Abstract
The ATP-binding cassette transport proteins (ABC transporters) represent important determinants of drug excretion. Protective or excretory tissues where these transporters mediate substrate efflux include the kidney proximal tubule. Regulation of the transport proteins in this tissue requires elaborate signaling pathways, including genetic, epigenetic, nuclear receptor mediated, posttranscriptional gene regulation involving microRNAs, and non-genomic (kinases) pathways triggered by hormones and/or growth factors. This review discusses current knowledge on regulatory pathways for ABC transporters in kidney proximal tubules, with a main focus on P-glycoprotein, multidrug resistance proteins 2 and 4, and breast cancer resistance protein. Insight in these processes is of importance because variations in transporter activity due to certain (disease) conditions could lead to significant changes in drug efficacy or toxicity.
Key words: breast cancer resistance protein, kidney disease, multidrug resistance protein, P-glycoprotein, transport regulation
INTRODUCTION
The kidneys largely contribute to the urinary excretion of drugs and metabolic waste products not only by filtering the blood from these potentially harmful compounds but also by contributing to their active tubular secretion. Therefore, the kidney proximal tubules possess a variety of transporters that cooperate in basolateral uptake and luminal secretion. Clinically, these xenobiotic transporters are often involved in significant interactions, which lead to unforeseen changes in drug plasma levels, uremia, and/or nephrotoxicity (1,2). Drugs may also interact with endogenous substrates and food components, leading to similar effects. In addition, the expression of transport systems and/or their function can be affected by disease circumstances. Under such conditions, xenobiotics and waste products produced can accumulate due to tubular dysfunction sometimes leading to a progression of kidney failure. Data obtained from animal models and human diseases showed that the expression level of drug transporters is tightly controlled as a key adaptation mechanism to such stress conditions (3). Insight in these mechanisms may provide therapeutic targets for clinical interventions in acute toxicity. In this issue of the AAPS Journal, the uptake transporters are described in other reviews, whereas here the function and regulation of an important class of efflux pumps belonging to the ATP-binding cassette (ABC) family of transporters are being discussed. The secretion of compounds into the urinary compartment via efflux transporters at the proximal tubule apical membrane is unidirectional and predominantly (but not exclusively) mediated by the ABC transporters.
The ABC protein family comprises a large number of transmembrane proteins expressed in almost all organisms ranging from prokaryotes to humans. ATP hydrolysis reveals the energy required for the translocation of substrates, for which each full transporter contains two cytoplasmic binding domains. The human ABC transporter family consists of 49 members divided in seven subfamilies ranging from A to G based on sequence and organization of the ABC domain(s) similarities. Members of the B, C, and G subfamilies have been associated with renal drug transport (Fig. 1) (1,2). Up to 20 ABC genes have, as yet, been linked to inherited diseases, including Tangier disease (ABCA1), sitosterolemia (ABCG5 or ABCG8), Dubin–Johnson syndrome (ABCC2), pseudoxanthoma elasticum (ABCC6), cystic fibrosis (ABCC7), and hyperinsulinemic hypoglycemia of infancy (ABCC8) (4). For disease modeling and/or drug target identification, ABC transport protein knockout animal models have offered considerable insights into the characteristics and function of the individual efflux pumps (see, e.g., (5,6)).
Fig. 1.
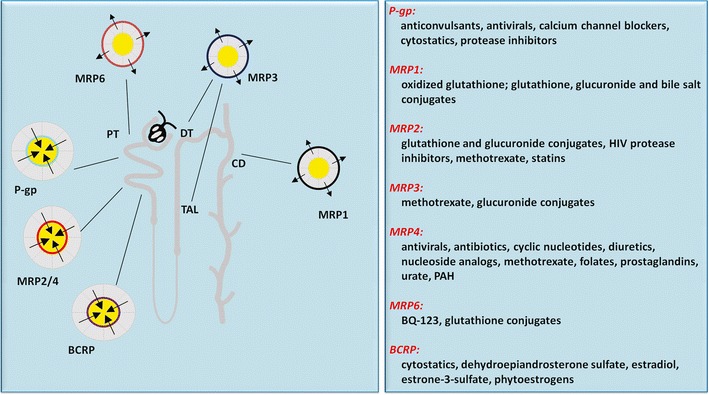
Localization of ABC transporters in the kidney and examples of their substrates. Note that P-gp, MRP2, MRP4, and BCRP are most important in renal drug handling and contribute to active tubular secretion and are localized at the proximal tubule (PT). Other nephron segments include thick ascending limb of Henle (TAL), distal tubule (DT), and collecting duct (CD). For details on specific substrates, the reader is referred to recently published reviews (2,7,8)
This review focuses on ABC proteins as xenobiotic efflux pumps in the kidney. These include P-glycoprotein (P-gp; ABCB1), the multidrug resistance (MRP)-associated protein family (MRP1–9, ABCC1–6, and ABCC10–12), and breast cancer resistance protein (BCRP; ABCG2). Moreover, the physiological and pathological regulatory pathways for P-gp, MRP2, MRP4, and BCRP, which are abundantly present in the contemplated tissue and mostly relevant for renal drug handling, are being discussed.
LOCALIZATION AND FUNCTION OF ABC TRANSPORTERS IN THE KIDNEY
P-glycoprotein
The best-characterized ABC transporter concerns P-gp, which was originally discovered for its over-expression in drug-resistant tumor cells. But later, the efflux pump was recognized as a crucial component of barrier tissues and a significant factor in drug disposition. In the kidney, expression of P-gp is confined to the proximal tubule apical membrane (9), a localization in agreement with a urinary efflux function. The resolved X-ray structure of mouse P-gp allows homology modeling by which insight into substrate handling can be obtained (10). P-gp has a preference for uncharged and cationic compounds. P-gp confers resistance not only to chemotherapeutics but also to a large number of other clinically and toxicologically relevant compounds (11). The problem of multidrug resistance is clinically counteracted by blocking P-gp function with compounds usually acting as competitive inhibitors (see for overview (11,12)). More specific substrates were identified using P-gp-deficient mice, the Mdr1a/1b(−/−) mice. Unlike humans, rodents contain two genes encoding for the efflux pump, Mdr1a and Mdr1b. Although no obvious phenotype, except for a larger susceptibility to neurotoxic drugs, was discovered in these mice at first sight (5), careful characterization revealed altered gastrointestinal and renal phenotypes. Spontaneous development of colitis and signs of severe chronic inflammation of the gut have been reported for Mdr1a-deficient mice when kept under specific pathogen-free conditions. Presumably, this was caused by defects in the epithelial cells lining the gut (13). More recently, a comparable phenotype in humans, associated with ABCB1 gene variants, was reported (14).
In the kidney, P-gp was reported to be inducible. After transplantation, an increased P-gp expression in renal tubule apical membrane was observed in patients treated with the immunosuppressive calcineurin inhibitor, cyclosporine A. However, patients with a 3,435 C−>T ABCB1 variant showed a higher sensitivity to nephrotoxicity associated with cyclosporine A therapy, possibly due to reduced apical expression of P-gp (15,16). Such mutations do not always lead to renal function problems but can have systemic effects as well. Recently, an association between ABCB1 polymorphisms and the resistance against steroid treatment of nephrotic syndrome in children was found (17), suggesting a gain of function in P-gp. In addition, we showed that the P-gp-deficient mice (Mdr1a/1b(−/−)) exhibit a generalized disturbed renal tubular function caused by decreased intracellular levels of ATP and altered morphology of mitochondria (18). This proximal tubular dysfunction showed prominent resemblances with renal Fanconi syndrome, with a diminished reabsorption of amino acids and low molecular weight proteins. In a further attempt to study the role of the efflux pump in tubular dysfunction, we investigated its role in the autosomal recessive disorder, cystinosis (19), which is associated with the development of renal Fanconi syndrome. We used conditionally immortalized proximal tubular epithelial cells obtained from urine from cystinotic patients and healthy volunteers. No differences in the functional expression of P-gp was found in cystinotic cells as compared with controls; however, inhibition of P-gp activity in control or cystinotic proximal tubular cells resulted in decreased uptake of phosphate, in agreement with the phenotype of P-gp-deficient mice and with that of patients with cystinosis (20).
Multidrug Resistance Proteins
Out of the nine MRP protein (ABCC) members, MRP2 and MRP4 are the most important drug efflux pumps in the kidney. Details on the function and role of MRPs can be found in previous reviews from our group and others (e.g., (2,7,21)). The MRPs mediate the transport of a wide variety of preferentially, but not exclusively, organic anions, including phase II metabolism products (2,7). MRP1 (ABCC1) is not expressed in the kidney proximal tubule but localizes to the basolateral membranes of the limb of Henle cells and the distal and collecting duct tubule cells (21), where it possibly protects against accumulating toxic compounds that can reach high concentrations due to hormone-controlled water reabsorption. In support, Abcc1 knockout mice developed polyuria and acquired nephrogenic diabetes insipidus when treated with etoposide (22).
