Abstract
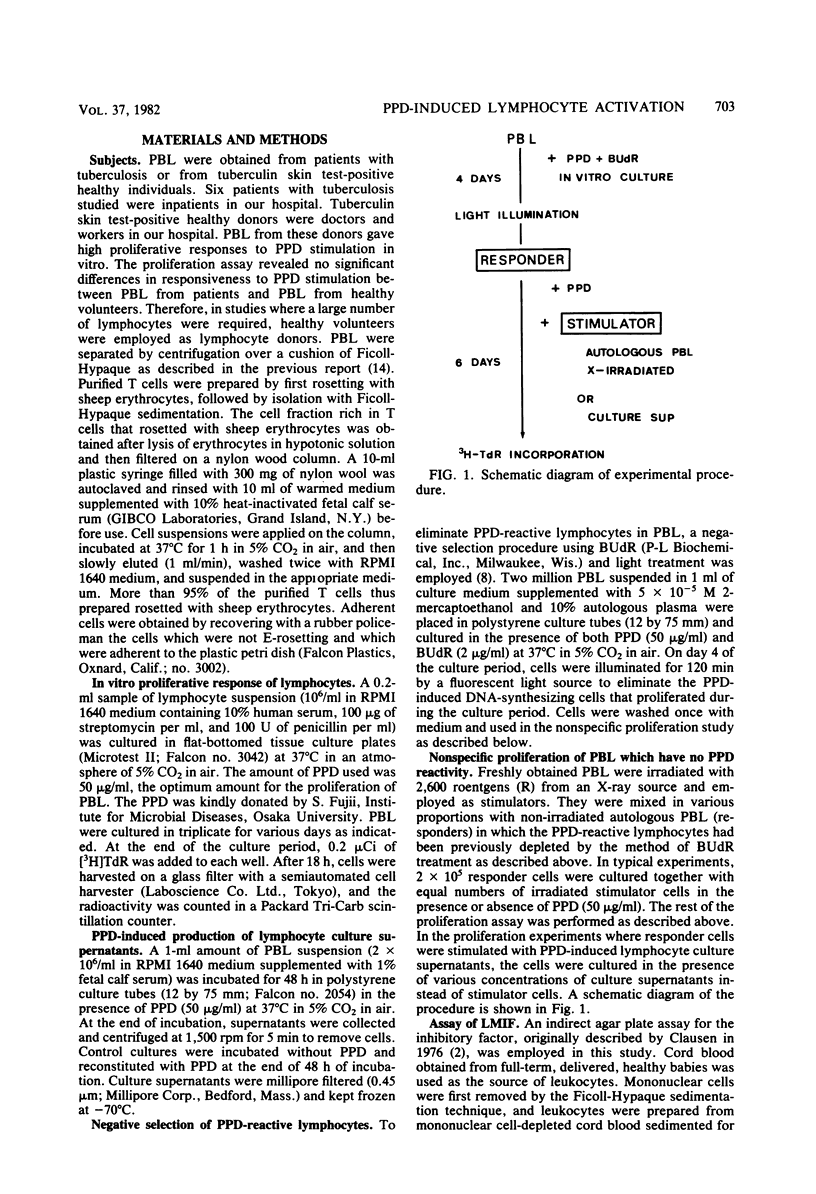
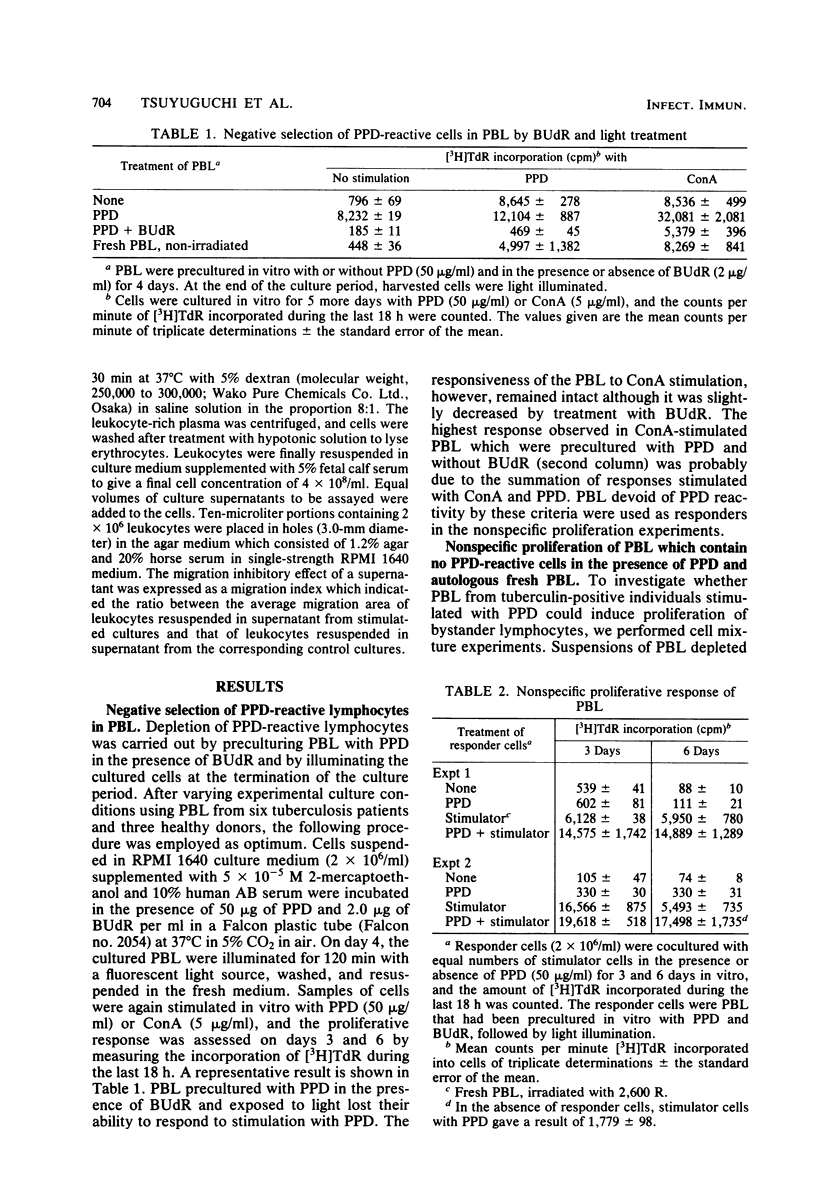
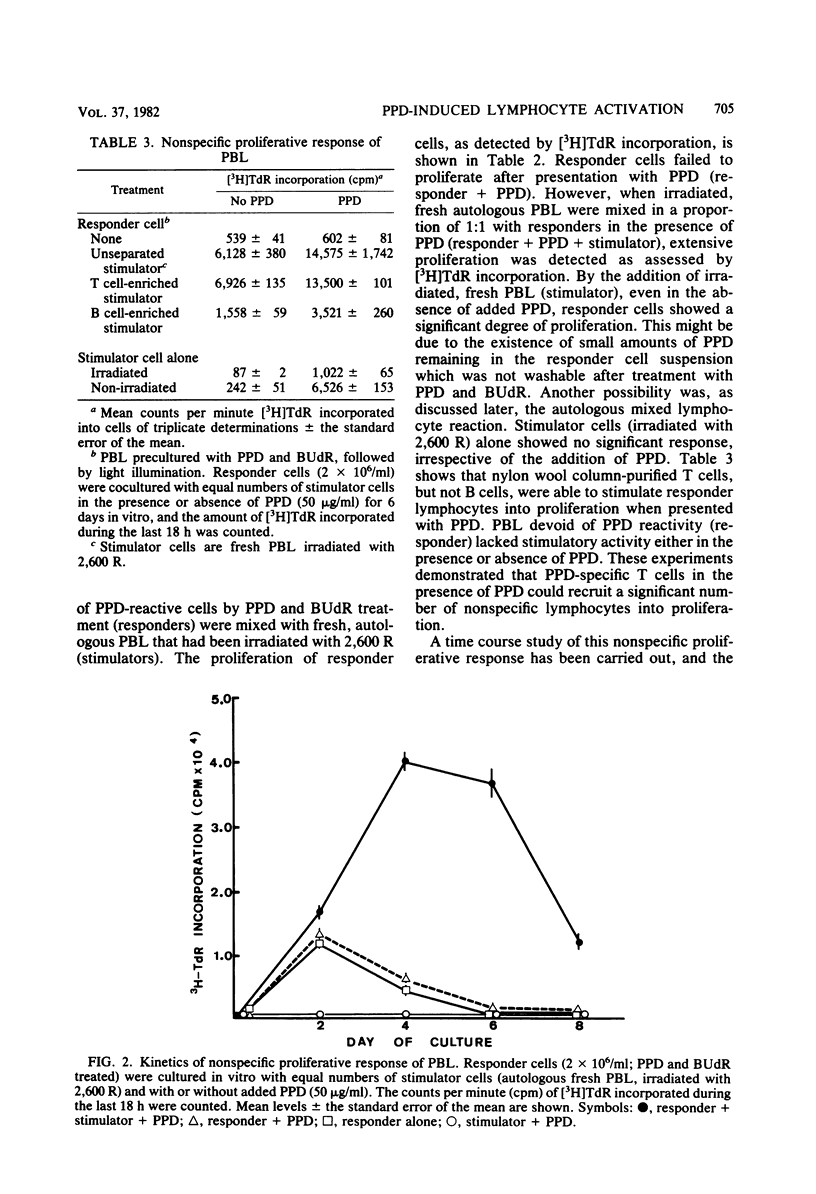
Peripheral blood lymphocytes (PBL) from tuberculosis patients were studied for their in vitro proliferative response stimulated with purified protein derivative (PPD)-tuberculin. Studies were designed to characterize the lymphocytes involved in the PPD-induced proliferation. PPD-responsive lymphocytes were eliminated in PBL by the procedure of cultivation of PBL with PPD in the presence of 5-bromodeoxyuridine and light illumination of the cultured cells. These PBL which lost PPD reactivity were no longer able to proliferate to PPD stimulation but were still capable of proliferation in the presence of both PPD and X-irradiated, autologous fresh PBL or upon addition of culture fluids from PPD-stimulated PBL. In addition, these nonspecifically activated lymphocytes released a soluble factor into the culture fluids which inhibited the migration of leukocytes. It was likely that large numbers of nonspecific T cells were induced to proliferate as a result of the presentation of specific T cells with the antigen PPD. It is suggested that a similar recruitment of lymphocytes by PPD-stimulated T cells takes place in vivo during the establishment of tuberculosis or antituberculous immunity or both.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustin A. A., Julius M. H., Cosenza H. Antigen-specific stimulation and trans-stimulation of T cells in long-term culture. Eur J Immunol. 1979 Sep;9(9):665–670. doi: 10.1002/eji.1830090903. [DOI] [PubMed] [Google Scholar]
