statistical tests (one-way between-group analysis of variance and paired t test to compare means) were used to investigate statistical differences between treated and untreated sites. A probability of p < 0.05 was considered statistically significant. All results are presented as mean and standard error of the mean.
RESULTS
TEWL Measurement
The baseline TEWL values of various anatomical sites are shown in Fig. 2. The TEWL values of the cheek were the highest (p > 0.05) compared with the other anatomical sites. This was followed by the TEWL value of the wrist then mid-ventral forearm and abdomen. There were no statistical differences between the TEWL value of the mid-ventral forearm and the abdomen (p > 0.1).
Fig. 2.
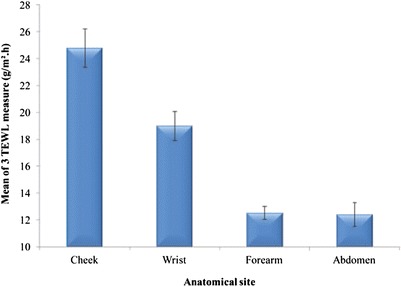
TEWL measurements for different anatomic sites (mean ± SEM, n = 22)
There were no statistical differences between males and females (p > 0.05). When considering ethnicity (data not shown), there were also no statistical differences in TEWL values between Black subjects and Caucasians at all the investigated anatomic sites (p > 0.1).
SC Protein Measurement
Figure 3 shows the mean protein content per strip (for tape strips 2–4) data for different anatomic sites. Apart from wrist versus abdomen (p < 0.05), no statistical differences were observed when comparing the remaining sites (p > 0.05). The data and SEM values are in line with previous values we have reported (11). There were no significant differences in the amount of protein removed for males compared with females (p > 0.1) when categorising the volunteers according to gender (data not shown).
Fig. 3.
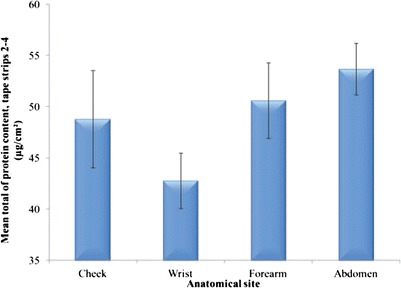
Mean SC protein content removed per strip from different anatomic sites, tape strips 2–4 (n = 22, mean ± SEM)
When the data were analysed by ethnic group (data not shown), similar trends were observed for both Caucasians and Black subjects, i.e. no significant differences in protein content removed from various anatomical sites were noted (p > 0.1).
Corneocyte Maturity and Surface Area
The results for corneocyte maturity, for tape strip 1, when analysed as a function of anatomic site, are shown in Fig. 4. There are no differences in overall corneocyte maturity between males and females or between ethnic groups (data not shown, p > 0.1).
Fig. 4.
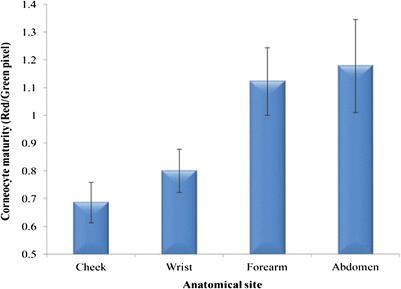
Corneocyte maturity at different anatomic sites, tape strip 1 (n = 22, mean ± SEM)
The corneocyte maturity of the cheek is the lowest followed by wrist then mid-ventral forearm and abdomen; however, cheek and wrist were not significantly different (p > 0.05). Values for cheek and wrist are significantly (p < 0.05) lower than mid-ventral forearm and abdomen.
Corneocyte surface area values (Fig. 5) measured using tape strip 1, mirrored the maturity results, i.e. less mature corneocytes are smaller than more mature cells. As for the maturity values, significant differences (p < 0.05) are evident between the cheek and wrist compared with mid-ventral forearm and abdomen. Values for cheek are not significantly different (p > 0.05) from the wrist. Similarly, values for mid-ventral forearm are not significantly different (p > 0.1) from the abdomen.
