Abstract
Endocrine emergencies pose unique challenges for the attending intensivist while managing critically ill patients. Besides taking care of primary disease state, one has to divert an equal attention to the possible associated endocrinopathies also. One of the common reasons for inability to timely diagnose an endocrinal failure in critically ill patients being the dominance of other severe systemic diseases and their clinical presentation. The timely diagnosis and administration of therapeutic interventions for these endocrine disorders can improve the outcome in critically ill patients. The timely diagnosis and administration of timely therapeutics in common endocrine disorders like severe thyroid disease, acute adrenal insufficiency and diabetic ketoacidosis significantly influence the outcome and prognosis. Careful evaluation of clinical history and a high degree of suspicion are the corner stone to diagnose such problems. Aggressive management of the patient is equally important as the complications are devastating and can prove highly fatal. The present article is an attempt to review some of the common endocrine emergencies in intensive care unit and the challenges associated with their diagnosis and management.
Keywords: Critically ill, diabetes mellitus, diabetic ketoacidosis, myxoedema coma, phaeochromocytoma, thyroid storm
INTRODUCTION
Various cellular and molecular functions of the body require endocrinal regulation. Any disturbance in the endocrine functions can lead to devastating sequelae in critically ill patients.[1–4] Although, newer diagnostic and therapeutic modalities have vastly helped in understanding, diagnosing and treatment of these endocrine failures but still the morbidity and mortality is high due to failure in timely diagnosis of an endocrinal disorder in critically ill patients which can significantly influence the outcome and prognosis.[5,6] The present article aims to discuss important endocrine emergencies such as diabetic hyperglycaemic states, adrenal insufficiency, myxoedema coma, thyroid storm, and pituitary apoplexy and their appropriate management in the intensive care setting.
EMERGENCY PRESENTATION OF DIABETES MELLITUS
This is the most common endocrinopathy observed in the critically ill patients. However, the exact figures of incidence of diabetes mellitus in ICU set up are not available but from sporadic reports it can be assumed that every 5th or 6th patient admitted in ICU suffer from diabetes mellitus (DM).[4] However, the life threatening complications of DM can be attributable to either severe hypoglycaemia or hyperglycaemia which is usually associated with deranged metabolic profile, electrolyte abnormalities, deranged renal functions, dehydration and depressed immunity. The presence of comorbid diseases such as cardiac diseases and end organ abnormalities may lead to impaired compensatory mechanisms during acute stressful conditions which are commonly encountered in ICU.[7–10] The presence of autonomic neuropathy in critically ill diabetic patients make them highly vulnerable to nerve injuries, bed sores, soft tissue trauma, delayed feeding and hemodynamic instability.[3–4] The most common diabetic emergencies encountered in the ICU include:
Diabetic ketoacidosis
Severe hypoglycaemia
Non-ketotic hyperosmolar coma
Diabetic ketoacidosis
It is an acute metabolic complication of insulin dependent (type 1) diabetes and occasionally of non insulin dependent (type 2) diabetes mellitus encountered during infection, trauma, cardiovascular injuries or other acute emergencies.[11] The most common mechanism is the deficiency of insulin combined with an infection but other potential causes may include but are not limited to pancreatitis and drugs (steroids, diuretics, vasopressors, antipsychotics, cocaine). Severe deficiency of insulin leads to reduction of glucose uptake and its utilisation by muscle, fat and liver while glycogenolysis and gluconeogenesis is enhanced. There is increased lipolysis, leading to formation of ketone bodies (acetone, beta-hydroxybutyric acid, and acetoacetic acid) and results in development of anion gap metabolic acidosis.[12] Proinflammatory cytokines and pro-coagulant factors (C-reactive protein and interleukin-6 and 8) are elevated predisposing the development of thrombosis in these critically ill patients.[13] Fluid loss, due to osmotic diuresis can occur to the extent of 5–7 L in critically ill adult patients and additionally may lead to depletion of sodium, potassium, chloride, magnesium and phosphate.
