Abstract
Introduction:
Celiac disease (CD) is a highly prevalent autoimmune disease. The symptoms of CD are varied and atypical, with many patients having no gastrointestinal symptoms. Metabolic bone disease (MBD) is a less recognized manifestation of CD associated with spectrum of musculoskeletal signs and symptoms, viz. bone pains, proximal muscle weakness, osteopenia, osteoporosis, and fracture. We here report five patients who presented with severe MBD as the only manifestation of CD.
Materials and Methods:
Records of 825 patients of CD diagnosed during 2002–2010 were retrospectively analyzed for clinical features, risk factors, signs, biochemical, and radiological parameters.
Results:
We were able to identify five patients (0.6%) of CD who had monosymptomatic presentation with musculoskeletal symptoms and signs in the form of bone pains, proximal myopathy, and fragility fractures without any gastrointestinal manifestation. All the five patients had severe MBD in the form of osteopenia, osteoporosis, and fragility fractures. Four of the five patients had additional risk factors such as antiepileptic drugs, chronic alcohol consumption, malnutrition, and associated vitamin D deficiency which might have contributed to the severity of MBD.
Conclusion:
Severe metabolic disease as the only presentation of CD is rare. Patients show significant improvement in clinical, biochemical, and radiological parameters with gluten-free diet, calcium, and vitamin D supplementation. CD should be looked for routinely in patients presenting with unexplained MBD.
Keywords: Celiac disease, fractures, metabolic bone disease, osteomalacia, osteoporosis
INTRODUCTION
Celiac disease (CD) is a highly prevalent autoimmune disease characterized by chronic intestinal inflammation with different degrees of intestinal atrophy that may be associated with malabsorption. In the classic presentation of the disease, seen mainly in children, there is a predominance of digestive symptoms with a clinical profile of malabsorption syndrome.[1] However, on many occasions, CD presents atypically with a predominance of extra-intestinal manifestations such as iron deficiency anemia, short stature,[2,3] recurrent stomatitis, bone pains, infertility, Graves’ disease, Type 1 diabetes mellitus,[4] and metabolic bone disease (MBD).[5]
MBD is a less well-recognized manifestation of CD. It could manifest as osteopenia, osteomalacia, osteoporosis, and rarely as pathological fractures. CD may be associated with low bone mineral density (BMD) even in the absence of classical abdominal symptoms.[5] Osteoporosis was seen in 6.8% of the CD patients who had presented with extraintestinal manifestations.[6] Osteoporosis is usually a complication of most severe form of CD and it is uncommon to have MBD as the only presentation of CD. We report severe MBD as the only manifestation of CD.
MATERIALS AND METHODS
Records of patients with CD (n = 825) seen at the Department of Gastroenterology and Endocrinology of Post Graduate Institute of Medical Education and Research, Chandigarh, with symptomatic MBD during January 2002 to December 2011 were reviewed. The study was approved by the Institute Ethics Committee and written consent from each patient was taken. Data retrieved included presenting symptoms, previous diagnosis, history of diarrhea, ingestion of drugs known to affect the BMD (steroids, anticonvulsants, anticoagulants, calcium supplements), any coexisting morbidities likely to affect BMD (chronic liver disease, chronic kidney disease, thyrotoxicosis , other malabsorption disorders), pregnancy, smoking, alcohol of ≥3 drinks/day, and prior treatment received. Physical examination findings of anthropometry, signs rickets/osteomalacia, and bony deformities were recorded.
The investigations details retrieved included hemogram, calcium profile, liver and renal function tests, 25 hydroxy vitamin D (25 OHD), and intact parathyroid hormone (iPTH), thyroid function tests, gonadal function tests, and serum cortisol. Serum iPTH was measured by immunochemiluminiscence assay and 25 (OH) vitamin D3 was estimated by radioimmunoassay in all patients. BMD was estimated on a Norland dual energy X-ray absortiometer 434D142 Rev. F in all patients at the lumbar spine and hip. A T-score of <-1 was considered as ospteopenic and T-score <-2.5 at any of these sites was considered osteoporosis.[7] Anti-tissue trans-glutaminase antibody was estimated by enzyme-linked immunosorbent assay (ELISA), (Blue Well; D-Tek, Mons, Belgium). Those positive for tTGAb were subjected to endoscopy, and mucosal details in the descending part of the duodenum were recorded, and two to four biopsies were taken. Histological interpretation was done by an experienced pathologist as per the modified Marsh classification.[8] Patients were diagnosed to have CD as per the modified European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) criteria.[9] CD was treated with a gluten-free diet, calcium (500 mg/day), vitamin D (600 000 IU cholecalciferol intramuscular stat followed by 60,000 IU monthly oral), iron, and multivitamin supplementation including folic acid and vitamin B12.[10] The patients were followed for a minimum period of 6 months. Relevant biochemical investigations and endoscopy findings of these patients are mentioned in Tables 1 and 2.
Table 1.