Fig. 5.
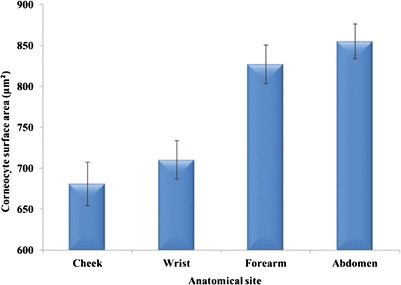
Corneocyte surface areas for different anatomic sites, tape strip 1 (n = 22, mean ± SEM)
For the two ethnic groups, a similar trend was observed, i.e. reduced corneocyte size for the cheek compared with the mid-ventral forearm or abdomen, but there were no significant differences in corneocyte surface area between the groups (p > 0.05).
A positive correlation between corneocyte maturity and surface area over the sites studied is also evident (Fig. 6), i.e. the greater the maturity at the specific site, the larger the corneocyte size. A similar correlation between corneocyte maturity and surface area was previously observed with respect to depth, i.e. large corneocytes on the outer layers of the stratum corneum are associated with more mature cells (11).
Fig. 6.
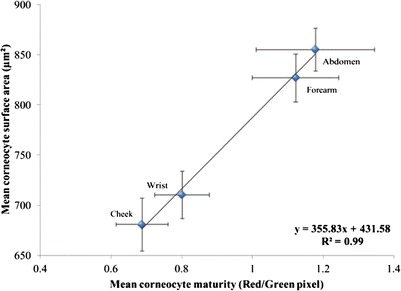
Correlation between corneocyte maturity and surface area (n = 22, mean ± SEM)
Protease Activity
For the three proteases studied (KLK5, KLK7 and tryptase), using tape strips 2–4, the activities of KLK5 and tryptase for the cheek were significantly higher than for all other sites (p < 0.001). There are no significant differences (p > 0.05) at any one site between males and females for the activity of any of the proteases measured. Similarly, when comparing the two ethnic groups no differences in the activity of proteases in the different layers are evident (p > 0.05).
DISCUSSION
TEWL Measurement
The TEWL values of the cheek were highest (p > 0.05) compared with the other sites. Values for the wrist were significantly lower (p < 0.05) than the cheek but significantly higher (p < 0.5) than mid-ventral forearm and abdomen. There were no statistical differences between the TEWL value of the mid-ventral forearm and the abdomen (p > 0.1). These results are consistent with the findings of Rougier et al. (2) who examined TEWL values of different anatomic sites and noted their order as follows: forearm [ventral elbow] < forearm [ventral-mid] < arm [upper–outer] < abdomen < forearm [ventral–wrist] < postauricular < forehead. Jang et al. (16) also investigated TEWL values at various anatomic sites and compared values for the left and right forearm in 24 volunteers [14 males, 10 females; 20–34 years]. The results were as follows: wrist > mid-forearm = proximal-forearm [near ventral elbow]. Schnetz et al. (17) determined TEWL values for selected facial sites and the mid-volar forearm in 11 volunteers [eight females, three males; 19–53 years]. The values were significantly lower for the forearm compared with facial sites and the highest value was detected for cheek; the TEWL for the forehead was significantly lower than cheek or chin (p < 0.05). Marrakchi and Maibach (18) reported TEWL values at seven different sites for 20 volunteers [24–83 years]; forehead, nose, cheek, nasolabial area, perioral area, chin and upper eyelid, forearm and neck. The TEWL value for the mid-ventral forearm was significantly lower (p < 0.05) compared with other sites. More recently, TEWL was evaluated for 90 Caucasian and Asian volunteers [males and females, 20–60 years] and reported as follows: forehead > wrist > ventral mid forearm adjacent to ventral wrist = ventral mid forearm adjacent toventral elbow = elbow = abdomen (6). Voegli et al. (14) also reported statistically significantly higher (p < 0.001) values of TEWL for the cheek compared with the forearm (Fig. 7).
