Abstract
Constitutional delay of growth and puberty is a transient state of hypogonadotropic hypogonadism associated with prolongation of childhood phase of growth, delayed skeletal maturation, delayed and attenuated pubertal growth spurt, and relatively low insulin-like growth factor-1 secretion. In a considerable number of cases, the final adult height (Ht) does not reach the mid-parental or the predicted adult Ht for the individual, with some degree of disproportionately short trunk. In the pre-pubertal male, testosterone (T) replacement therapy can be used to induce pubertal development, accelerate growth and relieve the psychosocial complaints of the adolescents. However, some issues in the management are still unresolved. These include type, optimal timing, dose and duration of sex steroid treatment and the possible use of adjunctive or alternate therapy including: oxandrolone, aromatase inhibitors and human growth hormone.
Keywords: Constitutional delay of growth and puberty, growth hormone, human chorionic gonadotropin, hypogonadotropic hypogonadism, oxandrolone, testosterone
INTRODUCTION
Although the commonest disorders of delayed puberty are idiopathic, concerns raised by delayed puberty are many and variably appreciated by different persons. These include possibility of underlying pathology, fear that puberty will never occur and emotional and psychosocial upset of immaturity, especially the associated short stature. In addition, concerns about long-term sequels comprising reduced final adult height (Ht), bone-mineralization and fertility may also be raised.[1–4]
Most specialists would not consider any treatment unless the boy is at least 14 years old and his bone age is at least 12 years old. However, many important questions are raised and their answers are briefly attempted in this review for better management of this condition.
THE NATURAL HISTORY OF CONSTITUTIONAL DELAY OF GROWTH AND PUBERTY
Inheritance pattern of constitutional delay of growth and puberty (CDGP) is consistent with autosomal dominant inheritance. It occurs twice as frequently in boys versus girls. The Ht and weight deficit and the short sitting Ht are usually evident at presentation and continue up to final adult Ht. The age of onset of puberty is delayed by an average of 2.5 years in girls and 3 years in boys. Ht deficit at onset of puberty correlates with final Ht. The time between onset of puberty and pubertal growth spurt is shorter than in normal children and the peak growth velocity is attenuated. Therefore, the mean final Ht is usually shorter than both target Ht and predicted adult Ht. The final Ht lies on average 1.85 SD below the mean of healthy adults, with large individual variations. Better final Ht appears to occur in children who had their slow growth during their late childhood, while those with early and progressive reduction of relative Ht in the early childhood have compromised final Ht. In addition, CDGP is combined with familial short stature in around 40% of short children with Ht < 3rd centile for age and gender.[5–9]
EARLY DIFFERENTIATION BETWEEN CONSTITUTIONAL DELAY OF GROWTH AND PUBERTY FROM PERMANENT HYPOGONADOTROPIC HYPOGONADISM
The question can be answered in many cases by following these steps:
A. Clinical assessment: This includes full physical and genital examination, studying the pattern of growth and skeletal maturation of the person as well as family history of puberty and measuring the Ht of parents. Analysis of these data can further divide these children into the following four categories:
-
According to family history and measurements of parents’ Ht into:
- CDGP with familial short stature (≈ 40% of patients)
- CDGP with normal parental stature (≈ 60% of patients)[10]
-
According to the pattern of Ht deficit will further divide these patients into:
- Those who have Ht deficit early in their childhood (less favorable prognosis for final Ht).
- Those who have Ht deficit late in childhood (better prognosis for final Ht.[11]
-
According to the size of the testes can further divide patients into two groups
- Those with a testicular volume = or > 4 mL (95% or more have CDGP)
- Those with testicular volume below 4 mL (40% of CDGP and all patients with hypogonadotropic hypogonadism (HH) have testicular volume < 4 ml)[12]
-
According to their predicted final adult Ht into:
- Those with predicted Ht near to mid-parental Ht with normal Ht standard deviation score (HtSDS).
- Those with predicted Ht below mid-parental Ht especially those with HtSDS < 2
Unfortunately, the prediction of final adult Ht in these patients proves to be inaccurate in many studies, which increase the difficulty of taking the decision for treatment.
B. Endocrine Assessment
This includes the measurements of:
-
Basal serum concentrations of morning testosterone (T). Again, this can separate boys with HH into two groups.[12]
- Those with basal T = or > 1.7 nmol/L (almost all of them have CDGP)
- Those with basal T < 1.7 nmol/L (all patients with HH and 45% of those with CDGP.[12]
Patients who have small testicular volume (<4 mL) and low T level (< 1.7 nmol/L) need further studies: including human chorionic gonadotropin (hCG) and/ or GnRH/GnRHa tests.
Table 1 presents different useful studies using these tests and their cut-point to differentiate CDGP and HH.
Table 1.
Different endocrine tests to differentiate constitutional delay of growth and puberty from hypogonadotropic hypogonadism [modified from 12-16]
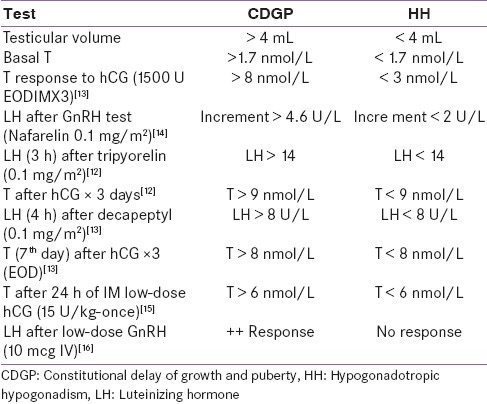
C. Response to a short therapeutic course of androgen
Increased testicular length during or over the following 4 months after stopping androgen treatment indicates normal gonadotropin secretion and pubertal progression.[17]
SHALL WE TREAT OR NOT?
