Abstract
Background:
Anaplastic cortical ependymomas are rare lesions with few cases reported in the literature.
Case Description:
We present a unique case of an anaplastic cortical ependymoma in a 51-year-old female presenting as a butterfly lesion with involvement of both frontal lobes. The patient underwent gross total resection of her tumor with further adjuvant treatment. We present the findings in our case and review the literature surrounding supratentorial ependymomas and their treatment outcomes.
Conclusion:
Rarely, cortical ependymoma can present as a butterfly lesion and should be considered in the differential diagnosis of such lesions in adults.
Keywords: Adult, Anaplastic, Cortical, Ependymoma, Supratentorial
INTRODUCTION
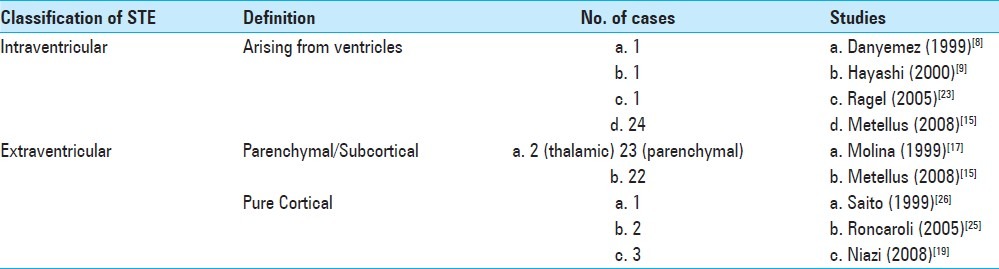
Ependymomas are tumors arising from the cells lining the ventricular system and the central canal of the spinal cord. In adults, ependymomas are uncommon, have a propensity to occur supratentorially and are associated with a higher incidence of anaplasticity.[18] Supratentorial ependymomas (STE) can be further divided into intraventricular and extraventricular types [Table 1]. Intraventricular ependymomas can be in either the third ventricle or lateral ventricles. Extraventricular ependymomas arise distant to the ventricles within the cerebral parenchyma and can even be purely cortical, arising purely within the cerebral cortex. The origin of the extraventricular STE is thought to be from fetal ependymal cell rests located in the periventricular region or at the angles of the ventricles.[28] Pure cortical ependymomas are the rarest, and we present, to our knowledge, a previously unreported case of an anaplastic ependymoma presenting as a butterfly lesion crossing the midline.
Table 1.
Classification of adult supratentorial ependymomas by tumor location
CASE REPORT
A 51-year-old Chinese female presented with the incidental finding of a large heterogeneously enhancing mass in the frontal lobes after a computer tomography (CT) scan for minor head injury following a fall [Figure 1]. Her past medical history included only adenomyosis and endometrial polyps. No neurological abnormalities were observed.
Figure 1.
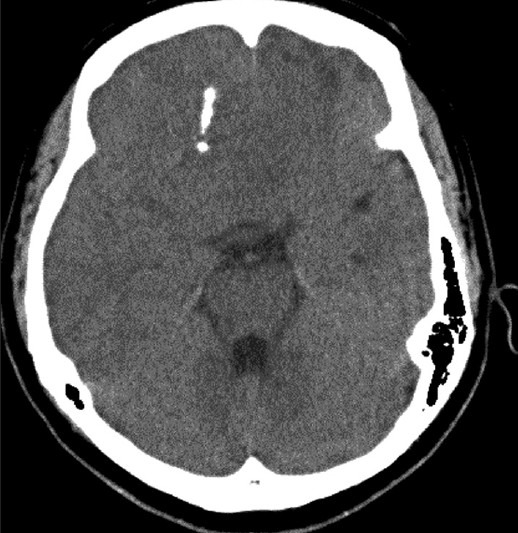
Noncontrasted CT brain showing bifrontal lestion with area of focal calcification
Magnetic resonance imaging (MRI) revealed a 76 × 70 × 54 mm mass in both frontal lobes with extension across the midline that was hypointense on T1-weighted, hyperintense on T2-weighted images, and demonstrated avid postgadolinium contrast enhancement [Figure 2]. The lesion showed foci of calcification and peripheral cystic components, with mild perilesional edema and significant mass effect. The corpus callosum was displaced posteriorly. Inferiorly, the lesion extended up to the olfactory bulb.
Figure 2.
