Historically, regulatory assessment of bioequivalence (BE) has relied upon the comparison of rate and extent of drug absorption between products (1). For drugs intended to be absorbed and systemically delivered to the site(s) of activity, this is generally achieved by measuring drug concentrations in an accessible biological fluid such as blood plasma. Typically, a single-dose, two-treatment, two-period, crossover design study is conducted in a limited number of healthy volunteers under fasting and fed conditions. Two products are deemed bioequivalent if the 90% confidence intervals of the geometric mean test/reference ratios for the pharmacokinetic (PK) parameters maximum plasma concentration, for rate (Cmax); area under the plasma concentration–time curve, for extent (AUC0-t); extrapolated total area under plasma curve to time infinity (AUC∞) fall within the limits of 80–125% (2). For the majority of systemically active drug products reviewed by the Office of Generic Drugs (OGD), the “traditional” metrics, AUC and Cmax, are effective measures of BE.
However, in cases where rapid onset of action as well as controlled duration of effect is needed for drug efficacy, a partial AUC metric (pAUC, a portion of the total AUC) may be needed to ensure therapeutic equivalency (3). For example, Ritalin LA® (methylphenidate HCl) is a mixed modified release (MMR) formulation designed to release a bolus of drug for rapid onset of activity and a delayed bolus of drug to sustain activity throughout the day (4). Ritalin LA® capsules contain half the dose as IR beads and half the dose as enteric-coated, DR beads. The Food and Drug Administration (FDA) currently recommends using two pAUC metrics, in addition to AUC∞ and Cmax, to assess BE of generics to Ritalin LA® (see Fig. 1) (5). To better understand the rationale for using pAUC metrics for Ritalin LA®, as well as the selection of the time intervals used for pAUCs, it is important to understand the interplay of the MMR drug release profile with the pharmacodynamic (PD) and PK properties of methylphenidate (MPH) (6).
Fig. 1.
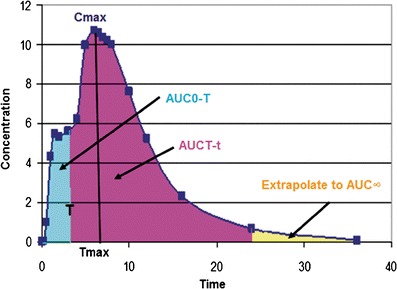
The first (early) pAUC metric, AUC0-T, compares test and reference systemic exposure responsible for early onset of response, while the second (late) pAUC metric, AUCT-t, compares test and reference systemic exposure responsible for sustaining the response later during the once-daily dosing interval
MPH is used to treat attention deficit disorder (ADD) and has a short half-life (2 h) and duration of efficacy (2–3 h) (4). According the FDA labeling, MPH MMR formulations (Ritalin LA®, Concerta®, and Metadate CD®) can be taken once a day, providing rapid onset of action in the morning and coverage during the day. MPH’s clinical effects can be assessed using the SKAMP (Swanson, Kotkin, Alger, M-Fynne, and Pelham) ratings (7). Thus, the time course of clinical outcome in ADD patients can be related to MPH pharmacokinetics by a PK/PD model. A PK/PD model was developed by comparing the time course of clinical (SKAMP) response to methylphenidate plasma concentrations following dosing with Concerta® and Metadate CD®. The model showed that clinical superiority is expected at any point in time for the formulation with the highest methylphenidate concentration (8). Therefore, a generic of Ritalin LA could potentially have an AUC and Cmax comparable to the RLD, but not be therapeutically equivalent due to differences in the time concentration profile.
As previously stated, two products are deemed bioequivalent if the 90% confidence intervals of the test/reference ratios for Cmax, AUC0-t, and AUC∞ fall within the BE limits of 80–125%. The parameter Tmax (the time of the peak plasma concentration) is also evaluated for similarity by the OGD, but not subject to statistical evaluation. If Tmax differs markedly between the test and reference products, OGD consults the relevant clinical division in the Center for Drug Evaluation and Research to determine whether such differences could result in a lack of therapeutic equivalence. However, the observed Tmax in patients taking Ritalin LA® does not occur in some subjects until the MPH from the DR portion of the formulations is being absorbed. Thus, Tmax will not always provide information about whether a generic of Ritalin LA will provide the same onset of activity as the RLD (1,9). In contrast, the PK parameters of the MPH IR products (e.g. Ritalin®) are characteristics of a single release phase.