There is an overlapping substrate specificity of MRP2 with that of MRP1 and MRP3, consistent with the high amino acid homology between these MRPs. Patients with Dubin–Johnson syndrome have a defect in MRP2, characterized by an impaired canalicular secretion of glutathione, glutathione conjugates, and bilirubin glucuronides. Two rat strains, the Groningen yellow/transport-deficient (GY/TR−) Wistar rat and the Esai hyperbilirubinaemic Sprague–Dawley rat (EHBR), are presented with a similar phenotype due to MRP2 deficiency (as reviewed in (21)). Also, the renal efflux of some larger MRP2 substrates was found to be impaired in the GY/TR− rat (23).
MRP3 (ABCC3) is expressed to a minor extent in the distal convoluted tubule basolateral membranes and, probably, cells lining the thick ascending loop of Henle (see for details (21)). In contrast, rat kidneys express Mrp3 in the basolateral membrane of proximal tubule cells. The efflux pump was found to be upregulated in hepatocytes of patients with the Dubin–Johnson syndrome, in agreement with MRP3 expression in EHBR rats that showed increased levels in the kidney as well. These findings suggest that MRP3 may function as a compensatory mechanism in MRP2 deficiency to reduce injury from accumulating toxic components, such as bilirubin glucuronide.
MRP4 shows a high expression in the kidney, where the efflux pump is expressed in the proximal tubule apical membrane (24). The wide range of drug substrates includes antibiotics, antivirals, and diuretics (8). In comparison to MRP2, MRP4 exhibits a higher expression in the kidney and a higher affinity for smaller organic anions (25). Polymorphisms in ABCC2 and ABCC4, leading to diminished MRP2 or MRP4 activity, respectively, are associated with increased sensitivity to nephrotoxicity or delayed graft function after kidney transplantation (26,27).
MRP5 (ABCC5) is ubiquitously expressed and located at the basolateral membrane in polarized epithelial cells (as reviewed in (21)). Like MRP4, MRP5 transports nucleoside and nucleotide drugs. The expression and localization of MRP5 along the nephron has yet to be determined.
MRP6 (ABCC6) is highly expressed in kidney and liver as compared with other tissues (as reviewed in (21)). In human and mouse kidney, the efflux pump is found in the cells lining the proximal tubules where the protein is localized to the basolateral plasma membrane and, to a lesser extent, in distal tubule cells of mouse. ABCC6 gene mutations or deletions were reported in patients suffering from pseudoxanthoma elasticum, a rare inherited connective tissue disease. Vitamin K was suggested as MRP6 substrate and essential element in this disease (28), but this hypothesis was recently rejected (29). Therefore, the physiological substrates of MRP6 potentially involved in pseudoxanthoma elasticum still need to be identified.
Although expression of MRP7 (ABCC10) seems low in human tissues (as reviewed in (21)), a recent study showed that polymorphisms in ABCC10 are associated with tenofovir-induced renal tubular toxicity (30). This suggests renal tubular apical membrane expression, which need to be proven. The localization and function (if any) of MRP8 (ABCC11) and MRP9 (ABCC12) in the kidney, also need to be confirmed.
BCRP
BCRP (ABCG2) was found to be localized at the apical surface of human proximal tubule cells (31). Gene expression analysis revealed also renal expression of Abcg1, Abcg5, and Abcg8, associated with the cholesterol transport (32); however, BCRP is the only member of the subfamily G proven to be involved in drug transport and not in cholesterol efflux. Hence, the activity of the efflux pump may be influenced by cholesterol through the plasma membrane lipids composition and a physical interaction between caveolin-1 and BCRP (33). G subfamily members are half-transporters, which need to homo- or heterodimerize to become a fully active efflux pump. A vast array of BCRP substrates have been identified, including small and large organic anions, uncharged or amphiphilic compounds. Abcg2 knockout mice showed lethal phototoxicity when subjected to an alfalfa-containing diet. This was attributed to pheophorbide A retention, indicating that BCRP excretes porphyrins (34). Besides, BCRP plays a role in the transport of or interactions with androgens, estrogens, and phytoestrogens (35).
A single nucleotide polymorphism (SNP) in ABCG2 was reported to be associated with increased systemic urate concentrations due to reduced urinary urate transport (36). Hyperuricemia coincides with features of gout, metabolic syndrome, and with cardiovascular and renal disease, which often results from urate-induced inflammatory and oxidative damage. In contrast, elevated plasma urate levels may be protective by the free radicals scavenging properties of the nitrogenous end product. For maintaining plasma urate at physiological levels, proper function of multiple renal transport proteins, including BCRP and MRP4, is a prerequisite (3,37). Often, SNPs result in a loss of function through affecting protein stability or by alterations in targeting the protein towards the plasma membrane. In contrast, also SNPs have been reported that resulted in a gain of function (38). With respect to ABC transporters in renal proximal tubule, the latter most likely contributes to reduced risk of tubulotoxicity but can have important pharmacodynamic effects.
ABC TRANSPORTER REGULATION IN KIDNEY PROXIMAL TUBULES
Clearly, distinct pathways are concerned with the regulation of ABC transporters, comprising genomic (genetic, epigenetic, and nuclear receptor mediated) pathways and non-genomic (kinases and the new emerging field of posttranscriptional target repression by microRNAs) regulation, triggered by hormones, growth factors, and exogenous factors, for which the time span of exposure and the stressor determine the effect. Additionally, the expression levels of transport proteins and their function can be altered by disease conditions, including renal or liver failure and inflammation. Although information on ABC transporter regulation in the kidney is sparse, the regulatory pathways revealed are discussed in more detail in the following sections, details of which can be found in Table I. An overview of potential mechanisms involved in genomic and non-genomic pathways in ABC transport protein regulation is presented in Fig. 2.
Table I.
Regulation of Expression and Transport Activity of the Renal ABC Transporters Present in Renal Proximal Tubules
| Treatment/compound | Mechanism of regulation | Transporter | Effect | Species | Tissue/cell type | References |
|---|---|---|---|---|---|---|
| Hormones | ||||||
| Steroid hormones | ||||||
| 5a-dihydroxytestosterone | Suppression of gene | Mrp4 (mRNA) | Inhibition | Mouse (males) | Kidney tissue | (41) |
| Estradiol | Suppression of gene | Bcrp (mRNA) | Inhibition | Mouse | Kidney tissue | (52) |
| Estradiol-17-β-d-glucuronide | Decreased protein biosynthesis and maturation | BCRP | Inhibition | Human transporter in different cell lines | LLC-PK1-BCRP | (53) |
| Corticosteroids/dexamethasone | Activation of glucocorticoid receptor, PXR, or other mechanisms | Mrp2 (protein) | Induction | Rat | Kidney tissue | (54) |
| Activation of glucocorticoid receptor, cMet tyrosine kinase, and downstream ERK1/2 | Mrp2 (activity) | Non-genomic increase | Killifish | Isolated proximal tubule | (46) | |
| P-gp | Protein inhibition | Rat | Kidney tissue | (54) | ||
| ET-1 | ||||||
| Aminoglycoside antibiotics, heavy metal salts, and radiocontrast agents | Activation ET-1 signaling pathway (ET-1 release, NO production by NOS, guanylyl cyclase activation, cGMP generation, and PKC activation) | MRP2 (protein and activity) | Inhibition (short term) and induction (long term) | Killifish/human transporter in canine cells | Isolated proximal tubule MDCKII + hMRP2 | (50,55,56) |
| Gentamicin | Activation ET-1 signaling pathway | P-gp (activity) | Inhibition (short-term) | Killifish | Isolated proximal tubule | (56) |
| Epidermal growth factor | Activation | BCRP | Stimulation protein expression | Human | LLC-PK1-BCRP | (57) |
| PTH | ||||||
| PTH and PTH-related protein | Activation through PKC signaling and reversal by stanniocalcin | Mrp2 (protein and activity) | Inhibition (short term) | Killifish | Isolated proximal tubule | (58) |