The challenging question is always shall we treat or not? This question becomes easier to answer if we consider some facts in the natural history of the condition.
Although in majority of cases spontaneous pubertal development will “kick in,” the pubertal onset is unpredictable and the average delay is around 3 years (i.e., a state of HH for a long time)
Many boys do not attain their mid-parental nor their predicted adult Ht and a good percentage of them end short for their parents and population
Many of them have combined CDGP and familial short stature
Methods for prediction of the final Ht are not reliable in CDGP
There is some evidence that this prolonged, although transient, state of hypogonadism might affect the bone mineral content or the total bone mass of adults who had CDGP, although this is not universally accepted
In addition; the effect of this state of hypogonadism on the onset and progression of spermatogenesis, follicle stimulating hormone (FSH and T are both required for initiation and maintenance of spermatogenesis in animal and human studies) and future fertility is still not studied
The association of CDGP associated with incompetence and vulnerability, impaired self-esteem, reluctance to participate in athletic activities, social isolation, impaired academic performance, substance abuse and disruptive and suicide behavior have been shown in many, but not all, studies to be significant. In fact, until now the most common reason behind treating these adolescents is psychological stress experienced by the child or his parents.[6,18–23]
ANDROGEN THERAPY, WHAT IS THE OPTIMAL TIMING, DOSE AND DURATION OF TREATMENT?
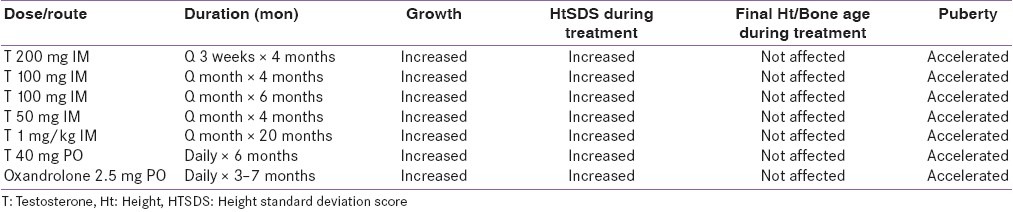
The most common drug used to treat this condition is depot T injections. Compared with other regimens, short- course low-dose depot T IM is an effective, practical, safe, well-tolerated and inexpensive regimen [Table 2]. However, some unresolved issues in the management include: the effect of androgen on inducing maturation of hypothalamic-pituitary axis, and the type, optimal timing, dose and duration of androgen treatment.[24]
Table 2.
Androgen therapy in constitutional delay of growth and puberty used in different studies [modified from 16-24]
Are we using androgens to sensitize/stimulate the hypothalamic-pituitary-gonadal axis to accelerate the onset of puberty? Or are we only replacing this transient, although long (2–3 years), hypogonadal state?
In fact, some good evidence of acceleration of the onset of central puberty in some boys with CDGP are proved with enlargement of the testes, during or in the 3–4 months following short course of T therapy (3–4 months) documenting the start of normal spontaneous gonadotropins secretion by the pituitary. Stimulation of true early puberty by excessive androgen in undertreated congenital adrenal hyperplasia is another example of T stimulation of puberty in boys and girls. However, some patients with CDGP require longer and/or repeated courses of T to induce onset of true puberty (18 months). It appears that some patients need prolonged replacement until maturation of their hypothalamic-pituitary-gonadal axis evolves.[25–30]
MONITORING ISSUES AND TAILORING TESTOSTERONE THERAPY TO SIMULATE PHYSIOLOGIC MATURATION
T, a natural androgen, is an acceptable physiologic replacement in this transient hypogonadal state. Short-term results of variable doses and duration of T therapy seem to be satisfactory [Table 2]. However, studying the pattern of normal T secretion in boys during normal puberty shall be our reference for the proper use of T therapy.
During normal puberty, normal T secretion has the following characteristics:
Gradual increase of circulating T levels with progression of puberty from Tanner II to Tanner V stages, correlated with testicular volume
The intra-testicular T, under the effect of luteinizing hormone (LH), is 50–100 times more than circulating T concentration
Episodic secretion of testicular T with a higher early morning circulating level 4. T secretion is associated with increasing secretion of FSH through puberty; both appear critical for the beginning of spermatogenesis.[31–35]
The clinical challenge is to tailor T replacement to induce puberty and optimize total skeletal growth without inducing premature fusion of the growth plate and to avoid any harmful effect on spermatogenesis. In other words, T therapy should be adjusted to simulate the gradually increasing circulating levels of T from Tanner II (average 60–150ng/dL) to Tanner III (150–250ng/ dL) to Tanner IV (250–500 ng/dL) to Tanner V (500–750 ng/ dL). In order to do that it is advisable to start with lower dose (e.g.,15 mg every 2 weeks monthly) of one of the long-acting esters (enanthate or cypionate) and gradually increasing the dose by 5 mg every 2 months.
Different T doses have been used by many authors with apparently satisfactory results.[17,36–42] The question is: can we achieve these levels of circulating T with the above used doses? Measurement of serum T levels is the most cost-effective way of monitoring T replacement therapy. In patients being treated with T enanthate or cypionate, serum T levels should be in the mid-normal range (as required) 1 week after the injection. If nadir levels 14 days after the injection are low, the interval between injections may be shortened.[36]
Monitoring T level at the end of the month or before injecting the next dose will help to decide when to stop the injection. Serum T = or > 100 ng/dL at that time points out to enough endogenous production of T and maturation of the hypothalamic-pituitary-gonadal axis. Most adolescents with CDGP need between 6 and 18 months of T therapy before maturation of their hypothalamic-pituitary-gonadal axis.[43–47]
Average circulating T concentrations after different doses of IM T doses are summarized in Table 3. It is clear that even with the low dose of T (50 mg per month), T blood level may exceed the physiological required dose (for 10– 15 days per month). Although most drug delivery devices may be appropriate for full adult androgen replacement, these doses are too large for the induction of puberty. Oral T (undecanoate), because of its short half-life and wide fluctuation of serum T level is not an ideal for androgen replacement. At present, the injectable form is the only one that is easily adaptable for the increasing amounts of androgen necessary for the various stages of pubertal development.