Clinical manifestations
Most patients usually present to emergency and ICU within 24 h of development of polyuria, polydipsia, weakness, and weight loss. There may be complaints of anorexia, abdominal pain, fatigue and muscle cramps. Intravascular volume depletion due to osmotic effects leads to dry mucous membranes, sunken eyeballs, tachycardia, orthostatic hypotension, and even supine hypotension. Tachypnea occurs to compensate the biochemical effects of metabolic acidosis. Infection and sepsis can aggravate the clinical effects of volume depletion. Altered sensorium ranging from mild confusion to coma may prevail.[6,14,15]
Laboratory profile
The diagnostic profile is typically associated with findings of hyperglycaemia with serum glucose levels usually between 500 and 800 mg/dl. High anion gap metabolic acidosis is invariably present; however, if frequent vomiting occurs, mixed metabolic acidosis and alkalosis picture may supervene. The clinical profile may also exhibit hyperosmolarity, increased serum and urinary ketone levels, mild hyponatremia, and hypocalcemia. Serum potassium levels are usually normal at presentation due to extracellular shift of potassium but once the treatment is initiated, hypokalemia may become clinically manifest.[14]
Therapeutic management
Management of diabetic ketoacidosis (DKA) is complex and requires frequent monitoring of hydration, electrolyte replacement, as well as acid–base status. Treatment comprises of five major components:
Volume replacement: The first goal of therapy is to correct tissue hypo-perfusion, improve glomerular filtration and reverse the insulin resistance and deficiency. Total body water deficit may be up to 10–12 L and replacement is usually started with 0.9% sodium chloride (NS).[16] Volume replacement can be given as 2 L 0.9% NS over first two hours followed by 2 L 0.9% NS or 0.45% saline over next 4 h and 8 L 0.9% NS or 0.45% saline over next 8 h while simultaneously monitoring the central venous pressures.[14]
Insulin therapy: Insulin therapy is started using a continuous infusion of 0.1 units/kg/h. A bolus dose of 0.15 units/ kg may be given while monitoring the blood glucose level every hour.[17] If the levels do not fall by approximately 75 mg/ dl/h, the infusion rate of insulin is doubled. Once the levels fall to 230-300 mg/dl, 5% dextrose infusion is started. When ketones levels disappear and the patient accepts oral intake, subcutaneous insulin therapy can be initiated with an overlap of about 30 minutes with insulin infusion.[14]
Potassium replacement: Potassium deficit in DKA is usually in the range of 300 mEq, but can go higher up to 1000 mEq and is attributed to osmotic renal losses and shifting of intracellular potassium to extracellular space. Potassium deficit may persist after DKA is treated and hence, oral replacement should continue.[14]
Phosphate replacement: This is usually not required, except in cases with initial serum phosphate concentration of 1.5 mg/dl. A further fall during treatment may lead to hypophosphatemia syndrome characterized by decreased myocardial contractility, respiratory muscle weakness and respiratory failure, hemolysis, and rhabdo-myolysis. Hypophosphatemic patients will require 500-1000 mg of elemental phosphate over 12–24 hours, i.e., 15–30 mmol of phosphate.[14]
Bicarbonate replacement: Acidosis generally does not require bicarbonate therapy. It usually resolves once insulin therapy is started. Bicarbonate replacement is initiated when pH < 7.0. The replacement is done using the base deficit by the following formula:
Bicarbonate (mmol) = base deficit × weight (kg) × 0.3
Half of the amount is given as bolus dose and rest as slow infusion over 8 hours period.
Mortality in DKA may result from: cerebral edema, particularly in children and adolescents; intravascular volume depletion; failure to recognize and replace electrolyte abnormalities; and the presence of comorbid conditions.
Hypoglycaemia
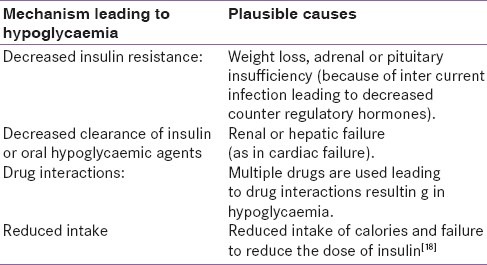
Hypoglycaemia is one of the common endocrine complications encountered in patients presenting to emergency or ICU. It can result from overenthusiastic and aggressive control of blood sugar in ICU. However, the pathophysiology of hypoglycaemia may involve multiple pathways [Table 1] which can be summarized as follows:
Table 1.