| Protein kinases | ||||||
| PKC | ||||||
| PMA | Activation of PKC | Mrp2 (protein and activity) | Inhibition (short term) | Killifish | Isolated proximal tubule | (56) |
| PKG | ||||||
| Guanylyl cyclase generation and cGMP activation (ET-1 signaling) | Activation of PKG | Mrp2 (protein and activity) | Inhibition (short term) | Killifish | Isolated proximal tubule | (50) |
| Nuclear receptors | ||||||
| PXR/SXR (NR1I2) | ||||||
| Rifampicin, clotrimazole, PCN, dexamethasone, and spironolactone | Activation of PXR | Mrp1 (mRNA and protein) | mRNA stimulation and protein no change | Porcine | LLC-PK1 cells | (59) |
| Activation of PXR | Mrp2 (mRNA and protein) | Stimulation or no effect | Porcine mouse | LLC-PK1 cells | (60,59) | |
| Activation of PXR | Mrp4 (mRNA and protein) | Inhibition | Mouse | Kidney tissue | (60) | |
| CAR (NR1I3) | ||||||
| Phenobarbital, diallyl sulfide, PCB 99, and TCPOBOP | Activation of CAR | Mrp2 (mRNA and protein) | Stimulation | Mouse/rat | Kidney tissue | (60) |
| MRP4 (mRNA) | Stimulation | Mouse | Kidney tissue | (60) | ||
| Disease conditions | ||||||
| Ischemia | ||||||
| Renal clamp | Adaptive response | P-gp | Stimulation | Mouse | Kidney tissue | (32) |
| Adaptive response | Bcrp | Stimulation | Mouse | Kidney tissue | (32) | |
| Adaptive response | Mrp4 | Downregulation | Mouse | Kidney tissue | (32) | |
| Inflammation | ||||||
| LPS | Adaptive response, probably via cytokine release and iNOS | Mrp2 (protein) | Stimulation | Rat | Kidney tissue | (51) |
| Adaptive response, probably iNOS and TNF alpha | P-gp (protein and activity) | Stimulation | Rat | Kidney tissue/rat proximal tubule cell line | (51,61) | |
| TNF alpha | Adaptive response via NF-kB activation | P-gp | Protein stimulation | Rat | Rat proximal tubule cell line | (51,61) |
| Toxicity | ||||||
| Cadmium | Adaptative response via NF-kB activation | P-gp | Human | Proximal tubule cells | (62) | |
| Cisplatin | Adaptative response via Nrf-2 activation | Mrp2 (mRNA and protein), MRP4 (mRNA and protein), and P-gp (mRNA and protein) | Increased expression | Mouse | Kidney tissue | (63,64) |
| Chronic renal failure | ||||||
| Subtotal nephrectomy | Adaptative response to circulating toxins | Mrp2 (mRNA and protein) | Stimulation | Rat | Kidney tissue | (65) |
| Liver failure | ||||||
| Bile duct ligation | Adaptive response | Mrp1 (protein) | Stimulation | Rat | Kidney tissue | (66) |
| Adaptive response | Mrp2 (mRNA, protein, and activity) | Stimulation | Rat | Kidney tissue | (67) | |
| Adaptive response and posttranscriptional regulation | Mrp4 (mRNA and protein) | Protein stimulation and mRNA no change | Rat/mouse | Kidney tissue | (68,69) | |
| Cholestasis | Adaptive response | Mrp2 (mRNA and protein) | Stimulation | Mouse | Kidney tissue | (70) |
BCRP/Bcrp breast cancer resistance protein, ET-1 endothelin-1, MRP2/Mrp2 multidrug resistance protein 2, MRP4/Mrp4 multidrug resistance protein 4, P-gp P-glycoprotein, PXR pregnane xenobiotic receptor, NO nitric oxide, NOS nitric oxide synthase, PTH parathyroid hormone, PMA phorbol myristate acetate, cGMP yclic guanosine monophosphate, PKC protein kinase C, PKG protein kinase C, SXR sensing nuclear receptor, PCN pregnenolone 16 α-carbonitrile, CAR constitutive androstane receptor, PCB polychlorinated biphenyl, LPS lipopolysaccharide, TNF tumor necrosis, iNOS inducible nitric oxide synthase, NF-kB nuclear factor-kappaB
Fig. 2.
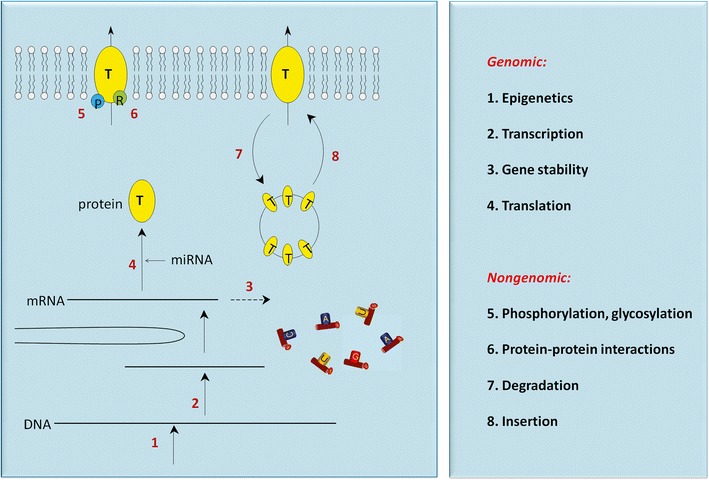
Regulation of ABC transporters in renal proximal tubule. Cellular processes in response to diverse signals resulting in ABC transport protein (T) modulation. These include epigenetic changes of DNA (1), modulation of transcription (2), stability of mRNA (3), translation of mRNA into ABC proteins (4), phosphorylation of the transporters by protein kinases (5), or allosteric control by regulatory proteins (6). Transporter abundancy in the membrane can also be regulated by modulating retrieval (7) or insertion (8) machineries for the ABC proteins. Scheme is adapted from (71)
Hormonal Regulation of ABC Transporters
Steroid Hormones
A few studies reported gender differences with respect to ABC transporter abundancy, suggesting a role for steroid hormones in their regulation. Abcb1b, but not Abcb1a, gene expression is somewhat higher in females than in male mice kidneys (39). This was confirmed for P-gp in rat livers, but not in kidneys, and after testosterone treatment P-gp levels in females reduced to male rat liver levels (40). The same study showed a similar effect on Mrp2 in rat livers, whereas Abcc2 gene expression levels were comparable in female and male kidney (39). For Mrp4, female predominant appearance is observed in murine kidney and liver (41). This contradicts to findings for rat where Mrp4 is more abundantly present in male than in female kidneys (42). Gonadectomy resulted in equal Mrp4 levels in male kidneys as compared with females (41), suggestive of a downregulation of Mrp4 by androgens. In contrast, androgens induced the abundance of MRP4 in prostate cancer cells (43). Whether this is related to interorgan and/or interspecies differences needs to be defined. BCRP expression was found to be sex specific as well (44) and its activity is influenced by sex steroids, suggesting a role for the efflux pump in steroid metabolism (45). We could recently support this hypothesis and showed presence and localization of BCRP in various steroid hormone producing organs together with potent inhibitory effects on BCRP activity by estradiol, progesterone, testosterone, and androstenedione (35).
Dexamethasone is often used as a ligand for nuclear hormone receptors, including pregnane xenobiotic receptor (PXR; NR1I2) and constitutive androstane receptor (CAR; NR1I3), which are discussed in the next section. However, the synthetic hormone can also act through the glucocorticoid receptor without activating transcription factors. Glucocorticoid signaling to Mrp2 was recently demonstrated in killifish renal proximal tubules through a rapid increase in Mrp2-mediated transport after dexamethasone exposure (46). Cortisol and triamcinolone acetonide exerted a similar effect, whereas cortisone, an inactive cortisol metabolite, was without effect. The glucocorticoid receptor mediated action was regulated through a non-genomic mechanism, because protein synthesis inhibition did not abolish the dexamethasone effect and neither did immunohistochemistry reveal any alterations in Mrp2 luminal membrane expression. This non-genomic action of dexamethasone corresponds to results from rat kidney where the glucocorticoid showed renoprotective effects against ischemia reperfusion injury (47). Furthermore, dexamethasone signaled to Mrp2 through c-Met, a receptor tyrosine kinase and its downstream effector pathway, MEK1/2, a protein ser/thr kinase. Blocking the receptor/ligand interaction and/or inhibition of tyrosine kinase activity are strategies for novel targeted therapies in oncology. The native ligand for c-Met, hepatocyte growth factor (HGF), showed identical effects, suggesting endogenous HGF is released upon dexamethasone treatment. HGF displays a complex program of proliferation and ontogenesis in a number of different cell types, including kidney. Moreover, HGF is involved in several in vivo and in vitro tumor cell invasions. Perturbation of HGF/c-Met signaling axis leads to enhanced signaling that occurs in a wide range of human cancers, and elevated c-Met signals are characteristic of aggressive tumors with poor prognosis (48). Inhibition of Mrp2 by tyrosine kinase inhibitors could aid in cancer therapy, because the efflux pump may contribute to drug resistance in chemotherapy (7). On the other hand, chemicals that influence the tyrosine kinase pathway, such as tyrosine kinase inhibitors that block c-Met, may increase the sensitivity to xenobiotics and lead to reduced renal function associated with tyrosine kinase inhibitor treatment (49). c-Met acts through the MEK1/2 signaling cascade, which in turn may phosphorylate and, thereby, activate ERK. It can be speculated that the increase in Mrp2 function caused by glucocorticoids and activating MEK1/2 and ERK1/2 signaling is an essential way by which renal cells cope with accumulating waste products of metabolism during stress.