Table 3.
Average serum concentrations of T after T depot injection
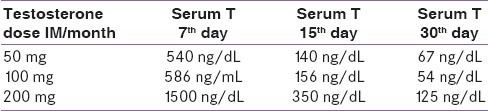
It appears that a protocol of twice monthly injections of low doses of T with a gradual and periodic increase in the dose would approach the physiological level of serum T appropriate for the early and middle stages of puberty in males with CDGP. A suggested protocol is as following:
T enanthate IM 15 mg Q 2 weeks for 2 months; then
T enanthate IM 20 mg Q 2 weeks for 2 months; then
T enanthate IM 25 mg Q 2 weeks for 2 months; then
T enanthate IM 30 mg Q 2 weeks for 2 months; then
T enanthate IM 35 mg Q 2 weeks for 2 months; then
T enanthate IM 40 mg Q 2 weeks for 2 months; then
T enanthate IM 45 mg Q 2 weeks for 2 months; then
T enanthate IM 50 mg Q 2 weeks for 2 months; then
T enanthate IM 60 mg Q 2 weeks for 2 months.
CONSTITUTIONAL DELAY OF GROWTH AND PUBERTY AND SPERMATOGENESIS
Normal spermarche occurs early in puberty before the peak growth spurt. It may occur with the early appearance or even before the appearance of pubic hair and testes growth. For spermatogenesis to be initiated, concentrations of T well in excess of those are needed to maintain androgen effects in other regions of the body. LH-induced T secretion is associated with age-appropriate gonadotropin production. In the normal testis, LH stimulates Leydig cells and this provides the testis with high local T to act directly on the androgen receptor AR to maintain sperm production. In the T-suppressed testis (using exogenous T), the low intratesticular T may not be able to initiate or maintain spermatogenesis. FSH is important for Sertoli cell function necessary for beginning of spermatogenesis. In rat, monkey, and human models, FSH and androgens act both separately and synergistically to support a range of key events in spermatogenesis, from spermatogonial stem cell division through to final sperm release. In addition, gonadotropins regulate germ cell survival in normal adult men.[48–55] Information about the effect of delayed puberty and/or androgen therapy on future (adult) spermatogenesis and fertility are lacking and need to be studied.
HUMAN CHORIONIC GONADOTROPIN VERSUS T THERAPY, ADVANTAGES AND DISADVANTAGES
Human chorionic gonadotropin (hCG), mainly with LH activity, can be used to induce puberty in CDGP. The half-life in serum is biphasic at 11 and 23 h with renal elimination; 10–12% within 24 h. In idiopathic hypogonadism, treatment with hCG 1500 U twice weekly, either SC or IM, for 6 months, keeps T level (and testicular size) in the normal level range similar to normal early puberty. In fact, hCG doses can be easily tailored (because of frequent injections) to give the required level of circulating T. During hCG therapy, there is diurnal variation in T, with peak serum levels in the morning falling to a nadir in the evening. In adult males with HH, hCG (5000 IU IM twice/week) can maintain normal adult level of serum T and may be sufficient to induce spermatogenesis without FSH. The use of hCG therefore appears to be more physiologic and potentially safer than T in the timely initiation of spermatogenesis and testicular growth in boys with CDGP. Short-term results of hCG and T therapy in CDGP revealed comparable results. However, HCG is more expensive and requires multiple injections.[33,48,56–62]
OTHER FORMS OF TREATMENT
Low-dose oxandrolone
Low-dose Oxandrolone (0.1 mg/kg/day, 2.5 mg/day for 3–12 months) is associated with mean increment of Ht growth velocity during treatment, which is maintained after treatment, with no significant change in Ht for bone age standard deviation scores and no change in final Ht. The possible use of oxandrolone earlier in the course of CDGP may be an advantage of this therapy and allows the use of other form of therapy later on if puberty is delayed.[63,64]
Addition of aromatase inhibitor
An aromatase inhibitor, e.g., letrozole (2.5 mg/PO) in addition to T (1 mg/kg/month for 6 months) appears to improve (increase) the final Ht to approach mid-parental Ht versus T + Placebo.[65,66] In fact, these results indirectly point out to a negative effect of using even low dose of T therapy on the final Ht of these patients.[65,66]
The use of dihydrotestosterone
Dihydrotestosterone (DHT) (50 mg IM every 2 weeks, for 4 months) is associated with appearance of secondary sex characteristics (Tanner II), increased lean body mass and decreased % body fat with no change in IGF-I, mean nocturnal GH, and estradiol (E2) concentrations.[67] In theory, lack of E2 effect may increase the potential for final Ht, as with adding aromatase inhibitor; however, these long-term studies about the effect on final adult Ht are lacking.