Showing the possible pathophysiology of hypoglycaemia
Clinical profile
Patients may exhibit sweating, trembling, pounding heart, anxiety and hunger. There may be visual disturbances, dizziness, confusion, headache, tingling, difficulty in speaking, and concentrating. The patient may suffer from ischemic changes of heart or may develop focal/ generalised seizures or deficit, stroke or coma.[14]
Laboratory investigations
Hypoglycaemia is arbitrarily defined as blood glucose level < 3.5 mmol/dl. However, serial glucose monitoring is very essential to diagnose and treat any such episode in the ICU.
Therapeutic management
In acutely ill patient the target level of glucose is 110 mg/dl. A number of factors interact to determine the management of a diabetic critically ill patient to prevent consequences of hyperglycemia as well as hypoglycaemia:
In patients kept nil orally: The blood glucose levels should be measured every 2–3 hours and insulin infusion used to titrate the blood glucose levels.
For patients on intermittent oral intake: longer acting insulin preparations (like NPH insulin) should be avoided. Rapid acting insulin preparations should be used after each meal and at midnight.[14]
Hyperglycaemic hyperosmolar coma (nonketotic hyperosmolar coma)
Clinical presentation and pathogenesis of nonketotic coma are similar to DKA. However, the patients are usually elderly who present with diabetes for the first time and are severely dehydrated, hyperglycaemic and often in coma. The presentation is delayed due to absence of ketosis but the management strategies are similar to that of DKA. The rate of fall of plasma glucose during management should be approximately 100 mg/dl/h and fluid replacement should be done with NS. Monitoring serum potassium levels is important for replacement therapy. Mortality is high because of associated concurrent illness, thrombotic events or infections.[14]
CHALLENGES OF DERANGED THYROID FUNCTIONS
In critically ill patients, thyroid functions play a significant role in maintaining the normal physiologic milieu and any disturbance surely affect the outcome and prognosis in these patients.[2] Administrations of exogenous thyroid hormones are sometimes necessary to cover up the failure of hypothalamus, pituitary and thyroid axis.[18,19]
Thyrotoxicosis/thyroid storm
It is defined as a life-threatening augmentation of the manifestations of hyperthyroidism. It is one of the most dreaded complications of thyroid gland occurring in less than 10% of patients hospitalized for thyrotoxicosis and is nine to ten times more common in women than in men. Thyroid storm can be precipitated by a number of factors such as severe infections, diabetic ketoacidosis, surgery, trauma, pulmonary thrombo-embolism, direct trauma or surgical manipulation of the thyroid gland, iodine administration or following discontinuation of anti-thyroid medications.[20] Also, salicylates by increasing the concentration of circulating free thyroid hormones to critical levels have been implicated in triggering thyroid storm.[21,22]
Clinical presentation of thyroid storm
The clinical symptomatology is dominated by involvement of multi-organ systems and may clinically present in the form of high grade fever (≥104° F), altered mental status (confusion, agitation, overt psychosis, and in extreme cases, even coma), cardiovascular complications (tachycardia that is out of proportion to fever, cardiac arrhythmias (including atrial fibrillation), and congestive heart failure), diffuse muscle weakness, tremor or fasciculations and neuropsychiatric syndromes. Gastrointestinal involvement is manifested as nausea, vomiting, diarrhoea and abdominal pain.[6,21] The clinical symptomatology is sometimes difficult to distinguish it from other medical emergencies such as Neurolept malignant syndrome, malignant hyperthermia and phaeochromocytoma.[20]
Diagnosis of thyroid storm
The diagnosis of thyroid storm in critically ill patients is essentially a clinical one and laboratory tests of thyroid function are only confirmatory. Total and free thyroxine (T4) and tri-iodothyronine (T3) are increased while thyrotropin (TSH) levels are reduced. However, T3 and T4 levels may be decreased by concurrent non-thyroidal illness. Therefore, levels of T4 and T3 may not correlate with the patient's clinical picture.[21–23]
Therapeutic management
The basic principles in the treatment of this medical emergency revolve around in providing:
Supportive therapy and symptomatic treatment that includes respiratory and hemodynamic support and measures to control hyperthermia. Oxygen therapy, management of hypertension, fluid and electrolyte replacement, antipyretics, environmental cooling, management of cardiac arrhythmias and heart failure should be initiated at the earliest.[21–23]
Treatment of hyperthyroidism using propylthiouracil (PTU) 600–1000 mg loading dose, followed by 1200 mg/day divided into doses given every 4–6 hours. Methimazole is as an alternative. Beta-blockers such as propanolol may be given intravenously initially in 1-mg increments every 10–15 minutes until symptoms are controlled or esmolol administered as a loading dose of 250–500 mcg/kg followed by an infusion of 50–100 mcg/kg/minute. Thyroid hormone release can be reduced by the administration of lithium (1200 mg/day divided into 3–4 doses), iodinated contrast (0.5 to 1 g every 12 h), and corticosteroids. In refractory cases, plasmapheresis, plasma exchange, and peritoneal hemodialysis can be used to remove circulating thyroid hormone.