Endothelin-1 and Calciotropic Hormones
An autocrine signaling pathway involving the vasoactive hormone, endothelin-1, that regulates P-gp and Mrp2 in response to several nephrotoxic agents in renal proximal tubules, was previously demonstrated (50,51). The signaling pathway was discovered originally in lower vertebrate, killifish renal tubules, but later demonstrated in rats in vivo and in MDCKII cells over-expressing human MRP2. Exposing killifish tubules to radiocontrast agents, aminoglycoside antibiotics, and certain heavy metals caused the following sequence of events: (1) release of endothelin (ET) to the medium, (2) activation of a basolateral ETB receptor, (3) activation of nitric oxide synthase (NOS), and generation of nitric oxide (NO), (4) activation of protein kinase C (PKC), and (5) reduction in P-gp- and Mrp2-mediated transport. Signaling appeared to be Ca2+ dependent, because ET release was blocked by the Ca2+-channel blocker, nifedipine, and it was activated (in the absence of nephrotoxicants) by elevated medium Ca2+. The calciotropic hormones, parathyroid hormone, PTH-related protein, and stanniocalcin, were also found to interfere with ET-regulated Mrp2 activity through PKC and cGMP signaling (58). Conversely, the results also pointed towards Ca2+-independent activation of NOS, suggesting involvement of iNOS that could be confirmed in rat studies (51). In the long-term, however, luminal Mrp2 and P-gp activities and protein expression are increased 24 h after transient exposure to ET-1 or nephrotoxicants. Blocking the efflux pumps resulted in an enhancement of cell damage and necrosis, indicating that their upregulation during toxic stress is a prerequisite for survival (51). An upregulation generally results from induced de novo synthesis of the transporter proteins or posttranscriptional regulation, which involves receptors and signaling pathways that alter their function (55,72). In support of a transcriptional regulation, increased gene expression levels of Abcb1b and Abcc2 were found in the kidney after nephrotoxic injury or in chronic renal failure (51,63,65).
Nuclear Factorial Regulation of ABC Transporters Expressed in Kidney
Nuclear hormone receptor superfamily members are vital regulators of the adaptive response in physiological alterations. Some members of this superfamily of transcriptional regulators are ligand inducible and triggered by either xenobiotics or endogenous, most often lipophilic, substances. Steroid hormones are natural ligands and act by binding DNA as a homodimer. Differences in transporter expression and its function between males and females are explained by binding of circulating sex hormones to nuclear steroid hormone receptors (see “Steroid Hormones”). Other nuclear receptor superfamily members bind DNA as heterodimers with retinoid X receptor alpha (RXRα; NR2B1), such as the farnesoid X receptor (FXR; NR1H4), PXR, CAR, and the peroxisome proliferator-activated receptor alpha (PPARα; NR1C1). In mammalian liver, P-gp and Mrp2 expressions are induced through activation of PXR and CAR (60). Although Mrp2 expression in the kidney can be regulated by PXR, the expression levels of this nuclear receptor in kidney appears to be low but with clear species differences (46). Treatment of rats with PXR ligands decreased the expression of Mrp4 in the kidney, suggesting a negative regulation of this efflux pump by PXR (42). No clear evidence was found for PXR, CAR, or FXR in regulating BCRP; neither was a role for PPARα found in regulating ABC transporters expressed in kidney.
The aryl hydrocarbon receptor (AhR) might be important in regulating MRP4 and BCRP transporters in the kidney (73,74). Binding of specific ligands to the AhR results in the formation of a heterodimer complex with the AhR nuclear translocator, which, in turn, binds to dioxin responsive element sequences located in the promoter regions of target genes, hence, regulating transcription (75). Activators of the AhR are diverse and include environmental contaminants (e.g., dioxins and polycyclic aromatic hydrocarbons), nutrients (e.g., flavanoids), endogenous metabolites (kynurenic acid and indoxyl sulphate), and drugs (statins). Just recently, AhR agonists have also been used in killifish renal proximal tubule to regulate protein expression of ABC transporters in kidney (76); however, information on AhR-mediated regulation of drug transporters in human kidney is as yet still absent. Species differences, such as reported for AhR regulation in rodent kidneys compared with humans (77), limit the ability to translate the findings across mammals, including humans. For instance the AhR-mediated BCRP regulation pathway in human intestinal cell lines could not be demonstrated in mouse cell lines (78).
Recent studies by Aleksunes et al. (64) revealed involvement of the nuclear factor E2-related factor 2 (Nrf2) in regulating Mrp2 and Mrp4 in cisplatin-induced nephrotoxicity. This pathway is activated in conditions of oxidative stress and is presumably the most important route to promote cell survival in stress conditions. Inhibitor of Nrf2 (INrf2), also called Kelch-like ECH-associated protein 1 (Keap1), links Nrf2 to the ubiquitin ligase, Cul3-Rbx1, complex that ubiquitinates, and degrades Nrf2. Physiologically, cytosolic INrf2/Cul3-Rbx1 is continuously degrading Nrf2, but during stress conditions Nrf2 translocates to the nucleus after dissociating from INrf2. Subsequently, Nrf2 binds to the antioxidant response element and activates a number of defensive genes, such as NAD(P)H/quinone oxidoreductase 1, glutathione-S-transferases (GST), heme oxygenase 1, ubiquitination enzymes, and drug transporters. A cross-reaction between Nrf2- and AhR-mediated signal transduction in regulating phase II enzymes have been reported (79), but whether a similar cross-talk between the transcription factors exist in regulating ABC transporters remain to be elucidated.
Regulation of ABC Transporters in Kidney Failure
Acute Kidney Injury
Acute kidney injury (AKI) is one of the most frequently occurring clinical problems concerning the kidney, which is associated with a high morbidity and mortality, and complicates 5% of all hospital admissions. The acute loss of kidney function is often a result of renal tubular necrosis. Differential expression of ABC transporters in the kidney was detected during pathological circumstances, such as nephrotoxicity or ischemic injury. Cadmium-, cisplatin-, gentamicin-, and endotoxin-mediated renal damage are associated with elevated levels of P-gp, Mrp2, and Mrp4 (51,72,63,62). In most drug-induced toxicities, oxidative stress pathways are involved as described under Nuclear factorial regulation of ABC transporters expressed in kidney.
Hartmann et al. (80) demonstrated increased urinary excretion of doxorubicin during endotoxemia in mice due to increased renal P-gp expression levels. The role of NO as an intermediate in the signaling pathways of P-gp (and also Mrp2) was derived from the endothelin-triggered pathways discovered in lower vertebrates. We suggested that generation of NO is a result of an induction in iNOS, which is under physiological conditions only weakly expressed in kidney proximal tubules. However, a remarkable induction in iNOS expression occurs in inflammatory conditions, e.g., in sepsis, endotoxemia, or in hypoxic renal injury (81). NO upregulates P-gp during endotoxemic AKI in rats, through an NF-kB-dependent pathway (61). Moreover, TNF-alpha-mediated upregulation in P-gp involved TLR4 activation and NF-kB translocation, which apparently is independent of NO. This indicates that at least two pathways are involved in the regulation of renal P-gp expression during endotoxemia. Whether similar regulatory pathways take place in renal MRP2 activity remains to be determined.
The effect of NO on renal function was investigated further in healthy human volunteers during endotoxemia (82). iNOS gene expression was highly induced after endotoxin administration accompanied by a marked increase in urinary NO metabolites excretion, which correlated strongly with the excretion of GST A1-1, a renal tubule injury marker. Blocking iNOS through aminoguanidine co-administration attenuated these effects, indicating that renal upregulation of iNOS and NO production result in nitrosative stress and renal injury. Whether drug transporters are functionally altered in human kidneys in inflammatory conditions needs to be defined.
Disturbances of homeostasis can induce coordinated changes of transporter expressions in remote organs as well. In liver injury, due to inflammation or ischemia-reperfusion injury, P-gp and Mrp2 expression levels were found to be reduced, whereas their expression levels in the kidney were upregulated (51,83). This adaptive interorgan response is possibly a compensatory mechanism to excrete detrimental waste products or stress factors during processes associated with liver failure. The differences in ABC transporter expressions between kidney and liver are likely to be related to variations in regulatory mechanisms. Sepsis-induced AKI for example, may cause clinical problems with drugs that depend on kidney function for their elimination and potentially are highly bound to plasma proteins. For some medicines, drug dosage adjustment will become mandatory in case of severe renal failure.