Fig. 7.
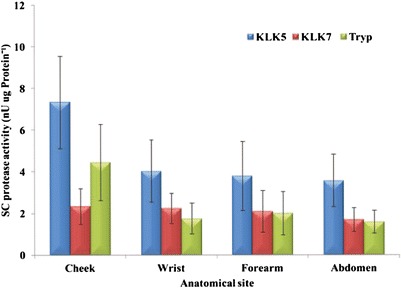
SC protease activity of different anatomic sites, pooled tape strips 2–4 (n = 22, mean ± SEM)
SC Protein Measurement
There were no statistical differences between the cheek, mid-ventral forearm and abdomen. The only significant difference observed was that between the wrist when compared to the abdomen. This observation of similar protein content within the removed baseline tapes is mainly due to the cohesiveness of the SC and water content within the SC layers. To investigate more fully any differences, more tape strips are required as shown in previous work (11,12).
SC protein content removed from the investigated sites showed no significant differences between males and females or Caucasian and Black subjects. This is in line with the findings of Voegeli et al. who reported no statistical difference in protein content between genders but noted that females generally showed a higher protein content in the forearm than males (13).
There are conflicting studies reporting SC hydration in Black and Caucasian subjects For example, Black skin is reported to require more tape strips to remove SC compared with White skin (19). This suggests a greater cohesiveness between the corneocytes of Black subjects compared with Caucasians therefore it is more difficult to remove SC from Black subjects than Caucasians. Manuskiatti et al. and colleagues reported that statistically there are no differences in SC hydration between Black and Caucasian skin in 22 female subjects (20). In contrast, Jungersted et al. (21) reported that 18 African origin subjects showed significantly less hydrated skin compared with 28 Danish-origin Caucasian subjects. The difference observed may be due to higher number of subjects in the study reported Jungersted et al. compared with the investigation conducted by Manuskiatti et al.
Corneocyte Maturity and Surface Area
The corneocyte maturity of the cheek is the lowest followed by wrist then mid-ventral forearm and abdomen. Although the literature is sparse in relation to this topic, it is encouraging to note that the data are consistent with Hirao et al. (15) who used similar methodology and reported that the corneocytes from the face are less mature than the upper arm.
There are no differences in overall corneocyte maturity between males and females at all sites. This agrees with the finding of Reichert et al., who stained corneocytes using ethyl rhodamine isothiocynate (22). Hirao et al. also observed no difference between males and females in the occurrence of involucrin-positive immature CEs in the face (15). Considering the different ethnic groups, the data show no significant differences between the groups at all the anatomical sites investigated. This is consistent with previous data from Hirao et al. (15) who evaluated facial SC maturity for a larger cohort (Japanese, Caucasian and African-American subjects; n = 20 for each ethnic group).
Comparing individual sites, mean corneocyte surface area of the cheek is significantly the smallest followed by the wrist then forearm and the abdomen. The changes in corneocyte surface area and maturity within different anatomical sites may be due to SC turnover. This is best explained by considering the work of Roberts and Marks (23). These authors reported that turnover time for the forehead was the slowest followed by the forearm, which was very similar to the values observed for the abdomen. Moreover, a relationship between corneocyte surface area and epidermal turnover rate has also been established (7,8). Kawai et al. reported that the corneocyte size from the face is much smaller than from the forearm. This may be correlated with SC turnover, which is twice as fast on the face when compared with the forearm (8). Corneocyte size was examined by Plewig and Marples (24) at nine different anatomic sites in males aged 21–31 years [two Black, two Caucasian subjects]. Larger corneocytes were observed for abdomen (average value 1,050 μm2) compared with forearm (850 μm2). The values in the present study are significantly lower (p < 0.05) for the abdomen but this may reflect differences the methodology used to size the corneocytes. Rougier et al. (2) collected corneocytes at different anatomic sites from a group of six to eight male subjects (20–30 year). Corneocyte size was ranked as follows: forearm [ventral elbow] = forearm [ventral-mid] = arm [upper–outer] = abdomen > forearm [ventral-wrist] > postauricular > forehead. The findings of Rougier et al. (2) suggested that the size of the cells of the stratum corneum is dependent on environmental exposure, i.e. those sites which are typically covered by clothing will have larger cell sizes compared with sites which are not.