Growth hormone treatment for constitutional delay of growth and puberty
According to FDA-approved guidelines, boys whose Ht predictions fall to 160 cm or less are considered for treatment with recombinant growth hormone (rechGH). However, none of the available methods of prediction are sufficiently sensitive to reliably recruit 14 to 16-year- old boys with CDGP whose final Ht will fall at or below 160 cm.[68–70] Before the onset of puberty, many boys with CDGP are short (HtSDS < -2) and have delayed bone age versus their chronological age. GH treatment for boys (n = 36) with non-familial short children with delayed bone age, started at an average age of 9.3 years (using 0.6 U/ kg/ week divided on daily doses) resulted in a Ht gain of 1.4 SD at the final Ht. In one study, oxandrolone (2.5 mg/day for 3 months) was compared to GH therapy (20 U/m2 /week for 12 months). Although both treated groups had accelerated growth velocity, the growth rate was better in the oxandrolone group. In this study, the older age of the boys (mean age = 13.9 years) and the short duration of therapy (12 months) could have affected the response to GH in these boys. Other short-term studies did not find significant effect of GH on final Ht in these boys. These data demonstrate the potential benefit of GH therapy if started early and used for longer duration in markedly short patients with CDGP. It may be an early useful adjunct to later androgen therapy in those children with poor prognosis for final Ht (i.e., those who had decelerated growth rate in early childhood, and those with short predicted adult Ht).[11]
Moreover, in a good number of boys, CDGP is associated with familial short stature.[10] The latter showed fairly good response to GH therapy. A meta-analysis of GH treatment for idiopathic short stature showed favorable response on the short-term growth rate as well as on the final Ht; this effect appears to be dose dependent.[71–74] The safety of GH treatment in pre-pubertal short boys on testicular size and T secretion was assessed and it appears that GH has no negative effect on both.[75]
Recently, it has been shown that boys with CDGP have increased total energy expenditure associated with relative deficiencies in T and lower IGF-I concentrations. Whether added nutritional supplements, alone or in combination with GH, which proved useful in many hypermetabolic states, could improve the growth pattern and final Ht of these children deserves further study.[76]
CONCLUSION AND MAIN POINTS
The differential diagnosis of delayed puberty in boys is essentially that of HH. The most common form of this is CDGP, which entails delayed onset of puberty but normal progression to normal adult. However, this diagnosis can only be reached after excluding other causes that lead to permanent HH, which cannot be diagnosed until late.
Diagnostic evaluation of an adolescent with delayed puberty includes detailed history and physical examination, including previous and present auxological parameters and bone maturation. Familial history is useful to support the diagnosis of CDGP. General hematological and biochemical parameters are useful to rule out some chronic systemic conditions that may present only by delayed growth and puberty. Basal hormonal study includes measurement of serum concentrations of gonadotropins (LH, FSH), T, prolactin, and thyroid hormones. Evaluation of GH- IGF-I axis may be indicated in some cases keeping in mind that IGF-I and IGFBP-3 levels are sex and age specific; they should be interpreted using skeletal age rather than chronologic age. Moreover, GH provocative testing may also yield falsely low values in some peri-pubertal individuals with CDGP unless they are primed with sex steroids.
An MRI of the hypothalamic-pituitary area may be necessary in subjects with rapid deceleration of growth rate, those with symptoms and/or signs suggesting increased intracranial pressure, or history of head trauma, and those with abnormal GH response to provocation. A karyotype is indicated in short girls with delayed puberty and in males with hypergonadotropic hypogonadism.[1–4]
HH is a lack of T in male patients secondary to hypothalamic or pituitary (low LH and FSH secretion), which may be transient (CDGP) or permanent. Boys aged 14 years or older should be suspected of being hypogonadal if they have underdeveloped testes and lack of secondary sexual characteristics. The benefits of T replacement therapy may include accelerating growth rate and inducing pubertal onset, but it shortens pubertal duration with apparently no adverse effect on final adult Ht. Androgen therapy appears to improve psychosocial function and assures normal bone mineral accretion during the critical period of adolescence and improves muscle mass and strength. Injection of low-dose of T enanthate or cypionate every 2–4 weeks or hCG twice weekly and gradual increase of doses to simulate physiological progression of puberty and maintain serum T levels in the mid-normal range (as required) 1 week after the injection appears to be a good goal . The use of low dose of oxandrolone between 12 and 14 years may offer an advantage of increasing growth rate without compromising adult Ht. The wise and early use of GH treatment with nutritional supplementation in some of these boys may be useful but remains to be proven.[69–76]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Martinez AS, Domené HM, Ropelato MG, Jasper HG, Pennisi PA, Escobar ME, et al. Estrogen priming effect on growth hormone (GH) provocative test: A useful tool for the diagnosis of GH deficiency. J Clin Endocrinol Metab. 2000;85:4168–72. doi: 10.1210/jcem.85.11.6928. [DOI] [PubMed] [Google Scholar]