Chronic Renal Failure
In rats with chronic kidney disease (CKD), an induction in the expression of Mrp2 was found in the kidney, whereas no changes in expression levels of P-gp were found (65). During CKD, human metabolism is highly disturbed leading to a progressive accumulation of metabolites that normally are excreted by the kidneys. Accumulation of predominantly small, protein-bound, organic molecules generates toxic concentrations of some of these so-called uremic toxins (84). These biologically active uremic retention solutes fully depend on residual renal function for their elimination through secretion (85). The basolateral uptake of uremic toxins in renal proximal tubule cells has been studied more intensely (3), however, far less is known about their urinary efflux for which MRP4 and BCRP may be important transporters. Both are involved in urinary secretion of urate (36,86), a uremic toxin responsible for the pathogenesis of gout and cardiovascular disease. The interaction of the uremic retention solutes hippuric acid, indoxyl sulfate and kynurenic acid with MRP4 and BCRP, and of indole-3-acetic acid and phenylacetic acid with MRP4 only was recently demonstrated (87). The inhibition of transport by the uremic toxins occurred at clinically relevant concentrations, suggesting that this mechanism may contribute to the progression and the many complications of CKD. Targeting efflux pumps through regulatory pathways in renal failure by increasing their abundance may be a challenging approach to limit the progression.
ABC Transporters in Kidney Regeneration
Kidney tissue has a natural ability to recover and regenerate after acute damage despite the high susceptibility of the proximal tubule epithelium. Drug transporters might play a role in repair by controlling proliferation, migration and morphogenesis. As reviewed in (12), ABC transport proteins were found to be upregulated in regeneration of diverse tissues, including liver, heart and skeletal muscle tissues, and actively protect cells via drug-independent mechanisms. In mice kidney tissue, a clear upregulation of P-gp and Bcrp was found after injury by ABC transporter expression profiling, suggesting a role for the efflux pumps in repair of damaged nephron segments (32). Both transporters are not only present in epithelia but also highly expressed on a population of primitive bone marrow-derived stem cells with long-term repopulating capacities, termed side population (SP) cells. When expression of P-gp and BCRP is reduced the cell undergoes a phenotypic change, usually towards a more differentiated cell. This indicates that P-gp and BCRP possibly determine tissue regeneration through their differential expression on SP cells, which have been implicated in the remodeling of various tissues and organs, such as skeletal muscle, liver, and heart (12). Contraditory reports on the involvement of SP cells in renal regeneration have been published, leaving a conclusive answer still open (88). A bone marrow reconstitution of wild-type, P-gp- or Bcrp-deficient mice origin in wild-type mice subjected to irradiation to destroy endogenous bone marrow, showed that transporter-deficient bone marrow transplantations protected wild type mice against ischemia-induced renal injury (18,88). This protection was likely a result of bone marrow-derived cells, because significantly more bone marrow-derived cells were detected in kidneys grafted with transporter-deficient bone marrow. Cell fusion of resident tubular cells with bone marrow cells was unlikely to have occurred, as shown by a gender mismatch study. It is likely that the protective mechanism resulted from an altered immune reaction, since bone marrow from P-gp- or Bcrp-deficient mice contained significantly more monocytes and granulocytes as well as early outgrowth endothelial progenitor cells, (88). As these findings in ABC transporter knockout mice contradict the findings in humans with polymorphisms that lead to reduced or nonfunctional efflux pump variants, more research is essential to fully understand the renal protective mechanism in P-gp- or Bcrp-deficiency.
CONCLUSIONS AND PERSPECTIVES
Information on ABC transporter regulation in the kidney is sparse but insight into the processes regulating P-gp, the MRPs and BCRP is of relevance to reduce nephrotoxicity often associated with drug therapy. Especially during kidney failure, a high demand is put on renal tubule cells to eliminate accumulating products, as incomplete clearance of drugs and other potentially toxic agents, including products from normal metabolism/uremic toxins, leads to enhanced nephrotoxicity and systemic problems. Targeting regulatory pathways as therapeutic strategy to enhance renal xenobiotic excretion and reduce accumulation offers a nephroprotective mechanism relevant in conditions of reduced renal function.
The assembly of the efflux transporters is influenced by the ubiquitin-proteasome pathway. Modulation of endosomal retrieval or ubiquitination can regulate the change in the degradation rate of apical-surface-resident ABC transporters, thereby inducing their cell-surface expression and increasing their activity (89–92).
Furthermore, epigenetic mechanisms, such as DNA hypermethylation and histone modifications, may influence diverse cellular processes through gene silencing, and are a characteristic of cancer. Transporter activities may be influenced by these processes (93), although nothing has been reported on epigenetic changes of ABC transporters expressed in the kidney.
Finally, a new emerging field in the regulation of not only transporter genes but also drug metabolizing enzymes and nuclear receptors, comprises the microRNAs, a family of short noncoding RNAs involved in the negative regulation of gene expression at the post-transcriptional level (94). MicroRNAs can induce mRNA degradation by binding to the 3′-untranslated region of the target gene or result in repression of protein translation. A few hundred microRNAs have been identified in various organisms, and it is estimated that 50% of the protein-coding genes is regulated through these microRNAs, with critical roles in the kidney (95). Although no specific examples have been reported for the regulation of ABC transporters in the kidney, microRNAs will undoubtedly have a major impact on the renal drug transporter field. In other tissues, regulation of P-gp, MRP2, MRP4, and BCRP by microRNAs, directly or indirectly through nuclear receptors or factors (including AhR, Nrf2, RXR, and PPARs), was convincingly demonstrated (94,96,97). MicroRNAs occasionally cause histone modification or DNA methylation of promoter sites as well, thereby affecting the target genes expression levels. The microRNAs are important in kidney development and regulate physiological processes and may also play a role in the pathogenesis of kidney diseases, such as drug-induced AKI and CKD (98). In addition, microRNAs can serve a role as biomarkers in kidney disease (99). Modulators of microRNAs, such as antagomirs, a novel class of chemically engineered oligonucleotides, have been developed to enable specific targeting, which influences the mechanisms that underlie disease initiation or progression (100). Clinical applications of this innovative approach are promising, including targeting kidney diseases but caution should be taken as microRNAs or their inhibition can become oncogenic.
