Abstract
Background:
Ependymoma has been described typically as an intramedullary tumor derived from ependymal cells. Intradural extramedullary presentation is rarely described and almost always as a unique lesion. Myxopapillary ependymoma is a histological variant that distinguishes from the ordinary type of ependymoma because of its generally better prognosis. We present two cases of multicentric extramedullary myxopapillary ependymomas.
Case Description:
Case 1 was a 30-year-old man with progressive paresthesia and paresis in the lower limbs, urinary sphincter disturbances, gait instability, ataxia, and chronic low back pain with multiple intradural extramedullary lesions at C2-C3, D2-D4-D5, and D12-L1. Case 2 was a 32-year-old man, presented with low back pain and mild paresthesia in the right lower limb. Magnetic resonance imaging (MRI) showed multiple intradural extramedullary lesions with homogeneous enhancement after gadolinium injection at C7, D2, D4, D5, D8, D10, D11, L1, L3, L5, S1, and S2. Complete tumor resection of the approached tumors was archived in both cases. Histological studies confirm myxopapillary ependymomas. Patient's neurologic outcome was good and no residual tumor was present at MRI control at 10 years in case 1 and 12 months in case 2.
Conclusions:
We report the first two cases of multicentric extramedullary myxopapillary ependymomas, this etiology must be taken into account in the differential diagnosis of intradural extramedullary tumors.
Keywords: Extramedullary ependymoma, multicentric ependymoma, myxopapillary ependymoma
INTRODUCTION
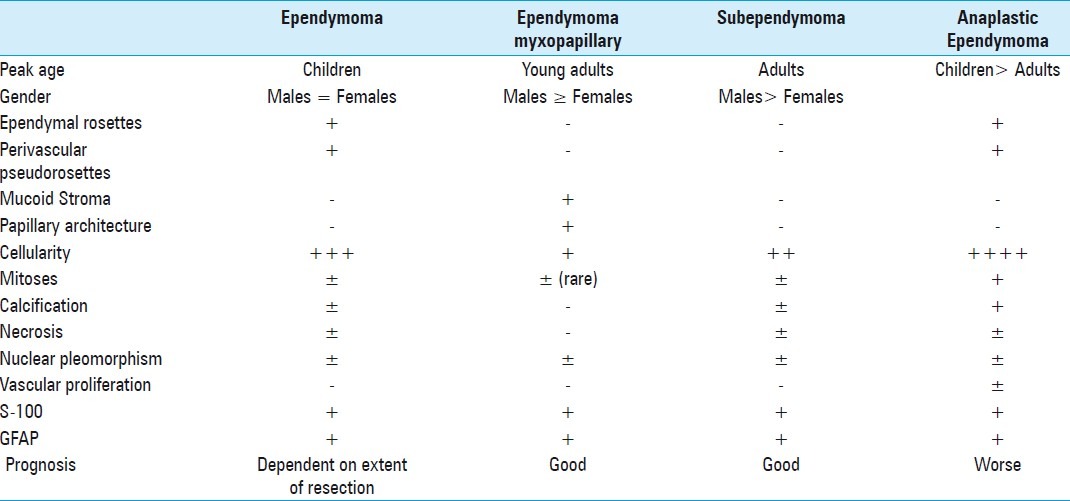
Ependymoma has been described typically as an intramedullary tumor derived from ependymal cells. Their location is most commonly proximal to the normal site of these cells. Intradural extramedullary presentation is rarely described except for those located at the terminal filum or conus medullaris. Myxopapillary ependymoma is a histological variant distinguishes from the ordinary type of ependymoma because of its generally better prognosis [2] [Table 1]. The authors present two cases of this unusual location and histological variant, and discuss the incidence, histopathogenesis, and treatment of this rare clinical presentation.
Table 1.