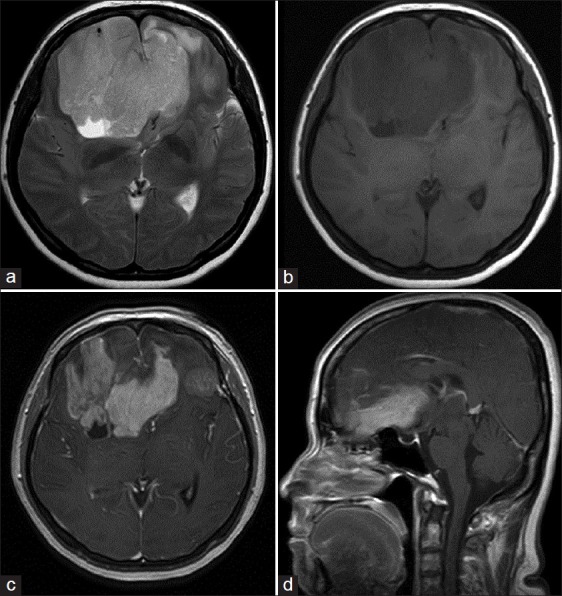
MRI showing bifrontal lesion crossing the midline and displacing the corpus callosum posteriorly (a) axial T2-weighted (b) axial T1-weighted (c) postcontrast axial T1-weighted, and (d) postcontrast sagittal T1-weighted images
The patient underwent gross total resection (GTR) of the tumor. Intraoperatively the tumor was not found to breach ventricles and the tumor was deemed to be entirely extraventricular. Intraoperative frozen section suggested the diagnosis of a high grade glioma. Histology showed a well demarcated cellulartumor [Figure 3] with prominent perivascular pseudo-rosettes and very occasional true (ependymal) rosettes. The tumor cells showed enlarged, hyperchromatic, pleomorphic nuclei, a granular ‘salt and pepper’ chromatin pattern, and fibrillary cytoplasm. Mitotic figures, including atypical forms were readily identified. There were areas of palisading necrosis and microvascular proliferation. The Ki-67 proliferative index was about 20%. These features were diagnostic of an anaplastic ependymoma (WHO grade III).
Figure 3.
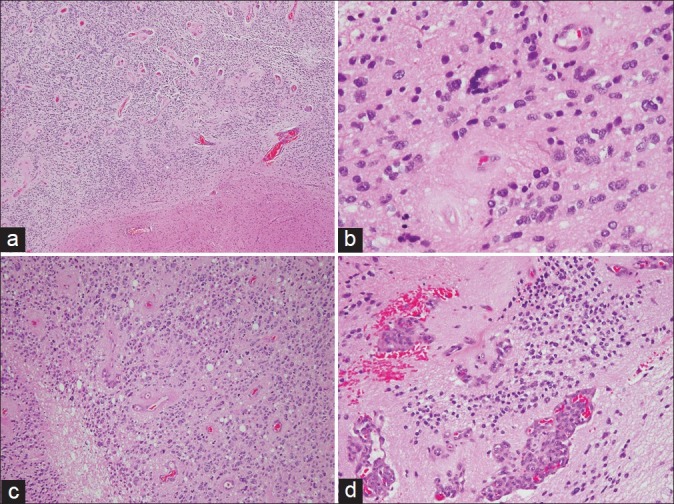
(a) Low power view showing a cellular tumor with perivascular pseudorosettes and a well demarcated border with the adjacent brain parenchyma (H and E, × 40). (b) An occasional true (ependymal) rosette is identified (H and E, × 400). (c) The tumor is mitotically active and shows areas of necrosis (H and E, × 200). (d) Microvascular proliferation is also present (H and E, × 200)
MRI taken on postoperative day 1 [Figure 4] showed no evidence of residual tumor. The patient's postoperative recovery was uneventful. Cerebrospinal fluid (CSF) studies were negative for malignant cells and no drop metastases were detected on neuroaxis MRI. The patient underwent adjuvant intensity- modulated radiation therapy of 60 Grays in 30 fractions to a region encompassing the tumor bed and a 2 cm margin around it over a period of 2 months. At follow up, the patient developed local recurrence in the frontal lobes within 2 months of completing radiotherapy. At 8 months postsurgery, progression of disease locally had caused her to become increasingly drowsy and by then she was wheelchair bound.
Figure 4.
