Abstract
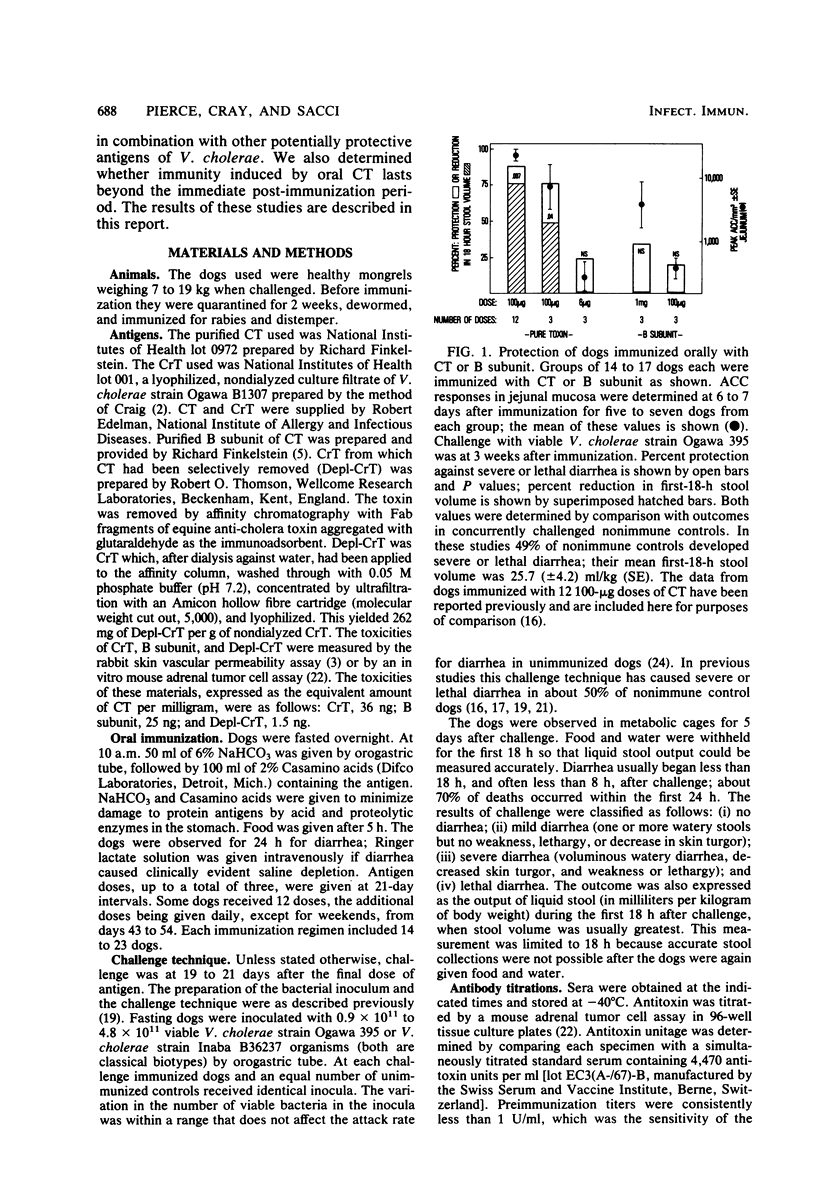
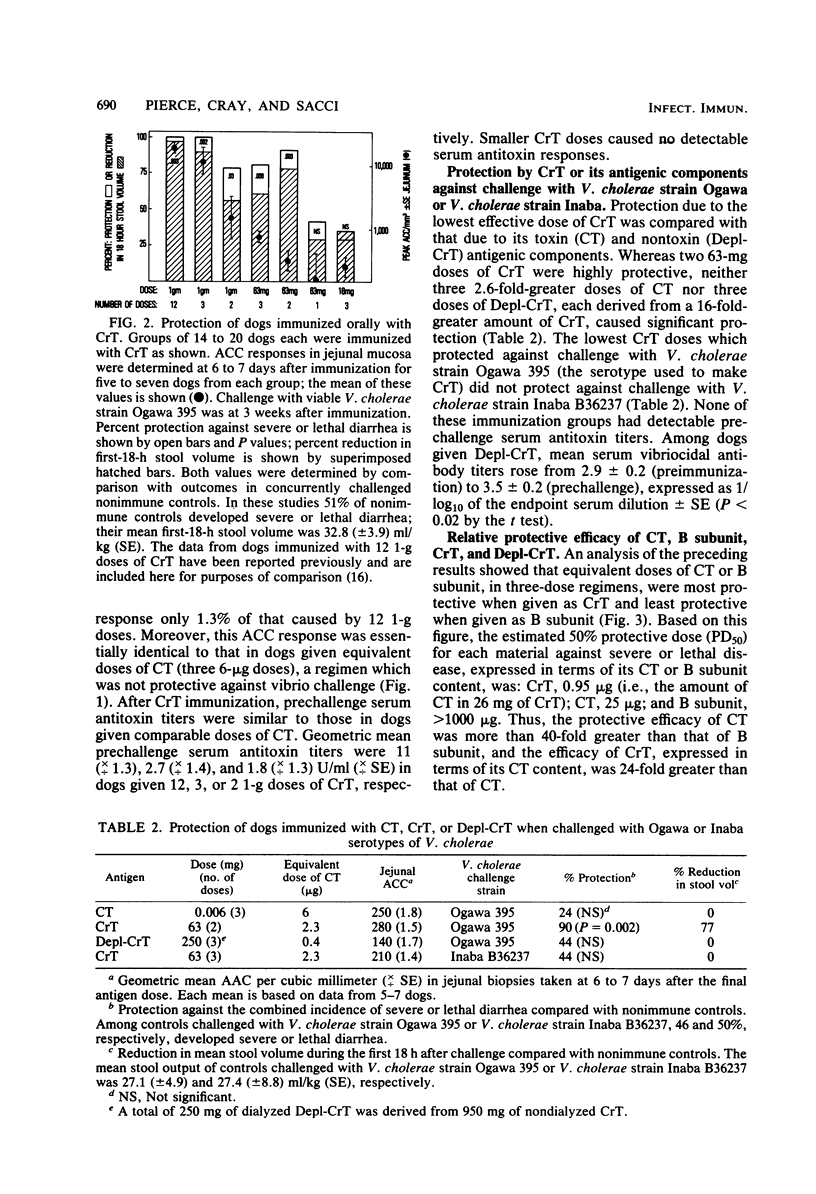
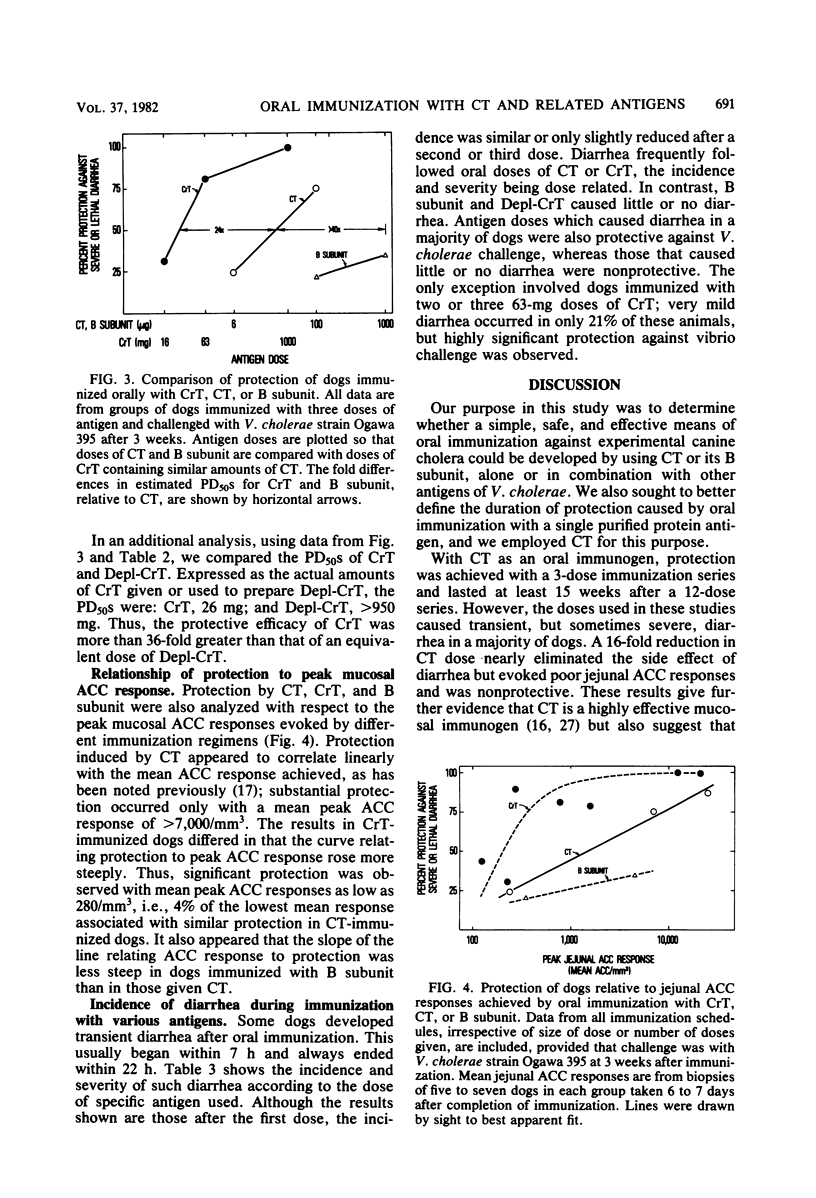
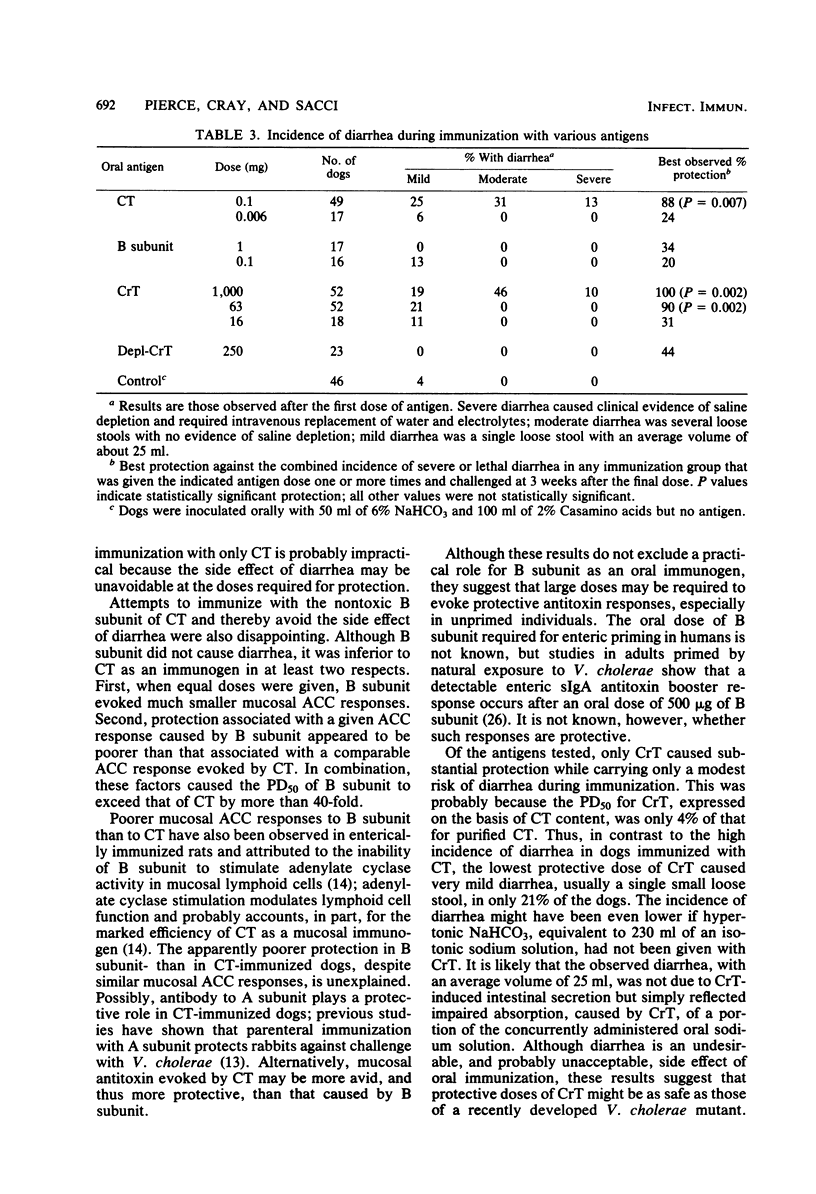
The immunogenicity and safety of purified cholera toxin (CT), its B subunit, and a crude culture filtrate of toxigenic Vibrio cholerae (CrT) were compared in dogs immunized orally and challenged with virulent V. cholerae. CT and CrT caused marked protection in two- or three-dose regimens. Protection due to CT occurred only with doses that caused transient, sometimes severe, diarrhea in most dogs; this protection was proportional to the peak antitoxin response in jejunal mucosa and lasted at least 15 weeks. In contrast, minimum protective doses of CrT contained much less cholera toxin, caused very mild diarrhea in only 21% of the dogs, and evoked protection that was greater than predicted from the modest jejunal antitoxin response. B subunit caused smaller jejunal antitoxin responses than did similar doses of CT and was poorly protective, the 50% protective dose being >40-fold greater than that of CT. Two observations indicated that protection due to CrT involved synergy between antibacterial and antitoxic immune responses. First, the 50% protective dose of CrT was 24-fold and >36-fold smaller than the 50% protective doses of its CT and non-CT antigenic components, respectively, when tested