We used the PK data from the IR MPH formulation to have an unbiased estimate of the Tmax of the IR component of the MMR formulation (10). The Tmax of MPH occurs at 2 h, which is also the time at which the peak PD effect of the early portion of Ritalin LA® is observed (4). We analyzed the PK data from FDA submissions and for IR only MPH products Tmax is about 2 ± 0.5 and 3 ± 0.5 h under fasting and fed conditions, respectively. Generally, approximately 95% of observations fall within two standard deviations of the mean. Therefore, pAUCs calculated to 3 h (pAUC0-3) in a fasting BE study and 4 h (pAUC0-4) for a fed BE study should ensure that 95% of the subjects should achieve their desired therapeutic response. Likewise, applying pAUC3-t and pAUC4-t to fasting and fed BE studies, respectively, should ensure that two products are therapeutically equivalent over the later part of the daily dosing interval. The variability of the early pAUC is higher than that of Cmax and AUC∞ under fasting and fed conditions, but reasonable for use in an equivalence test.
In conclusion, the use of two pAUCs metrics along with the AUC∞ and Cmax will ensure that the pharmacokinetic profiles and clinical effects of generics of Ritalin LA® are sufficiently similar to the brand name drug. For Ritalin LA®, the first (early) pAUC metric is designed to ensure that 90–95% of the subjects have achieved the optimal early onset of response, while the second (late) pAUC metric assesses the response due to the DR portion of the formulation.
References
- 1.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products–General Considerations. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070124.pdf. Accessed 23 Jan 2012.
- 2.Meeting of the Advisory Committee for Pharmaceutical Science and Clinical Pharmacology April 13, 2010 Briefing Information. http://www.fda.gov/ohrms/dockets/ac/01/transcripts/3804t2_03_Afternoon_Session.pdf. Accessed 23 Jan 2012
- 3.Chen ML, Shah VP, Ganes D, Midha KK, Caro J, Nambiar P, et al. Challenges and opportunities in establishing scientific and regulatory standards for assuring therapeutic equivalence of modified release products: workshop summary report. AAPS J. 2010;12(3):371–377. doi: 10.1208/s12248-010-9201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritalin LA Label. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=34566&CFID=104324036&CFTOKEN=7817fd5a1e259439-F14B296D-C81B-A59C-8039A19C3CD27924&jsessionid=84302ca9d80b916d5334116f7a46145b4247. Accessed 23 Jan 2012.
- 5.Draft Guidance on Methylphenidate Hydrochloride. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM281454.pdf. Accessed 23 Jan 2012.
- 6.FDA Meeting of the Advisory Committee for Pharmaceutical Sciences and Clinical Pharmacology. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AdvisoryCommitteeforPharmaceuticalScienceandClinicalPharmacology/UCM207955.pdf. Accessed 23 Jan 2012
- 7.Swanson J, Kinsbourne M, Roberts W, Zucker K. Time-response analysis of the effect of stimulant medication on the learning ability of children referred for hyperactivity. Pediatrics. 1978;61(1):21–29. [PubMed] [Google Scholar]
- 8.Swanson JM, Wigal SB, Wigal T, Sonuga-Barke E, Greenhill LL, Biederman J, et al. A comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (the Comacs Study) Pediatrics. 2004;113(3 Pt 1):e206–e216. doi: 10.1542/peds.113.3.e206. [DOI] [PubMed] [Google Scholar]
- 9.Chen ML, Davit B, Lionberger R, Wahba Z, Ahn HY, Yu LX. Using partial area for evaluation of bioavailability and bioequivalence. Pharm Res. 2011;28(8):1939–1947. doi: 10.1007/s11095-011-0421-x. [DOI] [PubMed] [Google Scholar]
- 10.Ritalin Label. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=34832. Accessed 23 Jan 2012