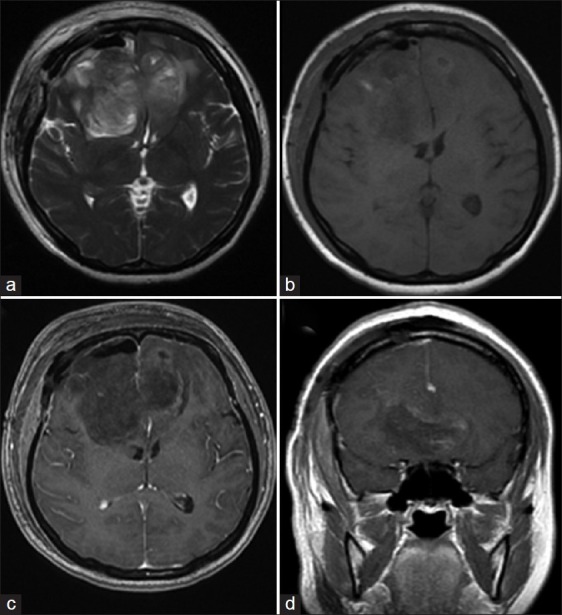
MRI on postoperative day 1 showing complete tumor resection (a) axial T2-weighted (b) axial T1-weighted (c) postcontrast axial T1-weighted, and (d) postcontrast coronal T1-weighted images
DISCUSSION
The term ‘butterfly lesion’ describes lesions that extend cross the midline, often via involvement of the corpus callosum. Lesions are typically aggressively infiltrative tumors such as glioblastoma multiforme (GBM) and B-cell lymphoma, and, given that the corpus callosum comprises primarily of myelinated axons, demyelinating disease such as multiple sclerosis.[5,6] In an immuno-compromised patient, primary central nervous system (PCNS) lymphoma, progressive multifocal leukoencephalopathy, and rarely, toxoplasmosis have been reported.[5,7,8] In our patient the corpus callosum was found to have been displaced posteriorly rather than infiltrated, a feature that helped to differentiate her tumor from an infiltrative process. As cortical STEs are rare and have not been reported to present as butterfly lesions, they were not considered in our pre-operative differential diagnosis.
STEs are usually large (≥4 cm) and cystic with their location classified as shown in Table 1. On CT they demonstrate moderate to intense contrast enhancement with homogeneous or ring-enhancement patterns. On MRI, they appear hypointense on T1-weighted and hyperintense on T2-weighted imaging, respectively. Peri-tumoral edema and hydrocephalus may be present and about one-third of tumors may harbor calcification.[2,29] Extraventricular tumors tended to present with seizures, focal neurological deficits, and behavioral disturbances while intraventricular lesions present with hydrocephalus and raised intracranial pressure.[15,30,31]
Although the clinical features in this case were highly suggestive of a GBM, this was later disproved by the classic histopathological features of an anaplastic ependymoma. There are also no known cases of GBM with ependymal differentiation reported in the literature.
Several prognostic factors have been identified with regard to survival outcome and tumor recurrence. Compared with infratentorial or spinal ependymomas, patients with STE tend to have poorer overall survival (OS) and progression-free survival (PFS) in patients.[1,13,14] Other predictors factors of poor prognosis include older age,[13,15] presence of microvascular proliferation, presence of necrosis, number of mitoses, degree of hypercellularity,[20] and greater Ki-67/MIB-1 proliferation index.[3,15] Extent of tumor resection was a positive predictor of OS and PFS,[1,14,15] and subtotal resection was correlated with subsequent CSF dissemination and metastasis. There is controversy about the prognostic value of the current histological grading system,[11,12,14] and a lack of consensus in establishing the criteria for classifying tumors according to the degree of anaplasticity.[12,14,15] However, overall histological grade and incompleteness of resection have been identified as significant predictors of tumor recurrence.[3,27]
Maximal safe resection with adjuvant radiotherapy (RT) is the current mainstay of treatment.[13,19,20,25] There has been interest in identifying patients in whom RT can be avoided without worsening treatment outcome with several reports showing treatment success with GTR without adjuvant RT for low grade ependymomas[4,21,24] and in some anaplastic STE in pediatric cases.[10,22] Studies have shown that prophylactic spinal irradiation has little role in the treatment of STE as the cortical lesions have a negligible risk of drop metastases and leptomeningeal seeding due to their distance from CSF pathways.[20] The overall outcome after surgery for STE is summarized in 2.
Our case is a unique case of an adult anaplastic supratentorial cortical ependymoma presenting as a butterfly lesion. This particular diagnosis should be considered in the differential diagnosis of such butterfly lesions in an adult.[Table 2]
Table 2.
Case series reporting on outcome in adult with supratentorial ependymomas following treatment
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/1/107/101001
Contributor Information
David W.K. Ng, Email: david.ng@mohh.com.sg.
Nicolas K.K. King, Email: Nicolas_kon@nni.com.sg.
Aaron S.C. Foo, Email: aaron.foo@mohh.com.sg.
Yih-Yian Sitoh, Email: Yih_Yian_Sitoh@nni.com.sg.