separately. Second, protection was greater in CrT-immunized dogs than in CT-immunized dogs for a given mucosal antitoxin response. Low doses of CrT evoked serotype-specific protection, indicating that the serotype-specific O somatic antigen contributed significatly to antibacterial protection. These results suggest that a simple, effective, nonliving oral vaccine for cholera based on combined antibacterial and antitoxic immunity can probably be achieved. However, further studies are needed to determine how a protective antitoxic response can be evoked without causing diarrhea during immunization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craig J. P. Preparation of the vascular permeability factor of Vibrio cholerae. J Bacteriol. 1966 Sep;92(3):793–795. doi: 10.1128/jb.92.3.793-795.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Fujita K., LoSpalluto J. J. Procholeragenoid: an aggregated intermediate in the formation of choleragenoid. J Immunol. 1971 Oct;107(4):1043–1051. [PubMed] [Google Scholar]
- Fuhrman J. A., Cebra J. J. Special features of the priming process for a secretory IgA response. B cell priming with cholera toxin. J Exp Med. 1981 Mar 1;153(3):534–544. doi: 10.1084/jem.153.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Ouchterlony O., Anderson A., Walletström G., Westerberg-Berndtsson U. Antitoxic immunity in experimental cholera: protection, and serum and local antibody responses in rabbits after enteral and parenteral immunization. Infect Immun. 