The size of cornecocytes from the eyelids, cheeks and nose [22 Japanese subjects; 7 males, 15 females; 20–40 years] was reported by Pratchyapruit et al. (25). In general, the surface area of corneocytes collected from the eyelid were largest (p < 0.05), followed by cheek and nose. The average value for cheek corneocyte surface area was 591.7 ± 72.0 μm2, consistent with the findings of the present study. Values reported by Machado et al. (6), indicate the smallest corneocyte surface area for the forehead [825 ± 76 μm2] and largest for the abdomen [1,236 ± 95 μm2]. Additionally, the rank order for surface area was: forehead < wrist < FA1 = FA2 = elbow < abdomen. For the abdomen, significantly larger cell sizes (p < 0.05) were observed compared with the remaining sites.
Protease Activity
The activities of KLK5 and tryptase were significantly higher on the cheek compared with all other investigated sites. However, the activity of KLK7 appears unchanged between the various anatomical sites studied. Elevated activities of the desquamatory and inflammatory enzymes are observed from the cheek in both males and female as well as Caucasian and Black subjects. This is in line with Voegeli et al. (14) who reported higher activity of these enzymes in the cheek compared with the forearm. The activity of KLK7 was unchanged between the various sites in contrast to the findings of Voegeli et al. (14) for this enzyme. The respective activities of KLK5, KLK7 and tryptase were not significantly different for the wrist, mid-ventral forearm and abdomen (p > 0.05).
Although the KLK5 and tryptase activity were highest in the cheek, activity values for both enzymes were similar in the wrist, mid-ventral forearm and the abdomen. On the other hand, KLK7 showed no change in its activity for any of the anatomic sites.
The increase in activity of KLK5 and the inflammatory serine protease, tryptase, for the cheek compared with other anatomic sites has been suggested to reflect subclinical micro-inflammatory conditions and desquamation induced by, for example, environmental influences (14). Other workers have also confirmed that inflammatory protease activity is present in normal skin and becomes higher when the skin barrier is damaged (14,26,27).
CONCLUSIONS
Sites with larger and more mature corneocytes are associated with lower TEWL values. We have previously (6) proposed an inverse relationship between TEWL and diffusional path length for water at various anatomic sites with TEWL values tending to zero when corneocytes are infinitely large. Skin sites with smaller corneocytes have fewer cell layers, with shorter permeation path lengths and higher TEWL values and thus TEWL may be used to characterise the permeation routes for different anatomic sites. Simple regression analysis of the relationship between corneocyte maturity and surface area resulted in a high correlation coefficient, consistent with previous work from our group. For the three proteases studied, KLK5 and tryptase were highest in the facial area compared with other sites, consistent with the hypothesis that this reflects subclinical inflammation at this site. Overall, the techniques used in this study provide rapid minimally non-invasive measures of the spatial distribution of corneocyte maturity and size as well as protease activity and protein content for various anatomic sites. This type of approach should be useful in determining the effect of formulations on the barrier properties of the skin and should, ultimately, aid the rational design of formulations. Equally, it is important to understand the variation in barrier function with site and how this is affected by simple parameters such as corneocyte size. This has toxicological implications and impacts on which medicines can be administered on which body site. For example, care has to be taken when steroids are applied to the face as unwanted systemic absorption can take place. Formulation efficacy as a function of anatomical site will be the focus of future studies.