Treatment of precipitating/underlying illness is very essential. The common causes such as fever, infection, stress, pain and others should be managed in the swiftest of manner.
Myxoedema coma
It is a severe form of hypothyroidism that is potentially fatal. It usually develops in a previously hypothyroid patient developing severe intercurrent illness or in inadequately treated and undiagnosed case of hypothyroidism.[19] The most common precipitating factors may include burns, trauma, surgery, severe infection, hypothermia, cardio-vascular event, medications, sepsis etc.[19,24] Though the exact incidence of mortality due to myxoedema coma in ICU patients is not available but mortality up to 30%–60% in patients with myxoedema coma has been documented in the literature.[25]
Clinical presentation
Clinical findings are similar to hypothyroidism but of greater magnitude.[19] The critically ill patients may develop hypothermia and altered mental status which may even lead to stupor, obtundation, or frank coma. Hypothermia is the hallmark; with core body temperature as low as 21°C. Haemodynamic and conduction disturbances may manifest in the form of bradycardia, prolonged QT interval, diminished cardiac output, pericardial effusion which may ultimately leads to cardio-vascular collapse. Central nervous system may show altered responses to hypercarbia and hypoxia and the associated respiratory muscle weakness may lead to hypoventilation and respiratory failure. Adrenal insufficiency may coexist and contribute to electrolyte and cardiac abnormalities. There may be generalized skin and soft tissue swelling, periorbital edema, ptosis, macroglossia, and the presence of cool, dry skin. Drug metabolism is significantly depressed. Usual doses of sedatives may lead to hypoventilation.[6,19]
Diagnosis of myxoedema coma
Though clinical picture of myxoedema coma is quite atypical in critically sick patients but the diagnosis can be confirmed by thyroid function tests. TSH is typically elevated with low T3 and T4 levels. The laboratory profile may reveal hyponatremia, hypoglycaemia, hypercapnia and respiratory acidosis.[6]
Therapeutic management
The basic principles of therapy in this medical emergency include[25]:
Rapid replacement of thyroid hormones with a loading dose of thyroxin 300–500 mcg, followed by 50–100 mcg daily depending on the patient's age, weight, and risk of complications. Intravenous tri-iodothyronine can be given as an initial bolus dose of 10–20 mcg, followed by 10 mcg every 4–24 hours, with taper to 10 mcg every 6 h. Hydrocortisone replacement 100 mg every 6–8 hours is required.
Treatment of the precipitating causes.
Supportive therapy includes oxygen therapy, non-invasive or invasive ventilation if required, passive warming, management of hypoglycaemia and hyponatremia and use of vasopressors to manage hypotension.[21,26]
Addisonian crisis: Acute adrenal insufficiency
The common causes of acute adrenal insufficiency include autoimmune disorders, infection, sudden withdrawal of adrenal replacement therapy, haemorrhage into adrenal glands, purpura fulminans or sudden failure of adrenal or pituitary functioning due to infection or trauma.[6,27–29]
Clinical presentation
Adrenal crisis occurs due to mineralocorticoid deficiency and usually presents with hypotension or hypotensive shock. Nonspecific symptoms such as anorexia, nausea, vomiting, abdominal pain, weakness, fatigue, lethargy, fever and confusion may be present in critically ill patients which can progress to coma. Electrolyte abnormalities may present in the form of hyponatremia as well as hyperkalemia.[21,30] There may be down regulation of glucocorticoid receptors which may warrant treatment with hydrocortisone.[31]
Laboratory profile
The usual laboratory findings may include hyponatremia, hyperkalemia, azotemia, hypoglycaemia and occasionally hypercalcemia and eosinophilia. The technical limitations of ICU discourage the performance of tests which suppresses the adrenal functions such as insulin response test and metyrapone inhibition.[27]