1975 Dec;12(6):1331–1340. doi: 10.1128/iai.12.6.1331-1340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Finkelstein R. A. Selection and characteristics of a Vibrio cholerae mutant lacking the A (ADP-ribosylating) portion of the cholera enterotoxin. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2052–2056. doi: 10.1073/pnas.76.4.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Cisneros L., Nalin D. R., Young C. R. Duration of infection-derived immunity to cholera. J Infect Dis. 1981 Jun;143(6):818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- Mosley W. H., Aziz K. M., Rahman A. S., Chowdhury A. K., Ahmed A. Field trials of monovalent Ogawa and Inaba cholera vaccines in rural Bangladesh--three years of observation. Bull World Health Organ. 1973;49(4):381–387. [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W. Protection against experimental cholera by oral or parenteral immunization. Infect Immun. 1979 Nov;26(2):594–598. doi: 10.1128/iai.26.2.594-598.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W. Synergistic protection against experimental cholera by immunization with cholera toxoid and vaccine. Infect Immun. 1979 Nov;26(2):528–533. doi: 10.1128/iai.26.2.528-533.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Engel P. F. Antitoxic immunity to cholera in dogs immunized orally with cholera toxin. Infect Immun. 1980 Feb;27(2):632–637. doi: 10.1128/iai.27.2.632-637.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sircar B. K. Induction of a mucosal antitoxin response and its role in immunity to experimental canine cholera. Infect Immun. 1978 Jul;21(1):185–193. doi: 10.1128/iai.21.1.185-193.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Kaniecki E. A., Northrup R. S. Protection against experimental cholera by antitoxin. J Infect Dis. 1972 Dec;126(6):606–616. doi: 10.1093/infdis/126.6.606. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Koster F. T. Priming and suppression of the intestinal immune response to cholera toxoid/toxin by parenteral toxoid in rats. J Immunol. 1980 Jan;124(1):307–311. [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B., Sircar B. K. Immunity to experimental cholera. III. Enhanced duration of protection after sequential parenteral-oral administration of toxoid to dogs. J Infect Dis. 1977 Jun;135(6):888–896. doi: 10.1093/infdis/135.6.888. [DOI] [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B., Barua D., Saxena R., Carpenter C. C. Vibriocidal and agglutinating antibody patterns in cholera patients. J Infect Dis. 1966 Dec;116(5):630–640. doi: 10.1093/infdis/116.5.630. [DOI] [PubMed] [Google Scholar]