- 2.Van den Broeck J, Hering P, Van de Lely A, Hokken-Koelega A. Interpretative difficulties with growth hormone provocative retesting in childhood-onset growth hormone deficiency. Horm Res. 1999;51:1–9. doi: 10.1159/000023304. [DOI] [PubMed] [Google Scholar]
- 3.Kanbur-Öksüz N, Derman O, Kınık E. Correlation of sex steroids with IGF-1 and IGFBP-3 during different pubertal stages. Turk J Pediatr. 2004;46:315–21. [PubMed] [Google Scholar]
- 4.Albanese A, Stanhope R. Investigation of delayed puberty. Clin Endocrinol. 1995;43:105–11. doi: 10.1111/j.1365-2265.1995.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 5.Wehkalampi K, Widén E, Laine T, Palotie A, Dunkel L. Patterns of inheritance of constitutional delay of growth and puberty in families of adolescent girls and boys referred to specialist pediatric care. J Clin Endocrinol Metab. 2007;93:723–8. doi: 10.1210/jc.2007-1786. [DOI] [PubMed] [Google Scholar]
- 6.Poyrazoğlu S, Günöz H, Darendeliler F, Saka N, Bundak R, Baş F. Constitutional delay of growth and puberty: From presentation to final height. J Pediatr Endocrinol Metab. 2005;18:171–9. doi: 10.1515/jpem.2005.18.2.171. [DOI] [PubMed] [Google Scholar]
- 7.Bierich JR. Constitutional delay of growth and adolescence. Baillieres Clin Endocrinol Metab. 1992;6:573–88. doi: 10.1016/s0950-351x(05)80113-2. [DOI] [PubMed] [Google Scholar]
- 8.Brämswig JH, Fasse M, Holthoff ML, von Lengerke HJ, von Petrykowski W, Schellong G. Adult height in boys and girls with untreated short stature and constitutional delay of growth and puberty: Accuracy of five different methods of height prediction. J Pediatr. 1990;117:886–91. doi: 10.1016/s0022-3476(05)80127-1. [DOI] [PubMed] [Google Scholar]
- 9.Crowne EC, Shalet SM, Wallace WH, Eminson DM, Price DA. Final height in boys with untreated constitutional delay in growth and puberty. Arch Dis Child. 1990;65:1109–12. doi: 10.1136/adc.65.10.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth Study: Growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994;125:29–35. doi: 10.1016/s0022-3476(94)70117-2. [DOI] [PubMed] [Google Scholar]
- 11.Wehkalampia K, Vangonena K, Lainea T, Dunkel L. Progressive reduction of relative height in childhood predicts adult stature below target height in boys with constitutional delay of growth and puberty. Horm Res. 2007;68:99–111. doi: 10.1159/000101011. [DOI] [PubMed] [Google Scholar]
- 12.Degros V, Cortet-Rudelli C, Soudan B, Dewailly D. The human chorionic gonadotropin test is more powerful than the gonadotropin-releasing hormone agonist test to discriminate male isolated hypogonadotropic hypogonadism from constitutional delayed puberty. Eur J Endocrinol. 2003;149:23–9. doi: 10.1530/eje.0.1490023. [DOI] [PubMed] [Google Scholar]
- 13.Kauschansky A, Dickerman Z, Phillip M, Weintrob N, Strich D. Use of GnRH agonist and human chorionic gonadotrophin tests for differentiating constitutional delayed puberty from gonadotrophin deficiency in boys. Clin Endocrinol (Oxf) 2002;56:603–7. doi: 10.1046/j.1365-2265.2002.01520.x. [DOI] [PubMed] [Google Scholar]
- 14.Ghai K, Cara JF, Rosenfield RL. Gonadotropin releasing hormone agonist (nafarelin) test to differentiate gonadotropin deficiency from constitutionally delayed puberty in teen-age boys--a clinical research center study. J Clin Endocrinol Metab. 1995;80:2980–6. doi: 10.1210/jcem.80.10.7559884. [DOI] [PubMed] [Google Scholar]
- 15.Martin MM, Martin AL. Constitutional delayed puberty in males and hypogonadotropic hypogonadism: A reliable and cost-effective approach to differential diagnosis. J Pediatr Endocrinol Metab. 2005;18:909–16. doi: 10.1515/jpem.2005.18.9.909. [DOI] [PubMed] [Google Scholar]
- 16.Zevenhuijzen H, K elnar CH, Crofton PM. Diagnostic utility of a low-dose gonadotropin-releasing hormone test in the context of puberty disorders. Horm Res. 2004;62:168–76. doi: 10.1159/000080324. [DOI] [PubMed] [Google Scholar]
- 17.Kaplowitz PB. Diagnostic value of testosterone therapy in boys with delayed puberty. Am J Dis Child. 1989;143:1001–2. doi: 10.1001/archpedi.1989.02150130126033. [DOI] [PubMed] [Google Scholar]
- 18.Albanese A, Stanhope R. Predictive factors in the determination of final height in boys with constitutional delay of growth and puberty. J Pediatr. 1995;126:545–50. doi: 10.1016/s0022-3476(95)70347-0. [DOI] [PubMed] [Google Scholar]
- 19.Crowne EC, Shalet SM, Wallace WH, Eminson DM, Price DA. Final height in boys with untreated constitutional delay in growth and puberty. Arch Dis Child. 1990;65:1109–12. doi: 10.1136/adc.65.10.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sperlich M, Butenandt O, Schwarz HP. Final height and predicted height in boys with untreated constitutional growth delay. Eur J Pediatr. 1995;154:627–32. doi: 10.1007/BF02079065. [DOI] [PubMed] [Google Scholar]
- 21.Crowne EC, Shalet SM, Wallace WH, Eminson DM, Price DA. Final height in girls with untreated constitutional delay in growth and puberty. Eur J Pediatr. 1991;150:708–12. doi: 10.1007/BF01958760. [DOI] [PubMed] [Google Scholar]
- 22.Albanese A, Stanhope R. Does constitutional delayed puberty cause segmental disproportion and short stature? Eur J Pediatr. 1993;152:293–6. doi: 10.1007/BF01956736. [DOI] [PubMed] [Google Scholar]
- 23.Brook CGD. Treatment of late puberty. Horm Res. 1999;51:101–3. doi: 10.1159/000053169. [DOI] [PubMed] [Google Scholar]