Demographic, histological and prognosis characteristic of ependymomas
CASE 1
A 30-year-old man was referred to our neurosurgical department presenting progressive paresthesia and paraparesis, urinary sphincter disturbances, gait instability, ataxia, and chronic low back pain. Neurological examination showed bilateral hypoesthesia at D2 and perineal region was not associated with radicular pain and hypereflexia in lower-limbs. Spinal magnetic resonance imaging (MRI) revealed multiple intradural extramedullary lesions, isointense on T1, hyperintense on T2, and fluid attenuated inversion recovery (FLAIR) sequences with intense contrast enhancement, at C2-C3, D2-D4-D5, and D12-L1 [Figure 1 shows no recurrence in T2. I, J, K and L, demonstrate no tumor at D12-L2 level in 10-year postoperative control”]. Cranial MRI did not reveal any abnormality. The D12-L1 tumor was located posteriorly in the spinal canal displacing anteriorly the conus medullaris and was removed through a D12-L2 laminectomy in first place. The D2 antero-medullary mass compress and displace posteriorly the spinal cord. After D1-D3 laminectomy and midline durotomy the left dental ligament was cut and retracted to improve surgical field. The tumor has soft consistence and a fleshy-gray appearance and was completely encapsulated by a thin fibrous capsule with a well delimitated cleavage plane. A microvascular attachment to the spinal cord was identified, coagulated, and divided. The tumor was debulked and completely resected using microsurgical techniques. Histological results inform a myxopapillary ependymoma. Patient's neurologic condition improved after surgery and he was discharged with a mild lower limb paresis and D2 left radicular pain, which resolved with rehabilitation 6 months after treatment. Adjuvant radiotherapy was performed and he had no tumor progression in 10 years follow up.
Figure 1.
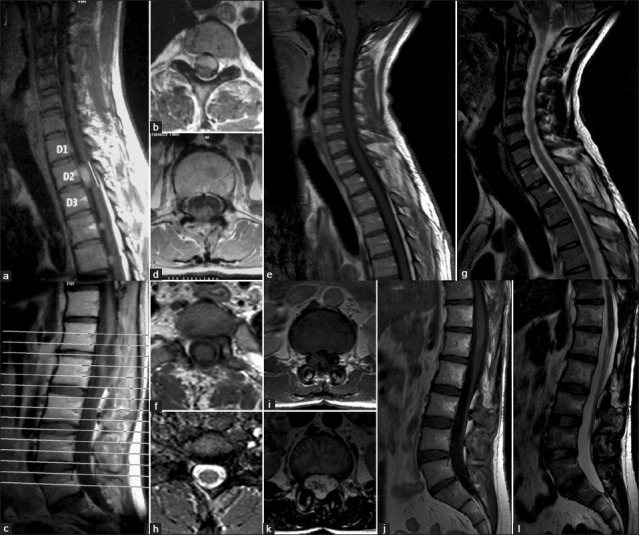
(a and b) Demonstrate D2 anteromedullary enhanced tumor on T1 sequence. (c and d) Show D12-L1 lesion posterior located in the spinal canal displacing anteriorly the conus medullaris. (e and f) 10-year postoperative control MRI showing no recurrence on T1. (g and h) Shows no recurrence in T2. (i, j, k and l) demonstrate no tumor at D12-L2 level in 10-year postoperative control
CASE 2
A 32-year-old man, presented with a 6-month history of low back pain and mild paresthesia in the right lower limb without dorsalgia or radicular pain. Neurological examination showed bilateral hypoesthesia at the T-8 level and hyperreflexia in lower limbs (right greater than left). MRI showed multiple intradural extramedullary lesions with homogeneous enhancement after gadolinium injection at C7, D2, D4, D5, D8, D10, D11, L1, L3, L5, S1, and S2 [Figure 2]. D10 tumor was “C” shaped occupying 60% of the spinal canal without foramen invasion or bone destruction. The tumor compresses anteriorly, right-lateral and posteriorly the spinal cord. Cranial MRI was normal. The tumor was approached through a D9-D10 laminectomy. Surgical findings include partially encapsulation, soft discretely hemorrhagic consistence with a well delimitated cleavage plane, and no medullary connection. The D10 right root was involved by the tumor but not infiltrated. After debulking complete tumor removal was archived under microscope magnification. Histological tumor diagnosis was also myxopapillary ependymoma [Figure 3]. Referred symptoms remainunchanged and control MRI at 12 months after surgery showed no recurrence or spinal cord damage. No adjuvant therapies were performed.
Figure 2.