Hwei Yee Lee, Email: Hwei_Yee_Lee@ttsh.com.sg.
Wai Hoe Ng, Email: Wai_Hoe_Ng@nni.com.sg.
REFERENCES
- 1.Amirian ES, Armstrong TS, Gilbert MR, Scheurer ME. Predictors of survival among older adults with ependymoma. J Neurooncol. 2012;107:183–9. doi: 10.1007/s11060-011-0730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armington WG, Osborn AG, Cubberley DA, Harnsberger HR, Boyer R, Naidich TP, et al. Supratentorial ependymoma: CT appearance. Radiology. 1985;157:367–72. doi: 10.1148/radiology.157.2.4048443. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong TS, Vera-Bolanos E, Bekele BN, Aldape K, Gilbert MR. Adult ependymal tumors: Prognosis and the M. D. Anderson Cancer Center experience. Neuro Oncol. 2010;12:862–70. doi: 10.1093/neuonc/noq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awaad YM, Allen JC, Miller DC, Schneider SJ, Wisoff J, Epstein FJ. Deferring adjuvant therapy for totally resected intracranial ependymoma. Pediatr Neurol. 1996;14:216–9. doi: 10.1016/0887-8994(96)00020-3. [DOI] [PubMed] [Google Scholar]
- 5.Bourekas EC, Varakis K, Bruns D, Christoforidis GA, Baujan M, Slone HW, et al. Lesions of the corpus callosum: MR imaging and differential considerations in adults and children. AJR Am J Roentgenol. 2002;179:251–7. doi: 10.2214/ajr.179.1.1790251. [DOI] [PubMed] [Google Scholar]
- 6.Bower RS, Burrus TM, Giannini C, Erickson BJ, Meyer FB, Pirko , et al. Teaching neuroimages: Demyelinating disease mimicking butterfly high-grade glioma. Neurology. 2010;75:e4–5. doi: 10.1212/WNL.0b013e3181e7ca1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhari VV, Yim CM, Hathout H, Lai A, Donovan SM. Atypical imaging appearance of toxoplasmosis in an HIV patient as a butterfly lesion. J Magn Reson Imaging. 2009;30:873–5. doi: 10.1002/jmri.21924. [DOI] [PubMed] [Google Scholar]
- 8.Daneyemez M, Can C, Izci Y, Beduk A, Timuckaynak E. The tanycytic ependymoma of the lateral ventricle: Case report. Minim Invasive Neurosurg. 1999;42:201–3. doi: 10.1055/s-2008-1053399. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi S, Kameyama S, Fukuda M, Takahashi H. Ganglioglioma with a tanycytic ependymoma as the glial component. Acta Neuropathol. 2000;99:310–6. doi: 10.1007/pl00007443. [DOI] [PubMed] [Google Scholar]
- 10.Hukin J, Epstein F, Lefton D, Allen J. Treatment of intracranial ependymoma by surgery alone. Pediatr Neurosurg. 1998;29:40–5. doi: 10.1159/000028683. [DOI] [PubMed] [Google Scholar]
- 11.Kawabata Y, Takahashi JA, Arakawa Y, Hashimoto N. Long-term outcome in patients harboring intracranial ependymoma. J Neurosurg. 2005;103:31–7. doi: 10.3171/jns.2005.103.1.0031. [DOI] [PubMed] [Google Scholar]
- 12.Korshunov A, Golanov A, Sycheva R, Timirgaz V. The histologic grade is a main prognostic factor for patients with intracranial ependymomas treated in the microneurosurgical era: An analysis of 258 patients. Cancer. 2004;100:1230–7. doi: 10.1002/cncr.20075. [DOI] [PubMed] [Google Scholar]
- 13.Mansur DB, Perry A, Rajaram V, Michalski JM, Park TS, Leonard JR, et al. Post operative radiation therapy for grade II and III intracranial ependymoma. Int J Radiat Oncol Biol Phys. 2005;61:387–91. doi: 10.1016/j.ijrobp.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Metellus P, Barrie M, Figarella-Branger D, Chinot O, Giorgi R, Gouvernet J, et al. Multicentric French study on adult intracranial ependymomas: Prognostic factors analysis and therapeutic considerations from a cohort of 152 patients. Brain. 2007;130(Pt 5):1338–49. doi: 10.1093/brain/awm046. [DOI] [PubMed] [Google Scholar]