References
- 1.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masereeuw R, Russel FG. Therapeutic implications of renal anionic drug transporters. Pharmacol Ther. 2010;126:200–216. doi: 10.1016/j.pharmthera.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Wu W, Dnyanmote AV, Nigam SK. Remote communication through solute carriers and ATP binding cassette drug transporter pathways: an update on the remote sensing and signaling hypothesis. Mol Pharmacol. 2011;79:795–805. doi: 10.1124/mol.110.070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moitra K, Dean M. Evolution of ABC transporters by gene duplication and their role in human disease. Biol Chem. 2011;392:29–37. doi: 10.1515/bc.2011.006. [DOI] [PubMed] [Google Scholar]
- 5.Schinkel AH, Mayer U, Wagenaar E, Mol CA, van Deemter L, Smit JJ, et al. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci USA. 1997;94:4028–4033. doi: 10.1073/pnas.94.8.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlaming ML, van Esch A, Pala Z, Wagenaar E, van de Wetering K, van Tellingen O, et al. Abcc2 (Mrp2), Abcc3 (Mrp3), and Abcg2 (Bcrp1) are the main determinants for rapid elimination of methotrexate and its toxic metabolite 7-hydroxymethotrexate in vivo. Mol Cancer Ther. 2009;8:3350–3359. doi: 10.1158/1535-7163.MCT-09-0668. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZS, Tiwari AK. Multidrug resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic diseases. FEBS J. 2011;278:3226–3245. doi: 10.1111/j.1742-4658.2011.08235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29:200–207. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Thiebaut F, Tsuro T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cascorbi I. P-glycoprotein: tissue distribution, substrates, and functional consequences of genetic variations. In: Fromm MF, Kim RB, editors. Drug transporters. Berlin: Springer; 2011. pp. 261–283. [DOI] [PubMed] [Google Scholar]
- 12.Huls M, Russel FG, Masereeuw R. The role of ATP binding cassette transporters in tissue defense and organ regeneration. J Pharmacol Exp Ther. 2009;328:3–9. doi: 10.1124/jpet.107.132225. [DOI] [PubMed] [Google Scholar]
- 13.Resta-Lenert S, Smitham J, Barrett KE. Epithelial dysfunction associated with the development of colitis in conventionally housed mdr1a−/− mice. Am J Physiol Gastrointest Liver Physiol. 2005;289:G153–G162. doi: 10.1152/ajpgi.00395.2004. [DOI] [PubMed] [Google Scholar]
- 14.Juyal G, Midha V, Amre D, Sood A, Seidman E, Thelma BK. Associations between common variants in the MDR1 (ABCB1) gene and ulcerative colitis among North Indians. Pharmacogenet Genomics. 2009;19:77–85. doi: 10.1097/FPC.0b013e32831a9abe. [DOI] [PubMed] [Google Scholar]
- 15.Hauser IA, Schaeffeler E, Gauer S, Scheuermann EH, Wegner B, Gossmann J, et al. ABCB1 genotype of the donor but not of the recipient is a major risk factor for cyclosporine-related nephrotoxicity after renal transplantation. J Am Soc Nephrol. 2005;16:1501–1511. doi: 10.1681/ASN.2004100882. [DOI] [PubMed] [Google Scholar]
- 16.Naesens M, Lerut E, de Jonge H, Van Damme B, Vanrenterghem Y, Kuypers DR. Donor age and renal P-glycoprotein expression associate with chronic histological damage in renal allografts. J Am Soc Nephrol. 2009;20:2468–2480. doi: 10.1681/ASN.2009020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi HJ, Cho HY, Ro H, Lee SH, Han KH, Lee H, et al. Polymorphisms of the MDR1 and MIF genes in children with nephrotic syndrome. Pediatr Nephrol. 2011;26:1981–1988. doi: 10.1007/s00467-011-1903-0. [DOI] [PubMed] [Google Scholar]
- 18.Huls M, Kramers C, Levtchenko EN, Wilmer MJ, Dijkman HB, Kluijtmans LA, et al. P-glycoprotein-deficient mice have proximal tubule dysfunction but are protected against ischemic renal injury. Kidney Int. 2007;72:1233–1241. doi: 10.1038/sj.ki.5002522. [DOI] [PubMed] [Google Scholar]
- 19.Wilmer MJ, Schoeber JP, van den Heuvel LP, Levtchenko EN. Cystinosis: practical tools for diagnosis and treatment. Pediatr Nephrol. 2011;26:205–215. doi: 10.1007/s00467-010-1627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peeters K, Wilmer MJ, Schoeber JP, Reijnders D, van den Heuvel LP, Masereeuw R, et al. Role of P-glycoprotein expression and function in cystinotic renal proximal tubular cells. Pharmaceutics. 2012;3:782–792. doi: 10.3390/pharmaceutics3040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Water FM, Masereeuw R, Russel FGM. Function and regulation of multidrug resistance proteins in the renal elimination of organic anions. Drug Metab Rev. 2005;37:457–485. doi: 10.1080/03602530500205275. [DOI] [PubMed] [Google Scholar]
- 22.Wijnholds J, Scheffer GL, van der Valk M, van der Valk P, Beijnen JH, Scheper RJ, et al. Multidrug resistance protein 1 protects the oropharyngeal mucosal layer and the testicular tubules against drug-induced damage. J Exp Med. 1998;188:797–808. doi: 10.1084/jem.188.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masereeuw R, Notenboom S, Smeets PH, Wouterse AC, Russel FG. Impaired renal secretion of substrates for the multidrug resistance protein 2 in mutant transport-deficient (TR-) rats. J Am Soc Nephrol. 2003;14:2741–2749. doi: 10.1097/01.ASN.0000094083.82845.FA. [DOI] [PubMed] [Google Scholar]
- 24.Van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol. 2002;13:595–603. doi: 10.1681/ASN.V133595. [DOI] [PubMed] [Google Scholar]
- 25.Smeets PH, Van Aubel RA, Wouterse AC, van den Heuvel JJ, Russel FG. Contribution of multidrug resistance protein 2 (MRP2/ABCC2) to the renal excretion of p-aminohippurate (PAH) and identification of MRP4 (ABCC4) as a novel PAH transporter. J Am Soc Nephrol. 2004;15:2828–2835. doi: 10.1097/01.ASN.0000143473.64430.AC. [DOI] [PubMed] [Google Scholar]
- 26.Kiser JJ, Aquilante CL, Anderson PL, King TM, Carten ML, Fletcher CV. Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47:298–303. doi: 10.1097/QAI.0b013e31815e7478. [DOI] [PubMed] [Google Scholar]
- 27.Grisk O, Steinbach AC, Ciecholewski S, Schluter T, Kloting I, Schmidt H, et al. Multidrug resistance-related protein 2 genotype of the donor affects kidney graft function. Pharmacogenet Genomics. 2009;19:276–288. doi: 10.1097/FPC.0b013e328328d4e9. [DOI] [PubMed] [Google Scholar]
- 28.Borst P, van de Wetering K, Schlingemann R. Does the absence of ABCC6 (multidrug resistance protein 6) in patients with pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery? Cell Cycle. 2008;7:1575–1579. doi: 10.4161/cc.7.11.6005. [DOI] [PubMed] [Google Scholar]
- 29.Fulop K, Jiang Q, Wetering KV, Pomozi V, Szabo PT, Aranyi T, et al. ABCC6 does not transport vitamin K3-glutathione conjugate from the liver: relevance to pathomechanisms of pseudoxanthoma elasticum. Biochem Biophys Res Commun. 2011;415:468–471. doi: 10.1016/j.bbrc.2011.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pushpakom SP, Liptrott NJ, Rodriguez-Novoa S, Labarga P, Soriano V, Albalater M, et al. Genetic variants of ABCC10, a novel tenofovir transporter, are associated with kidney tubular dysfunction. J Infect Dis. 2011;204:145–153. doi: 10.1093/infdis/jir215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huls M, Brown CD, Windass AS, Sayer R, van den Heuvel JJ, Heemskerk S, et al. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73:220–225. doi: 10.1038/sj.ki.5002645. [DOI] [PubMed] [Google Scholar]
- 32.Huls M, van den Heuvel JJ, Dijkman HB, Russel FG, Masereeuw R. ABC transporter expression profiling after ischemic reperfusion injury in mouse kidney. Kidney Int. 2006;69:2186–2193. doi: 10.1038/sj.ki.5000407. [DOI] [PubMed] [Google Scholar]
- 33.Storch CH, Ehehalt R, Haefeli WE, Weiss J. Localization of the human breast cancer resistance protein (BCRP/ABCG2) in lipid rafts/caveolae and modulation of its activity by cholesterol in vitro. J Pharmacol Exp Ther. 2007;323:257–264. doi: 10.1124/jpet.107.122994. [DOI] [PubMed] [Google Scholar]
- 34.Jonker JW, Buitelaar M, Wagenaar E, van der Valk MA, Scheffer GL, Scheper RJ, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci USA. 2002;99:15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dankers AC, Sweep FC, Pertijs JC, Verweij V, van den Heuvel JJ, Koenderink JB, et al. Localization of breast cancer resistance protein (BCRP) in endocrine organs and inhibition of its transport activity by steroid hormones. . Cell Tissue Res. 2012;349:551–563. doi: 10.1007/s00441-012-1417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, Kottgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci USA. 2009;106:10338–10342. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Aubel RA, Smeets PH, van den Heuvel JJ, Russel FG. Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am J Physiol Renal Physiol. 2005;288:F327–F333. doi: 10.1152/ajprenal.00133.2004. [DOI] [PubMed] [Google Scholar]