REFERENCES
- 1.Menon GK, Cleary GW, Lane ME. The structure and function of the stratum corneum. Int J Pharm. 2012;435(1):3–9. doi: 10.1016/j.ijpharm.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Rougier A, Lotte C, Corcuff P, Maibach HI. Relationship between skin permeability and corneocyte size according to anatomic site, age and sex in man. J Soc Cosmet Chem. 1988;39:15–26. [Google Scholar]
- 3.Oestmann E, Lavrijsen AP, Hermans J, Ponec M. Skin barrier function in healthy volunteers as assessed by transepidermal water loss and vascular response to hexyl nicotinate: intra- and inter-individual variability. Br J Dermatol. 1993;128:130–136. doi: 10.1111/j.1365-2133.1993.tb15141.x. [DOI] [PubMed] [Google Scholar]
- 4.Berardesca E, Maibach H. Racial differences in skin pathophysiology. J Am Acad Dermatol. 1996;34:667–672. doi: 10.1016/S0190-9622(96)80070-3. [DOI] [PubMed] [Google Scholar]
- 5.Nikolovski J, Stamatas GN, Kollias N, Wiegand BC. Barrier function and water-holding and transport properties of infant stratum corneum are different from adult and continue to develop through the first year of life. J Investig Dermatol. 2008;128:1728–1736. doi: 10.1038/sj.jid.5701239. [DOI] [PubMed] [Google Scholar]
- 6.Machado M, Salgado TM, Hadgraft J, Lane ME. The relationship between transepidermal water loss and skin permeability. Int J Pharm. 2010;384:73–77. doi: 10.1016/j.ijpharm.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 7.Holzle E, Plewig G. Effect of dermatitis, stripping, and steroid on the morphology of corneocytes. A new bioassay. J Investig Dermatol. 1977;68:350–356. doi: 10.1111/1523-1747.ep12496481. [DOI] [PubMed] [Google Scholar]
- 8.Kawai M, Imokawa G, Mizoguchi M. Physiological analysis of the facial skin by corneocyte morphology and stratum corneum turnover. Nippon Hifuka Gakkai Zasshi. 1989;99:999–1006. [PubMed] [Google Scholar]
- 9.Conti A, Schiavi ME, Seidenari S. Capacitance, transepidermal water loss and causal level of sebum in healthy subjects in relation to site, sex and age. Int J Cosmet Sci. 1995;17:77–85. doi: 10.1111/j.1467-2494.1995.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 10.Brancaleon L, Bamberg MP, Sakamaki T, Kollias N. Attenuated total reflection-Fourier transform infrared spectroscopy as a possible method to investigate biophysical parameters of stratum corneum in vivo. J Investig Dermatol. 2001;116:380–386. doi: 10.1046/j.1523-1747.2001.01262.x. [DOI] [PubMed] [Google Scholar]
- 11.Mohammed D, Matts PJ, Hadgraft J, Lane ME. Depth profiling of stratum corneum biophysical and molecular properties. Br J Dermatol. 2011;164:957–965. doi: 10.1111/j.1365-2133.2011.10211.x. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed D, Matts PJ, Hadgraft J, Lane ME. Influence of Aqueous Cream BP on corneocyte size, maturity, skin protease activity, protein content and transepidermal water loss. Br J Dermatol. 2011;164:1304–1310. doi: 10.1111/j.1365-2133.2011.10338.x. [DOI] [PubMed] [Google Scholar]
- 13.Voegeli R, Heiland J, Doppler S, Rawlings AV, Schreier T. Efficient and simple quantification of stratum corneum proteins on tape strippings by infrared densitometry. Skin Res Technol. 2007;13:242–251. doi: 10.1111/j.1600-0846.2007.00214.x. [DOI] [PubMed] [Google Scholar]