- Sack R. B., Carpenter C. C. Experimental canine cholera. I. Development of the model. J Infect Dis. 1969 Feb;119(2):138–149. doi: 10.1093/infdis/119.2.138. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J. Synergistic protective effect in rabbits of immunization with Vibrio cholerae lipopolysaccharide and toxin/toxoid. Infect Immun. 1976 Mar;13(3):735–740. doi: 10.1128/iai.13.3.735-740.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Lange S., Holmgren J. Intestinal immune response to cholera toxin: dependence on route and dosage of antigen for priming and boosting. Infect Immun. 1980 Nov;30(2):337–341. doi: 10.1128/iai.30.2.337-341.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Sack D. A., Holmgren J., Bardhan P. K. Intestinal antibody responses after immunisation with cholera B subunit. Lancet. 1982 Feb 6;1(8267):305–308. doi: 10.1016/s0140-6736(82)91568-9. [DOI] [PubMed] [Google Scholar]
- TOMASI T. B., Jr, TAN E. M., SOLOMON A., PRENDERGAST R. A. CHARACTERISTICS OF AN IMMUNE SYSTEM COMMON TO CERTAIN EXTERNAL SECRETIONS. J Exp Med. 1965 Jan 1;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley J. H., Keren D. F., Hamilton S. R., Brown G. D. Local (immunoglobulin A) immune response by the intestine to cholera toxin and its partial suppression with combined systemic and intra-intestinal immunization. Infect Immun. 1978 Feb;19(2):589–597. doi: 10.1128/iai.19.2.589-597.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