Adrenal function tests: In acute adrenal insufficiency diagnosis can be made reliably with ACTH stimulation test. Serum cortisol levels greater than 20 μg/dl makes the diagnosis of adrenal insufficiency unlikely. For ACTH stimulation test 250 μg cosyntropin is given intravenously and serum cortisol levels measured at 0, 30, and 60 minutes. An increment of more than 7 μg/dl or peak value greater than 17 μg/dl postadministration excludes adrenal insufficiency.[21]
Therapeutic management
The basic principle in management of this medical emergency involves the administration of corticosteroid replacement. Hydrocortisone 75–100 mg is given every 6–8 hours. In case of hypotension ACTH stimulation test is delayed and dexamethasone is used 3–4 mg every 6–8 hours. The dose of hydrocortisone is reduced to replacement level (10–20 mg morning and 5–10 mg evening) once the condition improves. Fluid and electrolyte replacement should be done at the earliest as timely aggressive management helps in elimination of vasopressor dependence, shortened ICU stay and better outcome.[32]
EMERGENCIES IN PITUITARY DISORDERS
The most common emergencies related to pituitary disorders in critically ill patients include:
Post-partum panhypopituitarism
Bleeding into pituitary tumour
Patients operated for pituitary tumours
Stress induced hypopituitarism
The most common acute complication observed with pituitary disorder, whether operated or non-operated, is the severe diabetes insipidus (DI). This is commonly observed in patients undergoing intracranial surgery for removal of pituitary tumour or severe head injury with bleed into the pituitary.[33] In majority of the cases, the disease resolves spontaneously but may leave residual effect in certain individuals with absolute ADH deficiency.[34]
Clinical profile and diagnosis
Clinical profile of such patients may reveal a typical picture of intravascular depletion of fluids, hyperosmolarity and hypernatremia which can progress to hypovolemic shock. The lab findings support the clinical picture and demonstrate lack of urine concentrating ability.
Management
Desmopressin (DDAVP) is the cornerstone of this clinical entity and is administered by careful titration of urine output, electrolytes and serum osmolarity. Though various routes such as intravenous, subcutaneous, intranasal and oral can be used for DDAVP administration, however intranasal which avoid the first pass metabolism in hepatic tissue and intravenous are the best.
PHAEOCHROMOCYTOMA
Mejorityof the patients with phaeochromocytoma gets admitted either for surgery of the phaeochromocytoma tumour itself or occasionally it can be an associated finding during some medical or surgical emergency. During the postoperative course these patients are highly susceptible to development of hypertension, hypotension and hypoglycaemia.[35] The hypertension during the postoperative period can be due to residual tumour left after excision while hypotension can result from rapid fall of catecholamines and pre-op adrenergic blockade.[36] Hypoglycaemia occurs due to significant rise of insulin levels during post-op period.[35,37] During pregnancy, maternal and fetal mortality and morbidity is extremely high during the management of phaeochromocytoma due to cardiac failure from catecholamine induced cardiomyopathy.[38,39] Surgical treatment aims at early caesarean section as well as removal of tumour whenever appropriate.[38,39]
Still many more endocrine emergencies can occur in ICU but their elaboration is out of scope of this review. These can be primary manifestations, arising from trauma or can occur secondary to surgical procedures which may involve phaeochromocytoma, pituitary tumour and craniopharyngioma and so on.[33,35,40,41]
CONCLUSION
Though relatively uncommon, endocrinal emergencies are life threatening and pose a diagnostic challenge for the physician and intensivist. They need to be promptly recognized and treated appropriately. Careful evaluation of clinical history and a high degree of suspicion are the corner stone to diagnose such problems. Aggressive management of the patient is equally important as the complications are devastating and can prove highly fatal.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Alarifi AA, van den Berghe GH, Snider RH, Becker KL, Mueller B, Nylen ES. Endocrine Markers and mediators in critical illness. 3rd ed. Philadelphia, PA, USA: J.B. Lippincott Co; 2001. [Google Scholar]