- 24.De Luca F, Argente J, Cavallo L, Crowne E, Delemarre-Van de Waal HA, De Sanctis C, et al. Management of puberty in constitutional delay of growth and puberty.International workshop on management of puberty for optimum auxological results. J Pediatr Endocrinol Metab. 2001;14(Suppl 2):953–7. doi: 10.1515/jpem-2001-s207. [DOI] [PubMed] [Google Scholar]
- 25.Kelly BP, Paterson WF, Donaldson MD. Final height outcome and value of height prediction in boys with constitutional delay in growth and adolescence treated with intramuscular testosterone 125 mg per month for 3 months. Clin Endocrinol (Oxf) 2003;58:267–72. doi: 10.1046/j.1365-2265.2003.01692.x. [DOI] [PubMed] [Google Scholar]
- 26.Soliman AT, AlLamki M, AlSalmi I, Asfour M. Congenital adrenal hyperplasia complicated by central precocious puberty: Linear growth during infancy and treatment with gonadotropin-releasing hormone analog. Metabolism. 1997;46:513–7. doi: 10.1016/s0026-0495(97)90186-4. [DOI] [PubMed] [Google Scholar]
- 27.Brown DC, Butler GE, Kelnar CJ, Wu FC. A double blind, placebo controlled study of the effects of low dose testosterone undecanoate on the growth of small for age, prepubertal boys. Arch Dis Child. 1995;73:131–5. doi: 10.1136/adc.73.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin MM, Martin AL, Mossman KL. Testosterone treatment of constitutional delay in growth and development: Effect of dose on predicted versus definitive height. Acta Endocrinol Suppl (Copenh) 1986;279:147–52. doi: 10.1530/acta.0.112s147. [DOI] [PubMed] [Google Scholar]
- 29.Butler GE, Sellar RE, Walker RF, Hendry M, Kelnar CJ, Wu FC. Oral testosterone undecanoate in the management of delayed puberty in boys: Pharmacokinetics and effects on sexual maturation and growth. J Clin Endocrinol Metab. 1992;75:37–44. doi: 10.1210/jcem.75.1.1619029. [DOI] [PubMed] [Google Scholar]
- 30.Crowne EC, Wallace WH, Moore C, Mitchell R, Robertson WR, Shalet SM. Degree of activation of the pituitary-testicular axis in early pubertal boys with constitutional delay of growth and puberty determines the growth response to treatment with testosterone or oxandrolone. J Clin Endocrinol Metab. 1995;80:1869–75. doi: 10.1210/jcem.80.6.7775634. [DOI] [PubMed] [Google Scholar]
- 31.McLachlan RI, O’Donnell L, Stanton PG, Balourdos G, Frydenberg M, de Kretser DM, et al. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. J Clin Endocrinol Metab. 2002c;87:546–56. doi: 10.1210/jcem.87.2.8231. [DOI] [PubMed] [Google Scholar]
- 32.Morse HC, Horike N, Rowley MJ, Heller CG. Testosterone concentrations in testes of normal men: Effects of testosterone propionate administration. J Clin Endocrinol Metab. 1973;37:882–6. doi: 10.1210/jcem-37-6-882. [DOI] [PubMed] [Google Scholar]
- 33.Coviello AD, Bremner WJ, Matsumoto AM, Herbst KL, Amory JK, Anawalt BD, et al. Intratesticular testosterone concentrations comparable with serum levels are not sufficient to maintain normal sperm production in men receiving a hormonal contraceptive regimen. J Androl. 2004;25:931–8. doi: 10.1002/j.1939-4640.2004.tb03164.x. [DOI] [PubMed] [Google Scholar]
- 34.Veldhuis JD, Kolp LA, Rogol AD, Johnson ML. Physiological attributes of episodic testosterone secretion. Ann NY Acad Sci. 1987;513:507. [Google Scholar]
- 35.Mitamura R, Yano K, Suzuki N, Ito Y, Makita Y, Okuno A. Diurnal rhythms of luteinizing hormone, follicle stimulating hormone, and testosterone secretion before the onset of male puberty. J Clin Endocrinol Metab. 1999;84:29–37. doi: 10.1210/jcem.84.1.5404. [DOI] [PubMed] [Google Scholar]
- 36.Bhasin S, Bremner WJ. Emerging issues in androgen replacement therapy. J Clin Endocrinol Metab. 1997;82:3–8. doi: 10.1210/jcem.82.1.3640. [DOI] [PubMed] [Google Scholar]
- 37.Wilson DM, Kei J, Hintz RL, Rosenfeld RG. Effects of testosterone therapy for pubertal delay. Am J Dis Child. 1988;142:96–9. doi: 10.1001/archpedi.1988.02150010106035. [DOI] [PubMed] [Google Scholar]
- 38.Soliman AT, Khadir MM, Asfour M. Testosterone treatment in adolescent boys with constitutional delay of growth and development. Metabolism. 1995;44:1013–5. doi: 10.1016/0026-0495(95)90098-5. [DOI] [PubMed] [Google Scholar]
- 39.Uruena M, Pantsiotou S, Preece MA, Stanhope R. Is testosterone therapy for boys with constitutional delay of growth and puberty associated with impaired final height and suppression of the hypothalamo-pituitary-gonadal axis? Eur J Pediatr. 1992;151:15–8. doi: 10.1007/BF02073882. [DOI] [PubMed] [Google Scholar]
- 40.Bergada I, Bergada C. Long term treatment with low dose testosterone in constitutional delay of growth and puberty: Effect on bone age maturation and pubertal progression. J Pediatr Endocrinol Metab. 1995;8:117–22. doi: 10.1515/jpem.1995.8.2.117. [DOI] [PubMed] [Google Scholar]
- 41.Albanese A, Kewley GD, Long A, Pearl KN, Robins DG, Stanhope R. Oral treatment for constitutional delay of growth and puberty in boys: A randomised trial of an anabolic steroid or testosterone undecanoate. Arch Dis Child. 1994;71:315–7. doi: 10.1136/adc.71.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malhotra A, Poon E, Tse WJ, Pringle PJ, Hindmarsh PC, Brook CG. The effects of oxandrolone on the growth hormone and gonadal axes in boys with constitutional delay of growth and puberty. Clin Endocrinol (Oxf) 1993;38:393–8. doi: 10.1111/j.1365-2265.1993.tb00520.x. [DOI] [PubMed] [Google Scholar]
- 43.Data Sheet. Primoteston® depot. [Last accessed on 2012 Feb 7]. Available from: http://www.bayerresources.com.au .