- 38.Jeong H, Herskowitz I, Kroetz DL, Rine J. Function-altering SNPs in the human multidrug transporter gene ABCB1 identified using a Saccharomyces-based assay. PLoS Genet. 2007;3:e39. doi: 10.1371/journal.pgen.0030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu H, Klaassen C. Gender differences in mRNA expression of ATP-binding cassette efflux and bile acid transporters in kidney, liver, and intestine of 5/6 nephrectomized rats. Drug Metab Dispos. 2008;36:16–23. doi: 10.1124/dmd.107.014845. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki T, Zhao YL, Nadai M, Naruhashi K, Shimizu A, Takagi K, et al. Gender-related differences in expression and function of hepatic P-glycoprotein and multidrug resistance-associated protein (MRP2) in rats. Life Sci. 2006;79:455–461. doi: 10.1016/j.lfs.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 41.Maher JM, Cheng X, Tanaka Y, Scheffer GL, Klaassen CD. Hormonal regulation of renal multidrug resistance-associated proteins 3 and 4 (MRP3 and MRP4) in mice. Biochem Pharmacol. 2006;71:1470–1478. doi: 10.1016/j.bcp.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Chen C, Klaassen CD. Rat multidrug resistance protein 4 (MRP4, Abcc4): molecular cloning, organ distribution, postnatal renal expression, and chemical inducibility. Biochem Biophys Res Commun. 2004;317:46–53. doi: 10.1016/j.bbrc.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Ho LL, Kench JG, Handelsman DJ, Scheffer GL, Stricker PD, Grygiel JG, et al. Androgen regulation of multidrug resistance-associated protein 4 (MRP4/ABCC4) in prostate cancer. Prostate. 2008;68:1421–1429. doi: 10.1002/pros.20809. [DOI] [PubMed] [Google Scholar]
- 44.Merino G, van Herwaarden AE, Wagenaar E, Jonker JW, Schinkel AH. Sex-dependent expression and activity of the ATP-binding cassette transporter breast cancer resistance protein (BCRP/ABCG2) in liver. Mol Pharmacol. 2005;67:1765–1771. doi: 10.1124/mol.105.011080. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Zhou L, Gupta A, Vethanayagam RR, Zhang Y, Unadkat JD, et al. Regulation of BCRP/ABCG2 expression by progesterone and 17beta-estradiol in human placental BeWo cells. Am J Physiol Endocrinol Metab. 2006;290:E798–E807. doi: 10.1152/ajpendo.00397.2005. [DOI] [PubMed] [Google Scholar]
- 46.Prevoo B, Miller DS, van de Water FM, Wever KE, Russel FG, Flik G, et al. Rapid, nongenomic stimulation of multidrug resistance protein 2 (Mrp2) activity by glucocorticoids in renal proximal tubule. J Pharmacol Exp Ther. 2011;338:362–371. doi: 10.1124/jpet.111.179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar S, Allen DA, Kieswich JE, Patel NS, Harwood S, Mazzon E, et al. Dexamethasone ameliorates renal ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20:2412–2425. doi: 10.1681/ASN.2008080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai AZ, Abella JV, Park M. Crosstalk in Met receptor oncogenesis. Trends Cell Biol. 2009;19:542–551. doi: 10.1016/j.tcb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Gafter-Gvili A, Ram R, Gafter U, Shpilberg O, Raanani P. Renal failure associated with tyrosine kinase inhibitors—case report and review of the literature. Leuk Res. 2010;34:123–127. doi: 10.1016/j.leukres.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Notenboom S, Miller DS, Smits P, Russel FG, Masereeuw R. Involvement of guanylyl cyclase and cGMP in the regulation of Mrp2-mediated transport in the proximal tubule. Am J Physiol Renal Physiol. 2004;287:F33–F38. doi: 10.1152/ajprenal.00443.2003. [DOI] [PubMed] [Google Scholar]
- 51.Heemskerk S, van Koppen A, van den Broek L, Poelen GJ, Wouterse AC, Dijkman HB, et al. Nitric oxide differentially regulates renal ATP-binding cassette transporters during endotoxemia. Pflugers Arch. 2007;454:321–334. doi: 10.1007/s00424-007-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. Tissue distribution and hormonal regulation of the breast cancer resistance protein (BCRP/Abcg2) in rats and mice. Biochem Biophys Res Commun. 2005;326:181–187. doi: 10.1016/j.bbrc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 53.Imai Y, Ishikawa E, Asada S, Sugimoto Y. Estrogen-mediated post transcriptional down-regulation of breast cancer resistance protein/ABCG2. Cancer Res. 2005;65:596–604. doi: 10.1158/0008-5472.CAN-05-1894. [DOI] [PubMed] [Google Scholar]
- 54.Demeule M, Jodoin J, Beaulieu E, Brossard M, Beliveau R. Dexamethasone modulation of multidrug transporters in normal tissues. FEBS Lett. 1999;442:208–214. doi: 10.1016/S0014-5793(98)01663-9. [DOI] [PubMed] [Google Scholar]
- 55.Aleksunes LM, Augustine LM, Scheffer GL, Cherrington NJ, Manautou JE. Renal xenobiotic transporters are differentially expressed in mice following cisplatin treatment. Toxicology. 2008;250:82–88. doi: 10.1016/j.tox.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masereeuw R, Terlouw SA, van Aubel RAMH, Russel FGM, Miller DS. Endothelin B receptor-mediated regulation of ATP-driven drug secretion in renal proximal tubule. Mol Pharmacol. 2000;57:59–67. [PubMed] [Google Scholar]
- 57.Takada T, Suzuki H, Gotoh Y, Sugiyama Y. Regulation of the cell surface expression of human BCRP/ABCG2 by the phosphorylation state of Akt in polarized cells. Drug Metab Dispos. 2005;33:905–909. doi: 10.1124/dmd.104.003228. [DOI] [PubMed] [Google Scholar]
- 58.Wever KE, Masereeuw R, Miller DS, Hang XM, Flik G. Endothelin and calciotropic hormones share regulatory pathways in multidrug resistance protein 2-mediated transport. Am J Physiol Renal Physiol. 2007;292:F38–F46. doi: 10.1152/ajprenal.00479.2005. [DOI] [PubMed] [Google Scholar]
- 59.Magnarin M, Morelli M, Rosati A, Bartoli F, Candussio L, Giraldi T, et al. Induction of proteins involved in multidrug resistance (P-glycoprotein, MRP1, MRP2, LRP) and of CYP 3A4 by rifampicin in LLC-PK1 cells. Eur J Pharmacol. 2004;483:19–28. doi: 10.1016/j.ejphar.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 60.Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, et al. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005;42:420–430. doi: 10.1002/hep.20784. [DOI] [PubMed] [Google Scholar]
- 61.Heemskerk S, Peters JG, Louisse J, Sagar S, Russel FG, Masereeuw R. Regulation of P-glycoprotein in renal proximal tubule epithelial cells by LPS and TNF-alpha. J Biomed Biotechnol. 2010;2010:525180. doi: 10.1155/2010/525180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thévenod F, Friedmann JM, Katsen AD, Hauser IA. Up-regulation of multidrug resistance P-glycoprotein via nuclear factor-kappaB activation protects kidney proximal tubule cells from cadmium- and reactive oxygen species-induced apoptosis. J Biol Chem. 2000;275:1887–1896. doi: 10.1074/jbc.275.3.1887. [DOI] [PubMed] [Google Scholar]
- 63.Notenboom S, Kuik LH, Peters B, Russel FGM, Masereeuw R. Up-regulation of the mulddrug resistance transporter 2 (MRP2) in renal proximal tubule cells by gentamicin. N Schmied Arch Pharmacol. 2005;371:R8. [Google Scholar]
- 64.Aleksunes LM, Goedken MJ, Rockwell CE, Thomale J, Manautou JE, Klaassen CD. Transcriptional regulation of renal cytoprotective genes by Nrf2 and its potential use as a therapeutic target to mitigate cisplatin-induced nephrotoxicity. J Pharmacol Exp Ther. 2010;335:2–12. doi: 10.1124/jpet.110.170084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laouari D, Yang R, Veau C, Blanke I, Friedlander G. Two apical multidrug transporters, P-gp and MRP2, are differently altered in chronic renal failure. Am J Physiol. 2001;280:F636–F645. doi: 10.1152/ajprenal.2001.280.4.F636. [DOI] [PubMed] [Google Scholar]
- 66.Pei QL, Kobayashi Y, Tanaka Y, Taguchi Y, Higuchi K, Kaito M, et al. Increased expression of multidrug resistance-associated protein 1 (MRP1) in hepatocyte basolateral membrane and renal tubular epithelia after bile duct ligation in rats. Hepatol Res. 2002;22:58–64. doi: 10.1016/S1386-6346(01)00110-3. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka Y, Kobayashi Y, Gabazza EC, Higuchi K, Kamisako T, Kuroda M, et al. Increased renal expression of bilirubin glucuronide transporters in a rat model of obstructive jaundice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G656–G662. doi: 10.1152/ajpgi.00383.2001. [DOI] [PubMed] [Google Scholar]