- 15.Metellus P, Figarella-Branger D, Guyotat J, Barrie M, Giorgi R, Jouvet A, et al. Supratentorial ependymomas: Prognostic factors and outcome analysis in a retrospective series of 46 adult patients. Cancer. 2008;113:175–85. doi: 10.1002/cncr.23530. [DOI] [PubMed] [Google Scholar]
- 16.Metellus P, Guyotat J, Chinot O, Durand A, Barrie M, Giorgi R, et al. Adult intracranial WHO grade II ependymomas: Long-term outcome and prognostic factor analysis in a series of 114 patients. Neuro Oncol. 2010;12:976–84. doi: 10.1093/neuonc/noq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molina OM, Colina JL, Luzardo GD, Mendez OE, Cardozo D, Velasquez HS, et al. Extraventricular cerebral anaplastic ependymomas. Surg Neurol. 1999;51:630–5. doi: 10.1016/s0090-3019(98)00118-9. [DOI] [PubMed] [Google Scholar]
- 18.Mork A, Loken A. Ependymoma. Cancer. 1977;40:907–15. doi: 10.1002/1097-0142(197708)40:2<907::aid-cncr2820400247>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Niazi TN, Jensen EM, Jensen RL. WHO Grade II and III supratentorial hemispheric ependymomas in adults: Case series and review of treatment options. J Neurooncol. 2009;91:323–8. doi: 10.1007/s11060-008-9717-z. [DOI] [PubMed] [Google Scholar]
- 20.Oya N, Shibamoto Y, Nagata Y, Negoro Y, Hiraoka M. Postoperative radiotherapy for intracranial ependymoma: Analysis of prognostic factors and patterns of failure. J Neurooncol. 2002;56:87–94. doi: 10.1023/a:1014442106111. [DOI] [PubMed] [Google Scholar]
- 21.Papadopoulos DP, Giri S, Evans RG. Prognostic factors and management of intracranial ependymomas. Anticancer Res. 1990;10:689–92. [PubMed] [Google Scholar]
- 22.Paulino AC. The local field in infratentorial ependymoma: Does the entire posterior fossa need to be treated? Int J Radiat Oncol Biol Phys. 2001;49:757–61. doi: 10.1016/s0360-3016(00)01353-5. [DOI] [PubMed] [Google Scholar]
- 23.Ragel BT, Townsend JJ, Arthur AS, Couldwell WT. Intraventricular tanycytic ependymoma: Case report and review of the literature. J Neurooncol. 2005;71:189–93. doi: 10.1007/s11060-004-1371-5. [DOI] [PubMed] [Google Scholar]
- 24.Reni M, Brandes AA, Vavassori V, Cavallo G, Casagrande F, Vastola F, et al. A multicenter study of the prognosis and treatment of adult brain ependymal tumors. Cancer. 2004;100:1221–9. doi: 10.1002/cncr.20074. [DOI] [PubMed] [Google Scholar]
- 25.Roncaroli F, Consales A, Fioravanti A, Cenacchi G. Supratentorial cortical ependymoma: Report of three cases. Neurosurgery. 2005;57:E192. doi: 10.1227/01.neu.0000164171.29292.d6. [DOI] [PubMed] [Google Scholar]
- 26.Saito T, Oki S, Mikami T, Kawamoto Y, Yamaguchi S, Kuwamoto K, et al. Supratentorial ectopic ependymoma: A case report. No Shinkei Geka. 1999;27:1139–44. [PubMed] [Google Scholar]
- 27.Schwartz TH, Kim S, Glick RS, Bagiella E, Balmaceda C, Fetell MR, et al. Supratentorial ependymoma in adult patients. Neurosurgery. 1999;44:721–31. doi: 10.1097/00006123-199904000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Shuangshoti S, Rushing EJ, Mena H, Olsen C, Sandberg GD. Supratentorial extraventricular ependymal neoplasms: A clinicopathologic study of 32 patients. Cancer. 2005;103:2598–605. doi: 10.1002/cncr.21111. [DOI] [PubMed] [Google Scholar]
- 29.Spoto GP, Press GA, Hesselink JR, Solomon M. Intracranial ependymoma and subependymoma: MR manifestations. AJNR Am J Neuroradiol. 1990;11:83–91. [PMC free article] [PubMed] [Google Scholar]
- 30.Van Gompel JJ, Koeller KK, Meyer FB, Marsh WR, Burger PC, Roncaroli F, et al. Cortical ependymoma: An unusual epileptogenic lesion. J Neurosurg. 2011;114:1187–94. doi: 10.3171/2010.12.JNS10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yadav YR, Neha , Chandrakar SK. Pure cortical supratentorial extraventricular ependymoma. Neurol India. 2009;57:213–5. doi: 10.4103/0028-3886.51301. [DOI] [PubMed] [Google Scholar]