- 44.Aycan Z, Öcal G, Berberoğlu M, Evliyaoğlu O, Adıyaman P. Monitoring serum testosterone levels on androgen therapy with long-acting testosterone esters in the prepubertal and pubertal age groups. Turk J Endocrinol Metab. 2004;3:113–6. [Google Scholar]
- 45.Yilmaz D, Ersoy B, Bilgin E, Gümüşer G, Onur E, Pinar ED. Bone mineral density in girls and boys at different pubertal stages: Relation with gonadal steroids, bone formation markers, and growth parameters. J Bone Miner Metab. 2005;23:476–82. doi: 10.1007/s00774-005-0631-6. [DOI] [PubMed] [Google Scholar]
- 46.Schubert M, Minnemann T, Hübler D, Rouskova D, Christoph A, Oettel M, et al. Intramuscular iestosterone undecanoate: Pharmacokinetic aspects of a novel testosterone formulation during long-term treatment of men with hypogonadism. J Clin Endocrinol Metab. 2004;89:5429–34. doi: 10.1210/jc.2004-0897. [DOI] [PubMed] [Google Scholar]
- 47.Nieschlag E, Behre HM. Pharmacology and clinical uses of testosterone. In: Nieschlag E, Behre HM, editors. Testosterone-action, deficiency, substitution. 2nd ed. Berlin: Springer-Verlag; 1998. pp. 93–328. [Google Scholar]
- 48.Coviello AD, Matsumoto AM, Bremner WJ, Herbst KL, Amory JK, Anawalt BD, et al. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab. 2005;90:2595–602. doi: 10.1210/jc.2004-0802. [DOI] [PubMed] [Google Scholar]
- 49.Matthiesson KL, McLachlan RI, O’Donnell L, Frydenberg M, Robertson DM, Stanton PG, et al. The relative roles of follicle-stimulating hormone and luteinizing hormone in maintaining spermatogonial maturation and spermiation in normal men. J Clin Endocrinol Metab. 2006;91:3962–9. doi: 10.1210/jc.2006-1145. [DOI] [PubMed] [Google Scholar]
- 50.Pak TR, Lynch GR, Ziegler DM, Lunden JB, Tsai PS. Disruption of pubertal onset by exogenous testosterone and estrogen in two species of rodents. Am J Physiol Endocrinol Metab. 2003;284:E206–12. doi: 10.1152/ajpendo.00352.2002. [DOI] [PubMed] [Google Scholar]
- 51.Matsumoto AM, Karpas AE, Bremner WJ. Chronic human chorionic gonadotropin administration in normal men: Evidence that follicle-stimulating hormone is necessary for the maintenance of quantitatively normal spermatogenesis in man. J Clin Endocrinol Metab. 1986;62:1184–92. doi: 10.1210/jcem-62-6-1184. [DOI] [PubMed] [Google Scholar]
- 52.Jarow JP, Zirkin BR. The Androgen microenvironment of the human testis and hormonal control of spermatogenesis. Ann N Y Acad Sci. 2005;1061:20820. doi: 10.1196/annals.1336.023. [DOI] [PubMed] [Google Scholar]
- 53.McLachlan RI, O’Donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, et al. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res. 2002;57:149–79. doi: 10.1210/rp.57.1.149. [DOI] [PubMed] [Google Scholar]
- 54.Ruwanpura SM, McLachlan RI, Matthiesson KL, Meachem SJ. Gonadotrophins regulate germ cell survival, not proliferation, in normal adult men. Hum Reprod. 2008;23:403–11. doi: 10.1093/humrep/dem376. [DOI] [PubMed] [Google Scholar]
- 55.Sluka P, O’Donnell L, Bartles JR, Stanton PG. FSH regulates the formation of adherens junctions and ectoplasmic specialisations between rat Sertoli cells in vitro and in vivo. J Endocrinol. 2006;189:381–95. doi: 10.1677/joe.1.06634. [DOI] [PubMed] [Google Scholar]
- 56.Gordon D, Lauder JC, Cohen HN, O’Donnell AM, Semple CG, Thomson JA. An Evaluation of human chorionic gonadotrophin (HCG) therapy in boys with delayed puberty. Q J Med. 1990;74:247–56. [PubMed] [Google Scholar]
- 57.Soliman A, Nasr I, Thabet A, Rizk M, El Matary W. Human chorionic gonadotropin therapy in adolescent boys with constitutional delayed puberty vs those with -thalassemia major. Metabolism. 2005;54:15–23. doi: 10.1016/j.metabol.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto AM, Bremner WJ. Stimulation of sperm production by human chorionic gonadotropin after prolonged gonadotropin suppression in normal men. J Androl. 1985;6:137–43. doi: 10.1002/j.1939-4640.1985.tb00829.x. [DOI] [PubMed] [Google Scholar]
- 59.Sheckter CB, Matsumoto AM, Bremner WJ. Testosterone administration inhibits gonadotropin secretion by an effect directly on the human pituitary. J Clin Endocrinol Metab. 1989;68:397–401. doi: 10.1210/jcem-68-2-397. [DOI] [PubMed] [Google Scholar]
- 60.Liu PY, Turner L, Rushford D, McDonald J, Baker HW, Conway AJ, et al. Efficacy and safety of recombinant human follicle stimulating hormone (Gonal-F) with urinary human chorionic gonadotrophin for induction of spermatogenesis and fertility in gonadotrophin-deficient men. Hum Reprod. 1999;14:1540–45. doi: 10.1093/humrep/14.6.1540. [DOI] [PubMed] [Google Scholar]