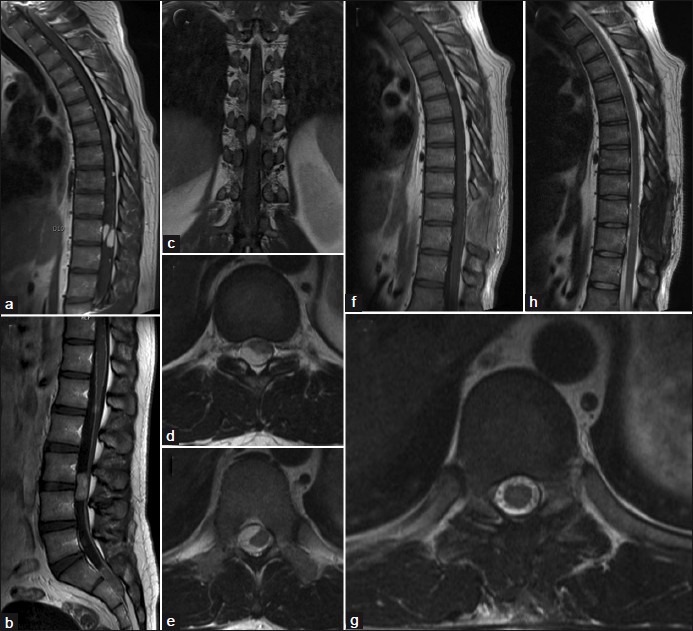
(a and b) Shows sagittal multiple spine enhanced tumours. (c) Demonstrate coronal view of D10 tumor on T1-weighted sequence. (d and e) Presents axial view of “C” shaped D10 lesion. (f) Present 12-month postoperative T1-weighted contrast sequence. (g and h)Demonstrate 12-month postoperative control on T2-weighted images
Figure 3.
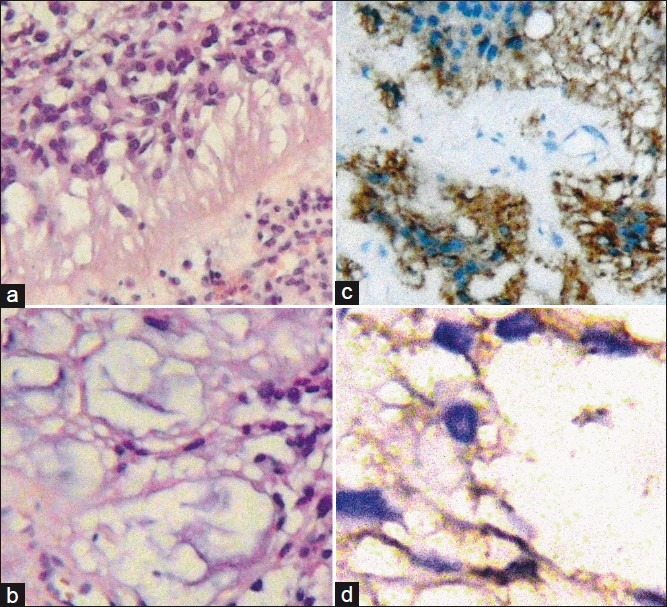
(a) Neoplastic proliferation of ependymal cells, eosinophilic cytoplasm and hyperchromatic nuclei with mild anisokaryosis and papillary structures. (b) Abundant myxoid matrix. (c and d) Immunohistochemistry were performed with antigen retrieval methods informing positive stain to GFAP and S-100 protein
DISCUSSION
Extramedullary ependymomas appers to be more frequent in the 5th decade of life [3] and females and only 20 cases of this unusual location have being described in literature.[3–10] The myxopapillary histological variant has a slight male predominance and a peak age in young adults at 3rd decade.[3] Myxopapillary ependymomas often arise from filum terminale and are encountered in the conus medullaris area, this location “technically speaking” is also extramedulary, and the subpial medullary location is the result of its cerebrospinal fluid (CSF) seeding.[10] So far only one case was reported in a focal extramedullary locations [5] besides the conus and filum terminale. It has been postulated that intradural extramedullary ependymomas develop from ectopic ependymal cells that migrates from the neural tube to the extramedullary space during its closure,[12] this neuroaxis invagination is evidenced by the tumor encapsulation of the pia mater and arachnoid membrane in almost all cases.[3] Some authors describe CSF dissemination as a result of aggressive tumor behavior [10] or anaplastic transformation of initial benign lesions.[17] Although most of these extramedullary tumors were described in a single level, Iunes[11] described the first report of a mutifocal intradural extramedullary grade II ependymoma. This spread could be the result of drops metastasis from these primary lower spinal lesions. We present two cases of this extremely rare multicentric dissemination of a grade I, myxopapillary ependymomas.