- Clausen J. E. Migration inhibitory effect of cell-free supernatants from tuberculin-stimulated cultures of human mononuclear leukocytes demonstrated by two-step MIF agarose assay. J Immunol. 1973 Feb;110(2):546–551. [PubMed] [Google Scholar]
- Geha R. S., Schneeberger E., Rosen F. S., Merler E. Interaction of human thymus-derived and non-thymus-derived lymphocytes in vitro. Induction of proliferation and antibody synthesis in B lymphocytes by a soluble factor released from antigen-stimulated T lymphocytes. J Exp Med. 1973 Nov 1;138(5):1230–1247. doi: 10.1084/jem.138.5.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Indiveri F., Wilson B. S., Russo C., Quaranta V., Pellegrino M. A., Ferrone S. Ia-like antigens on human T lymphocytes: relationship to other surface markers, role in mixed lymphocyte reactions, and structural profile. J Immunol. 1980 Dec;125(6):2673–2678. [PubMed] [Google Scholar]
- Kuntz M. M., Innes J. B., Weksler M. E. Lymphocyte transformation induced by autologous cells. IV. Human T-lymphocyte proliferation induced by autologous or allogeneic non-T lymphocytes. J Exp Med. 1976 May 1;143(5):1042–1054. doi: 10.1084/jem.143.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Nishikawa S. I., Hirata T., Izumi T. PPD-induced immunoglobulin production in human peripheral blood lymphocytes. II. Separation of PPD-reactive helper T cells from PWM-reactive helper T cells in polyclonal immunoglobulin production of human peripheral blood lymphocytes. J Immunol. 1979 Sep;123(3):1106–1109. [PubMed] [Google Scholar]