- 68.Denk GU, Soroka CJ, Takeyama Y, Chen WS, Schuetz JD, Boyer JL. Multidrug resistance-associated protein 4 is up-regulated in liver but down-regulated in kidney in obstructive cholestasis in the rat. J Hepatol. 2004;40:585–591. doi: 10.1016/j.jhep.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 69.Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, et al. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825–838. doi: 10.1016/S0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- 70.Zollner G, Fickert P, Fuchsbichler A, Silbert D, Wagner M, Arbeiter S, et al. Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J Hepatol. 2003;39:480–488. doi: 10.1016/S0168-8278(03)00228-9. [DOI] [PubMed] [Google Scholar]
- 71.Terlouw SA, Masereeuw R, Russel FG. Modulatory effects of hormones, drugs, and toxic events on renal organic anion transport. Biochem Pharmacol. 2003;65:1393–1405. doi: 10.1016/S0006-2952(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 72.Notenboom S, Wouterse AC, Peters B, Kuik LH, Heemskerk S, Russel FG, et al. Increased apical insertion of the multidrug resistance protein 2 (MRP2/ABCC2) in renal proximal tubules following gentamicin exposure. J Pharmacol Exp Ther. 2006;318:1194–1202. doi: 10.1124/jpet.106.104547. [DOI] [PubMed] [Google Scholar]
- 73.Wang H, Wu X, Hudkins K, Mikheev A, Zhang H, Gupta A, et al. Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: effects of pregnancy and correlations with nuclear receptors. Am J Physiol Endocrinol Metab. 2006;291:E1295–E1304. doi: 10.1152/ajpendo.00193.2006. [DOI] [PubMed] [Google Scholar]
- 74.Xu S, Weerachayaphorn J, Cai SY, Soroka CJ, Boyer JL. Aryl hydrocarbon receptor and NF-E2-related factor 2 are key regulators of human MRP4 expression. Am J Physiol Gastrointest Liver Physiol. 2010;299:G126–G135. doi: 10.1152/ajpgi.00522.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beischlag TV, Luis MJ, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–250. doi: 10.1615/CritRevEukarGeneExpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahringer A, Seymour A, Miller DS, Fricker G. Aryl hydrocarbon receptor-dependent regulation of the ABC transporters in kidney tubules from killifish (Fundulus heteroclitus) MDIBL Bulletin. 2009;48:102. [Google Scholar]
- 77.Flaveny CA, Murray IA, Perdew GH. Differential gene regulation by the human and mouse aryl hydrocarbon receptor. Toxicol Sci. 2010;114:217–225. doi: 10.1093/toxsci/kfp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan KP, Wang B, Yang M, Boutros PC, Macaulay J, Xu H, et al. Aryl hydrocarbon receptor is a transcriptional activator of the human breast cancer resistance protein (BCRP/ABCG2) Mol Pharmacol. 2010;78:175–185. doi: 10.1124/mol.110.065078. [DOI] [PubMed] [Google Scholar]
- 79.Ma Q, Kinneer K, Bi Y, Chan JY, Kan YW. Induction of murine NAD(P)H:quinone oxidoreductase by 2,3,7,8-tetrachlorodibenzo-p-dioxin requires the CNC (cap ‘n’ collar) basic leucine zipper transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2): cross-interaction between AhR (aryl hydrocarbon receptor) and Nrf2 signal transduction. Biochem J. 2004;377:205–213. doi: 10.1042/BJ20031123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hartmann G, Cheung AK, Piquette-Miller M. Inflammatory cytokines, but not bile acids, regulate expression of murine hepatic anion transporters in endotoxemia. J Pharmacol Exp Ther. 2002;303:273–281. doi: 10.1124/jpet.102.039404. [DOI] [PubMed] [Google Scholar]
- 81.Liang M, Knox FG. Production and functional roles of nitric oxide in the proximal tubule. Am J Physiol. 2000;278:R1117–R1124. doi: 10.1152/ajpregu.2000.278.5.R1117. [DOI] [PubMed] [Google Scholar]
- 82.Heemskerk S, Pickkers P, Bouw MPWJ, Draisma A, van der Hoeven JG, Peters WHM, et al. Upregulation of renal inducible nitric oxide synthase during human endotoxemia and sepsis is associated with proximal tubule injury. Clin J Am Soc Nephrol. 2006;1:853–862. doi: 10.2215/CJN.00490206. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka Y, Chen C, Maher JM, Klaassen CD. Ischemia-reperfusion of rat livers decreases liver and increases kidney multidrug resistance associated protein 2 (Mrp2) Toxicol Sci. 2008;101:171–178. doi: 10.1093/toxsci/kfm261. [DOI] [PubMed] [Google Scholar]
- 84.Vanholder R, Baurmeister U, Brunet P, Cohen G, Glorieux G, Jankowski J. A bench to bedside view of uremic toxins. J Am Soc Nephrol. 2008;19:863–870. doi: 10.1681/ASN.2007121377. [DOI] [PubMed] [Google Scholar]
- 85.Lee CT, Kuo CC, Chen YM, Hsu CY, Lee WC, Tsai YC, et al. Factors associated with blood concentrations of indoxyl sulfate and p-cresol in patients undergoing peritoneal dialysis. Perit Dial Int. 2010;30:456–463. doi: 10.3747/pdi.2009.00092. [DOI] [PubMed] [Google Scholar]
- 86.Van Aubel RA, Smeets PH, van den Heuvel JJ, Russel FG. Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am J Physiol Renal Physiol. 2005;288:F327–F333. doi: 10.1152/ajprenal.00133.2004. [DOI] [PubMed] [Google Scholar]
- 87.Mutsaers HA, van den Heuvel LP, Ringens LH, Dankers AC, Russel FG, Wetzels JF, et al. Uremic toxins inhibit transport by breast cancer resistance protein and multidrug resistance protein 4 at clinically relevant concentrations. PLoS One. 2011;6:e18438. doi: 10.1371/journal.pone.0018438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huls M, Schoeber JP, Verfaillie CM, Luttun A, Ulloa-Montoya F, Menke AL, et al. Deficiency of either P-glycoprotein or breast cancer resistance protein protect against acute kidney injury. Cell Transplant. 2010;19:1195–1208. doi: 10.3727/096368910X504478. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Z, Wu JY, Hait WN, Yang JM. Regulation of the stability of P-glycoprotein by ubiquitination. Mol Pharmacol. 2004;66:395–403. doi: 10.1124/mol.104.001966. [DOI] [PubMed] [Google Scholar]
- 90.Wakabayashi-Nakao K, Tamura A, Furukawa T, Nakagawa H, Ishikawa T. Quality control of human ABCG2 protein in the endoplasmic reticulum: ubiquitination and proteasomal degradation. Adv Drug Deliv Rev. 2009;61:66–72. doi: 10.1016/j.addr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 91.Hayashi H, Naoi S, Nakagawa T, Nishikawa T, Fukuda H, Imajoh-Ohmi S, et al. Sorting Nexin 27 interacts with multidrug resistance-associated protein 4 (MRP4) and mediates internalization of MRP4. J Biol Chem. 2012;287:15054–15065. doi: 10.1074/jbc.M111.337931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hayashi H, Mizuno T, Horikawa R, Nagasaka H, Yabuki T, Takikawa H, et al. 4-phenylbutyrate modulates ubiquitination of hepatocanalicular MRP2 and reduces serum total bilirubin concentration. J Hepatol. 2012;56:1136–1144. doi: 10.1016/j.jhep.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 93.Klaassen CD, Lu H, Cui JY. Epigenetic regulation of drug processing genes. Toxicol Mech Methods. 2011;21:312–324. doi: 10.3109/15376516.2011.562758. [DOI] [PubMed] [Google Scholar]
- 94.Koturbash I, Beland FA, Pogribny IP. Role of microRNAs in the regulation of drug metabolizing and transporting genes and the response to environmental toxicants. Expert Opin Drug Metab Toxicol. 2012;8:597–606. doi: 10.1517/17425255.2012.673587. [DOI] [PubMed] [Google Scholar]
- 95.Ho J, Kreidberg JA. The long and short of microRNAs in the kidney. J Am Soc Nephrol. 2012;23:400–404. doi: 10.1681/ASN.2011080797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haenisch S, Laechelt S, Bruckmueller H, Werk A, Noack A, Bruhn O, et al. Down-regulation of ATP-binding cassette C2 protein expression in HepG2 cells after rifampicin treatment is mediated by microRNA-379. Mol Pharmacol. 2011;80:314–320. doi: 10.1124/mol.110.070714. [DOI] [PubMed] [Google Scholar]
- 97.Turrini E, Haenisch S, Laechelt S, Diewock T, Bruhn O, Cascorbi I. MicroRNA profiling in K-562 cells under imatinib treatment: influence of miR-212 and miR-328 on ABCG2 expression. Pharmacogenet Genomics. 2012;22:198–205. doi: 10.1097/FPC.0b013e328350012b. [DOI] [PubMed] [Google Scholar]
- 98.Chandrasekaran K, Karolina DS, Sepramaniam S, Armugam A, Wintour EM, Bertram JF, et al. Role of microRNAs in kidney homeostasis and disease. Kidney Int. 2012;81:617–627. doi: 10.1038/ki.2011.448. [DOI] [PubMed] [Google Scholar]
- 99.Lorenzen JM, Thum T. Circulating and urinary microRNAs in kidney disease. Clin J Am Soc Nephrol. 2012;(in press) [DOI] [PubMed]
- 100.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]