Histologically, ependymal rosettes and perivascular pseudorosettes that characteristically mark ependymomas are generally absent or are not well formed in the myxopapillary subtype, although mucoid stroma, which is a distinctive feature of myxopapallyillary ependymoma, is not observed in classic ependymomas or subependymomas. Parietal hyalinization of vessels is frequently seen in myxopapillary subtypes. Mitotic figures, calcifications, necrosis, and cyst formations are not usually present [Table 1]. Myxopapillary ependymoma is histopathologically benign, grade I in the World Health Organization (WHO) classification, however, malignant transformation can occur, leading to recurrence or intradural dissemination.[10,14–20] Outcome is strongly influenciated by the extent of surgical resection, which is the best predictor of good prognosis. Sonneland et al.[21] reported a mean survival of 19 years in patients who underwent gross total resection as compared with 14 years for patients who underwent subtotal resection. In the case of multilevel compromise, asymptomatic lesions of this histopathological variant can be observed at appropriate time intervals due to lack or slow growth during follow-up as shown in Case 1. Otherwise, rare cases of aggressive behavior have been reported involving dissemination within and outside the central nervous system.[22,23] A close follow-up is recommended because of the potential for anaplastic transformation, recurrence, or metastasis.[13]The utilization of adjunctive radiotherapy has shown a significant improvement in overall survival even posterior to gross total resection.[1,21] In our 1 case we use it, presenting no tumor recurrence in 10 years.
CONCLUSIONS
We report the first two cases of multicentric extramedullary myxopapillary ependymomas. This etiology must be taken into account in the differential diagnosis of intradural extramedullary tumors, a complete imaging study of the entire neuroaxis should be perform always in order to exclude the presence of tumor dissemination. In multifocal presentation, asymptomatic lesions of this histopathological variant may be observed at appropriate time intervals due to lack or slow growth.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/1/102/100859
Contributor Information
Federico Landriel, Email: fedelandriel@gmail.com.
Pablo Ajler, Email: pablo.ajler@hospitalitaliano.org.ar.
Nicolas Tedesco, Email: nicolas.tedesco@hospitalitaliano.org.ar.
Damián Bendersky, Email: damian.bendersky@hospitalitaliano.org.ar.
Eduardo Vecchi, Email: eduardo.vecchi@hospitalitaliano.org.ar.
REFERENCES
- 1.Akyurek S, Chang EL, Yu TK, Little D, Allen PK, McCutcheon I, et al. Spinal myxopapillar outcomes in patients treated with surgery and radiotherapy at M.D. J Neurooncol. 2006;80:177–83. doi: 10.1007/s11060-006-9169-2. [DOI] [PubMed] [Google Scholar]
- 2.Benzagmout M, Boujraf S, Oulali N, Chbani L, Amarti A, Chakour K, et al. Intradural extramedullary ependymoma: Is there constantly a hormonal relationship? Surg Neurol. 2008;70:536–8. doi: 10.1016/j.surneu.2007.05.055. discussion 538. [DOI] [PubMed] [Google Scholar]
- 3.Cerase A, Venturi C, Oliveri G, De Falco D, Miracco C. Intradural extramedullary spinal anaplastic ependymoma.Case illustration. J Neurosurg Spine. 2006;5:476. doi: 10.3171/spi.2006.5.5.476. [DOI] [PubMed] [Google Scholar]
- 4.Cheng CH, Lee TC, Huang HY, Lui CC. Extramedullary thoracic myxopapillary ependymoma—a case report of a rare tumour. Ann Acad Med Singapore. 1996;25:869–72. [PubMed] [Google Scholar]
- 5.Davis C, Barnard RO. Malignant behavior of myxo papillary ependymoma. J Neurosurg. 1985;62:925–9. doi: 10.3171/jns.1985.62.6.0925. [DOI] [PubMed] [Google Scholar]