- 61.Finkel DM, Phillips JL, Snyder PJ. Stimulation of spermatogenesis by gonadotropins in men with hypogonadotropic hypogonadism. N Engl J Med. 1985;313:651–5. doi: 10.1056/NEJM198509123131102. [DOI] [PubMed] [Google Scholar]
- 62.Zhang FP, Pakarainen T, Poutanen M, Toppari J, Huhtaniemi I. The low-gonadotropin-independent constitutive production of testicular testosterone is sufficient to maintain spermatogenesis. Proc Natl Acad Sci U S A. 2003;100:13692–7. doi: 10.1073/pnas.2232815100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joss EE, Schmidt HA, Zuppinger KA. Oxandrolone in constitutionally delayed growth, a longitudinal study up to final height. J Clin Endocrinol Metab. 1989;69:1109–15. doi: 10.1210/jcem-69-6-1109. [DOI] [PubMed] [Google Scholar]
- 64.Crowne EC, Wallace WH, Moore C, Mitchell R, Robertson WH, Holly JM, et al. Effect of low dose oxandrolone and testosterone treatment on the pituitary-testicular and GH axes in boys with constitutional delay of growth and puberty. Clin Endocrinol (Oxf) 1997;46:209–16. doi: 10.1046/j.1365-2265.1997.t01-1-1150928.x. [DOI] [PubMed] [Google Scholar]
- 65.Wickman S, Sipila I, Ankarberg-Lindgren C, Norjavaara E, Dunkel L. A specific aromatase inhibitor and potential increase in adult height in boys with delayed puberty: A randomised controlled trial. Lancet. 2001;357:1743–8. doi: 10.1016/S0140-6736(00)04895-9. [DOI] [PubMed] [Google Scholar]
- 66.De Ronde W. Therapeutic uses of aromatase inhibitors in men. Curr Opin Endocrinol Diabetes Obes. 2007;14:235–40. doi: 10.1097/MED.0b013e328121aad2. [DOI] [PubMed] [Google Scholar]
- 67.Saad RJ, Keenan BS, Danadian K, Lewy VD, Arslanian SA. Dihydrotestosterone treatment in adolescents with delayed puberty: Does it explain insulin resistance of puberty? J Clin Endocrinol Metab. 2001;86:4881–6. doi: 10.1210/jcem.86.10.7913. [DOI] [PubMed] [Google Scholar]
- 68.Krajewska-Siuda E, Malecka-Tendera E, Krajewski-Siuda K. Are short boys with constitutional delay of growth and puberty candidates for rhGH therapy according to FDA recommendations? Horm Res. 2006;65:192–6. doi: 10.1159/000092120. [DOI] [PubMed] [Google Scholar]
- 69.Wit JM. Growth hormone treatment of idiopathic short stature in KIGS. In: Ranke MB, Wilton P, editors. Growth hormone therapy in KIGS-10 years Experience. Heidelberg Lipzig: Johann Ambrosius Barth Verlag; 1999. pp. 225–43. [Google Scholar]
- 70.Bierich JR, Nolte K, Drews K, Brügmann G. Constitutional delay of growth and adolescence.Results of short-term and long-term treatment with GH. Acta Endocrinol. 1992;127:392–6. doi: 10.1530/acta.0.1270392. [DOI] [PubMed] [Google Scholar]
- 71.Buyukgebiz A, Hindmarsh PC, Brook CG. Treatment of constitutional delay of growth and puberty with oxandrolone compared with growth hormone. Arch Dis Child. 1990;65:448–9. doi: 10.1136/adc.65.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Finkelstein BS, Imperiale TF, Speroff T, Marrero U, Radcliffe DJ, Cuttler L. Effect of growth hormone therapy on height in children with idiopathic short stature. Arch Pediatr Adolesc Med. 2002;156:230–40. doi: 10.1001/archpedi.156.3.230. [DOI] [PubMed] [Google Scholar]
- 73.Leschek EW, Rose SR, Yanovski JA, Troendle JF, Quigley CA, Chipman JJ, et al. National Institute of Child Health and Human Development-Eli Lilly & Co. Growth Hormone Collaborative Group. Growth Hormone Collaborative Group.Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: A randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:3138–9. doi: 10.1210/jc.2003-031457. [DOI] [PubMed] [Google Scholar]
- 74.Wit JM, Rekers-Mombarg LT, Cutler DG, Jr, Crowe B, Beck TB, Roberts K, et al. Growth hormone (GH) treatment to final height in children with idiopathic short stature: Evidence for a dose effect. J Pediatr. 2005;146:45–53. doi: 10.1016/j.jpeds.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 75.Ankarberg-Lindgren C, Norjavaara E, Wikland KA. Short boys treated with growth hormone show normal progression of testicular size and achieve normal serum testosterone concentrations. Eur J Endocrinol. 2002;146:681–5. doi: 10.1530/eje.0.1460681. [DOI] [PubMed] [Google Scholar]
- 76.Han JC, Balagopal P, Sweeten S, Darmaun D, Mauras N. Evidence for hypermetabolism in boys with constitutional delay of growth and maturation. J Clin Endocrinol Metab. 2006;91:2081–6. doi: 10.1210/jc.2005-2762. [DOI] [PubMed] [Google Scholar]