- 2.Nylen ES, Alarifi AA. Humoral markers of severity and prognosis of critical illness. Best Pract Res Clin Endocrinol Metab. 2001;15:553–73. doi: 10.1053/beem.2001.0169. [DOI] [PubMed] [Google Scholar]
- 3.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 4.Bajwa SS. Intensive care management of critically sick diabetic patients. Indian J Endocrinol Metab. 2011;15:349–50. doi: 10.4103/2230-8210.85603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turina M, Christ-Crain M, Polk HC., Jr Impact of diabetes mellitus and metabolic disorders. Surg Clin North Am. 2005;85:1153–61. doi: 10.1016/j.suc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg PA, Inzucchi SE. Critical issues in endocrinology. Clin Chest Med. 2003;24:583–606. doi: 10.1016/s0272-5231(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 7.Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350:1629–38. doi: 10.1056/NEJMoa020266. [DOI] [PubMed] [Google Scholar]
- 8.Webster JI, Sternberg EM. Role of the hypothalamic-pituitary-adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. J Endocrinol. 2004;181:207–21. doi: 10.1677/joe.0.1810207. [DOI] [PubMed] [Google Scholar]
- 9.Prigent H, Maxime V, Annane D. Science review: Mechanisms of impaired adrenal function in sepsis and molecular actions of glucocorticoids. Crit Care. 2004;8:243–52. doi: 10.1186/cc2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho JT, Al-Musalhi H, Chapman MJ, Quach T, Thomas PD, Bagley CJ, et al. Septic shock and sepsis: A comparison of total and free plasma cortisol levels. J Clin Endocrinol Metab. 2006;91:105–14. doi: 10.1210/jc.2005-0265. [DOI] [PubMed] [Google Scholar]
- 11.Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, et al. Management of hyperglycemic crises in patients with diabetes mellitus (technical review) Diabetes Care. 2001;24:131–53. doi: 10.2337/diacare.24.1.131. [DOI] [PubMed] [Google Scholar]
- 12.Kitabchi AE, Nyenwe EA. Hyperglycemic crises in diabetes mellitus: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinol Metab Clin North Am. 2006;35:725–51. doi: 10.1016/j.ecl.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53:2079–86. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 14.Lpp E, Huang C. Diabetes mellitus, hyperglycemia and critically ill patient. In: Bongard FS, Sue DY, Vintch JRE, editors. Current diagnosis and treatment critical care. 3rd ed. New York: McGraw-Hill; 2008. pp. 581–97. [Google Scholar]
- 15.Trachtenbarg DE. Diabetic ketoacidosis. Am Fam Physican. 2005;71:1705–14. [PubMed] [Google Scholar]
- 16.Dhatariya KK. Diabetic ketoacidosis. BMJ. 2007;334:1284–5. doi: 10.1136/bmj.39237.661111.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Position statement: Hyperglycemic crises in patients with diabetes mellitus. Diabetes Care. 2001;24:154–61. doi: 10.2337/diacare.24.1.154. [DOI] [PubMed] [Google Scholar]
- 18.Lynn Loriaux D. Adrenocortical insufficiency. In: Becker KL, editor. Principles and practice of endocrinology and metabolism. 3rd ed. Philadelphia, PA: Lippincott Williams ' Wilkins; 2001. pp. 739–43. [Google Scholar]
- 19.Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, et al. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol Endocrinol. 2002;16:184–99. doi: 10.1210/mend.16.1.0759. [DOI] [PubMed] [Google Scholar]
- 20.Chong HW, See KC, Phua J. Thyroid storm with multiorgan failure. Thyroid. 2010;20:333–6. doi: 10.1089/thy.2009.0181. [DOI] [PubMed] [Google Scholar]
- 21.Bhasin S, Ballani P, Mac RP. Endocrine problems in critically ill. In: Bongard FS, Sue DY, Vintch JR, editors. Current diagnosis and treatment critical care. New York: McGraw-Hill; 2008. pp. 566–80. [Google Scholar]