- 6.Duffau H, Gazzaz M, Kujas M, Fohanno D. Primary intradural extramedullary ependymoma: Case report and review of the literature. Spine (Phila Pa 1976) 2000;25:1993–5. doi: 10.1097/00007632-200008010-00021. [DOI] [PubMed] [Google Scholar]
- 7.Fuentes Rodríguez N, de la Paz Rivero M, Prince López JA, Salas Rubio JH. Ependimoma intradural extramedular primario. Rev Cub Med Mil. 2004;33(1):0–0. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0138 65572004000100009&lng=es&nrm=iso. ISSN 1561-3046 . [Google Scholar]
- 8.Gonzalez Feria L, Fernandez Martin F, Ginoves Sierra M, Galera Davidson H. Giant dorsal extramedullary ependymoma. Arch Neurobiol (Madr) 1971;34:325–32. [PubMed] [Google Scholar]
- 9.Graca J, Gultasli N, D'Haene N, Brotchi J, Salmon I, Baleriaux D. Cystic extramedullary ependymoma. AJNR Am J Neuroradiol. 2006;27:818–21. [PMC free article] [PubMed] [Google Scholar]
- 10.Hentschel SJ, McCutcheon IE, Ginsberg L, Weinberg JS. Exophytic ependymomas of the spinal cord. Acta Neurochir (Wien) 2004;146:1047–50. doi: 10.1007/s00701-004-0275-7. [DOI] [PubMed] [Google Scholar]
- 11.Iunes EA, Stávale JN, Caldas Pessoa RC, Ansai R, Onishi FJ, de Paiva Neto MA, et al. Multifocal intradural extramedullary ependymoma. Case report J Neurosurg Spine. 2011;14:65–70. doi: 10.3171/2010.9.SPINE09963. [DOI] [PubMed] [Google Scholar]
- 12.Katoh S, Ikata T, Inoue A, Takahashi M. Intradural extramedullary ependymoma.A case report. Spine (Phila Pa 1976) 1995;20:2036–8. doi: 10.1097/00007632-199509150-00017. [DOI] [PubMed] [Google Scholar]
- 13.Kinsman MJ, Callahan JD, Hattab EM, Cohen-Gadol AA. Extramedullary spinal ependymoma: A diagnostic challenge and review of the literature. Clin Neurol Neurosurg. 2011;113:661–4. doi: 10.1016/j.clineuro.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Li MH, Holtås S. MR imaging of spinal intramedullary tumors. Acta Radiol. 1991;32:505–13. [PubMed] [Google Scholar]
- 15.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliver B, de Castro A, Sarmiento MA, Arguello C, Blazquez MG. Dorsal extramedullary ependymoma. Arch Neurobiol (Madr) 1981;44:215–24. [PubMed] [Google Scholar]
- 17.Patterson RH, Campbell WG, Parsons H. Ependymoma of the cauda equina with multiple visceral metastases: Report of a case. J Neurosurg. 1961;18:145–50. doi: 10.3171/jns.1961.18.2.0145. [DOI] [PubMed] [Google Scholar]
- 18.Payer M, Yonekawa Y, Imhof HG. Solitary thoracic intradural extramedullary ependymoma. J Clin Neurosci. 1999;6:344–5. doi: 10.1054/jocn.1997.0076. [DOI] [PubMed] [Google Scholar]
- 19.Robles SG, Saldana C, Boto GR, Martinez A, Zamarron AP, Jorquera M, et al. Intradural extramedullary spinal ependymoma: A benign pathology? Spine (Phila Pa 1976) 2005;30:E251–4. doi: 10.1097/01.brs.0000161008.13441.7b. [DOI] [PubMed] [Google Scholar]
- 20.Schuurmans M, Vanneste JA, Verstegen MJ, van Furth WR. Spinal extramedullary anaplastic ependymoma with spinal and intracranial metastases. J Neurooncol. 2006;79:57–9. doi: 10.1007/s11060-005-9114-9. [DOI] [PubMed] [Google Scholar]
- 21.Sonneland PR, Scheithauer BW, Onofrio BM. Myxopapillary ependymoma.A clinicopathologic and immunohistochemical study of 77 cases. Cancer. 1985;56:883–93. doi: 10.1002/1097-0142(19850815)56:4<883::aid-cncr2820560431>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Wagle WA, Jaufman B, Mincy JE. Intradural extramedullary ependymoma: MRpathologic correlation. J Comput Assist Tomogr. 1988;12:705–7. doi: 10.1097/00004728-198807000-00045. [DOI] [PubMed] [Google Scholar]
- 23.Wolfla CE, Azzarelli B, Shah MV. Primary extramedullary ependymoma of the thoracic spine.Case illustration. J Neurosurg. 1997;87:643. doi: 10.3171/jns.1997.87.4.0643. [DOI] [PubMed] [Google Scholar]