- Philp J. R., McCormack J. G., Moore A. L., Johnson J. E., 3rd A lymphokine resembling transfer factor that stimulates MIF production by nonsensitive lymphocytes. J Immunol. 1981 Apr;126(4):1469–1472. [PubMed] [Google Scholar]
- Sheldon P. J., Holborow E. J. Human erythrocyte rosette formation with mitogen-stimulated human lymphocytes--a marker for the demonstration of activated T-cells. J Immunol Methods. 1975 Jul;7(4):379–386. doi: 10.1016/0022-1759(75)90046-0. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Gillis S., Baker P. E., McKenzie D., Ruscetti F. W. T-cell growth factor-mediated T-cell proliferation. Ann N Y Acad Sci. 1979;332:423–432. doi: 10.1111/j.1749-6632.1979.tb47136.x. [DOI] [PubMed] [Google Scholar]
- Tse H. Y., Schwartz R. H., Paul W. E. Cell-cell interactions in the T cell proliferative response. I. Analysis of the cell types involved and evidence for nonspecific T cell recruitment. J Immunol. 1980 Aug;125(2):491–500. [PubMed] [Google Scholar]
- Tsuyuguchi I., Shiratsuchi H., Fujiwara H. Increase in rosette-forming T cells with autologous human erythrocytes in lymphocytes of patients with tuberculosis by in vitro stimulation with purified protein derivative. Int Arch Allergy Appl Immunol. 1982;67(2):161–168. doi: 10.1159/000233008. [DOI] [PubMed] [Google Scholar]
- Tsuyuguchi I., Shiratsuchi H., Teraoka O., Hirano T. Increase in T cells bearing IgG Fc receptors in peripheral blood of patients with tuberculosis by in vitro stimulation with purified protein derivative. Am Rev Respir Dis. 1980 Jun;121(6):951–957. doi: 10.1164/arrd.1980.121.6.951. [DOI] [PubMed] [Google Scholar]