- 14.Voegeli R, RawlingS AV, Doppler S, Heiland J, Schreier T. Profiling of serine protease activities in human stratum corneum and detection of a stratum corneum tryptase-like enzyme. Int J Cosmet Sci. 2007;29:191–200. doi: 10.1111/j.1467-2494.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- 15.Hirao T, Denda M, Takahashi M. Identification of immature cornified envelopes in the barrier-impaired epidermis by characterization of their hydrophobicity and antigenicities of the components. Exp Dermatol. 2001;10:35–44. doi: 10.1034/j.1600-0625.2001.100105.x. [DOI] [PubMed] [Google Scholar]
- 16.Jang HY, Park CW, Lee CH. A study of transepidermal water loss at various anatomical sites of the skin. Br J Dermatol. 1993;128:130–136. doi: 10.1111/j.1365-2133.1993.tb15141.x. [DOI] [PubMed] [Google Scholar]
- 17.Schnetz E, Kuss O, Schmitt J, Diepgen TL, Kuhn M, Fartasch M. Intra-and inter-individual variations in transepidermal water loss on the face: facial locations for bioengineering studies. Contact Dermatitis. 1999;40:243–247. doi: 10.1111/j.1600-0536.1999.tb06057.x. [DOI] [PubMed] [Google Scholar]
- 18.Marrakchi S, Maibach HI. Biophysical parameters of skin: map of human face, regional, and age-related differences. Contact Dermatitis. 2007;57:28–34. doi: 10.1111/j.1600-0536.2007.01138.x. [DOI] [PubMed] [Google Scholar]
- 19.Weigand DA, Haygood C, Gaylor JR. Cell layers and density of Negro and Caucasian stratum corneum. J Investig Dermatol. 1974;62:563–568. doi: 10.1111/1523-1747.ep12679412. [DOI] [PubMed] [Google Scholar]
- 20.Manuskiatti W, Schwindt DA, Maibach HI. Influence of age, anatomic site and race on skin roughness and scaliness. Dermatology. 1998;196:401–407. doi: 10.1159/000017932. [DOI] [PubMed] [Google Scholar]
- 21.Jungersted JM, Høgh JK, Hellgren LI, Jemec GB, Agner T. Ethnicity and stratum corneum ceramides. Br J Dermatol. 2010;163:1169–1173. doi: 10.1111/j.1365-2133.2010.10080.x. [DOI] [PubMed] [Google Scholar]
- 22.Reichert U, Michel S, Schmidt R. The cornified envelope: a key structure of terminally differentiating keratinocytes. In: Darmon M, Blumenberg M, editors. Molecular biology of the skin: the keratinocyte. NY: Academic; 1993. pp. 107–150. [Google Scholar]
- 23.Roberts D, Marks R. The determination of regional and age variations in the rate of desquamation: a comparison of four techniques. J Investig Dermatol. 1980;74:13–16. doi: 10.1111/1523-1747.ep12514568. [DOI] [PubMed] [Google Scholar]
- 24.Plewig G, Marples RR. Regional differences of cell sizes in the human stratum corneum. Part I. J Investig Dermatol. 1970;54:13–18. doi: 10.1111/1523-1747.ep12551482. [DOI] [PubMed] [Google Scholar]
- 25.Pratchyapruit W, Kikuchi K, Gritiyarangasan P, Aiba S, Tagami H. Functional analyses of the eyelid skin constituting the most soft and smooth area on the face: contribution of its remarkably large superficial corneocytes to effective water-holding capacity of the stratum corneum. Skin Res Technol. 2007;13:169–175. doi: 10.1111/j.1600-0846.2007.00183.x. [DOI] [PubMed] [Google Scholar]
- 26.Voegeli R, Rawlings AV, Doppler S, Schreier T. Increased basal transepidermal water loss leads to elevation of some but not all stratum corneum serine proteases. Int J Cosmet Sci. 2008;30:435–442. doi: 10.1111/j.1468-2494.2008.00472.x. [DOI] [PubMed] [Google Scholar]
- 27.Voegeli R, Rawlings AV, Breternitz M, Doppler S, Schreier T, Fluhr JW. Increased stratum corneum serine protease activity in acute eczematous atopic skin. Br J Dermatol. 2009;161:70–77. doi: 10.1111/j.1365-2133.2009.09142.x. [DOI] [PubMed] [Google Scholar]