- 22.Pimentel L, Hansen K. Thyroid disease in the emergency department: A clinical and laboratory review. J Emerg Med. 2005;28:201–9. doi: 10.1016/j.jemermed.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Ringel MD. Management of hypothyroidism and hyperthyroidism in the intensive care unit. Crit Care Clin. 2001;17:59–74. doi: 10.1016/s0749-0704(05)70152-4. [DOI] [PubMed] [Google Scholar]
- 24.Wartofsky L. Myxedema coma. In: Braverman LE, Utiger RD, editors. Werner & Ingbar's The Thyroid: A fundamental and clinical text. Philadelphia: Lippincott Williams &; 2000. pp. 843–7. [Google Scholar]
- 25.Wall CR. Myxedema coma: Diagnosis and treatment. Am Fam Physician. 2000;62:2485–90. [PubMed] [Google Scholar]
- 26.Savage MW, Mah PM, Weetman AP, Newell-Price J. Endocrine emergencies. Postgrad Med J. 2004;80:506–5. doi: 10.1136/pgmj.2003.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steele BW, Wang E, Palmer-Toy DE, Killeen AA, Elin RJ, Klee GG. Total long-term within-laboratory precision of cortisol, ferritin, thyroxine, free thyroxine, and thyroid-stimulating hormone assays based on a College of American Pathologists fresh frozen serum study: Do available methods meet medical needs for precision? Arch Pathol Lab Med. 2005;129:318–22. doi: 10.5858/2005-129-318-TLWPOC. [DOI] [PubMed] [Google Scholar]
- 28.Zaloga GP, Marik P. Hypothalamic-pituitary-adrenal insufficiency. Crit Care Clin. 2001;17:25–41. doi: 10.1016/s0749-0704(05)70150-0. [DOI] [PubMed] [Google Scholar]
- 29.Christ-Crain M, Jutla S, Widmer I, Couppis O, König C, Puder J, et al. Measurement of serum free cortisol shows discordant responsivity to stress and dynamic evaluation. J Clin Endocrinol Metab. 2007;92:1729–935. doi: 10.1210/jc.2006-2361. [DOI] [PubMed] [Google Scholar]
- 30.Bouillon R. Acute adrenal Iinsufficiency. Endocrinol Metab Clin North Am. 2006;35:767–75. doi: 10.1016/j.ecl.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283:1038–45. doi: 10.1001/jama.283.8.1038. [DOI] [PubMed] [Google Scholar]
- 32.Widmer IE, Puder JJ, Konig C, Pargger H, Zerkowski HR, Girard J, et al. Cortisol response in relation to the severity of stress and illness. J Clin Endocrinol Metab. 2005;90:4579–86. doi: 10.1210/jc.2005-0354. [DOI] [PubMed] [Google Scholar]
- 33.Bajwa S, Bajwa SK. Anesthesia and Intensive care implications for pituitary surgery: Recent trends and advancements. Indian J Endocrinol Metab. 2011;15:224–32. doi: 10.4103/2230-8210.84872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickstein G. On the term “relative adrenal insufficiency”–or what do we really measure with adrenal stimulation tests? J Clin Endocrinol Metab. 2005;90:4973–4. doi: 10.1210/jc.2005-1196. [DOI] [PubMed] [Google Scholar]
- 35.Bajwa SS, Bajwa SK. Implications and considerations during pheochromocytoma resection: A challenge to the anesthesiologist. Indian J Endocrinol Metab. 2011;15:337–44. doi: 10.4103/2230-8210.86977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinney MA, Narr BJ, Warner MA. Perioperative Management of Pheochromocytoma. J Cardiothorac Vasc Anesth. 2002;16:359–69. doi: 10.1053/jcan.2002.124150. [DOI] [PubMed] [Google Scholar]
- 37.Schif RL, Welsh GA. Perioperative evaluation and management of the patient with endocrine dysfunction. Med Clin N Am. 2003;87:175–92. doi: 10.1016/s0025-7125(02)00150-5. [DOI] [PubMed] [Google Scholar]
- 38.Lyman DJ. Paroxysmal hypertension, pheochromocytoma, and pregnancy. J Am Board Fam Pract. 2002;15:153–8. [PubMed] [Google Scholar]
- 39.Hudsmith JG, Thomas CE, Browne DA. Undiagnosed pheochromocytoma mimicking severe preeclampsia in a pregnant woman at term. Int J Obstet Anesth. 2006;15:240–5. doi: 10.1016/j.ijoa.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Bajwa SJ, Kulshrestha A. Renal endocrine manifestations during polytrauma: A cause of concern for the anesthesiologist. Indian J Endocrinol Metab. 2012;16:252–7. doi: 10.4103/2230-8210.93744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bajwa SJ, Bajwa SK, Bindra GS. The anesthetic, critical care and surgical challenges in the management of craniopharyngioma. Indian J Endocrinol Metab. 2011;15:123–6. doi: 10.4103/2230-8210.81944. [DOI] [PMC free article] [PubMed] [Google Scholar]