Biochemical and hormonal profile (before and after gluten-free diet)
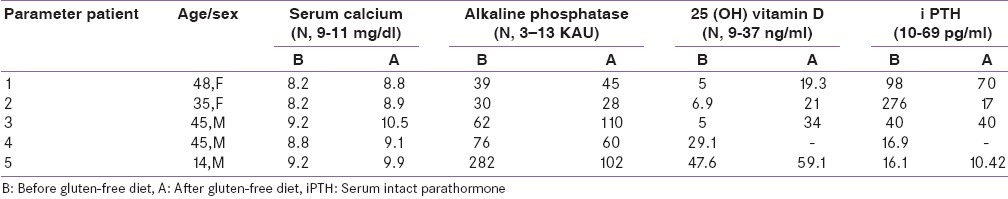
Table 2.
Serum tissue transglutaminase levels and doudenoscopy findings of patients

RESULTS
A total of 825 patients of CD were seen during this time period, out of which 5 (0.6%) had monosymptomatic presentation as symptomatic MBD without any gastrointestinal complaints. Salient presenting features of each case are given below briefly along with the investigative build up in Tables 1 and 2.
Case 1: A 48-year-old postmenopausal lady presented with diffuse body aches and pains for past 2 years. She developed progressively, increasing difficulty in rising from the squatting posture and walking, and sustained pathological fracture of right forearm 2 months before admission. She had no history of chronic diarrhea, pain abdomen, hepatic, or renal impairment and no history of consumption of antitubercular, antiepileptics, or glucocorticoids. She was confined to bed for 1 year before admission and had poor sunlight exposure. She had received intermittent calcium and vitamin D supplements without significant improvement.
On examination, she was bed bound, appeared thin, frail, and had generalized bony tenderness. She had kyphosis and shrunken pelvis. The right upper limb was immobilized in a plaster cast and there was limitation of movements of left elbow and wrist. She had generalized atrophy of muscles with decrease in muscle strength (power was 3/5). Skeletal survey showed generalized severe osteopenia with tufting of phalanges. She had fracture of multiple ribs [Figure 1], wedge collapse of upper and lower thoracic vertebrae, triradiate pelvis with coxa-vara and protrusio acetabuli [Figure 2], fracture of inferior ramii of pelvis, right humerus, and upper end of left ulna. Her 99mTc methylene-diphosphonate (MDP) bone scan showed generalized increase in metabolic activity over the skeleton. Dual energy X-ray absorptiometry (DEXA) showed T-score of -4.9 at neck of femur and -7.1 at lumbar spine (L1-L4). She gradually improved and during follow-up of 1 year with the CD treatment protocol, her fractures had healed [Figure 3], and she was able to stand with support. Subsequently, at the end of 3 years of follow-up, she is performing her all house hold work.
Figure 1.
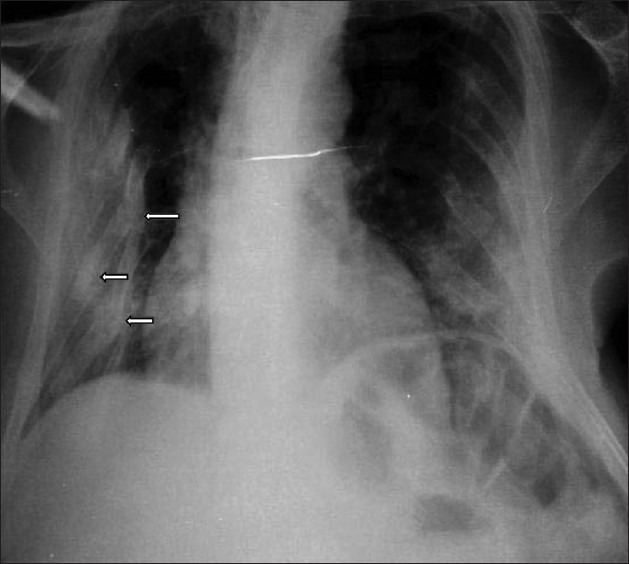
Chest X-ray P-A view showing fracture of multiple ribs (arrows)
Figure 2.
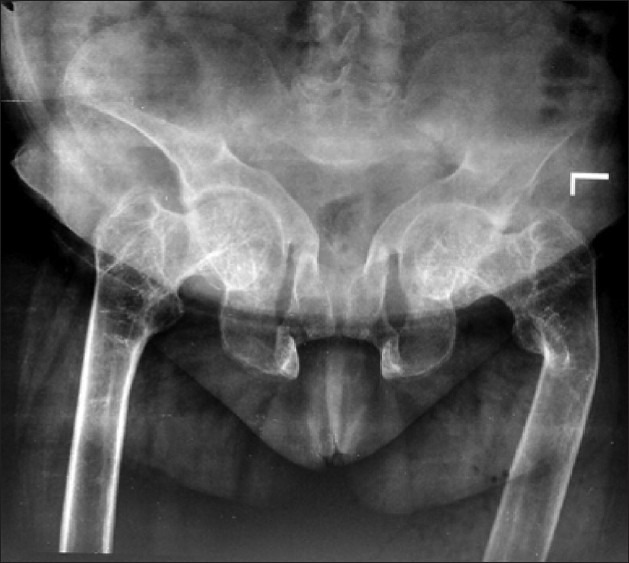
X-ray of pelvis showing triradiate pelvis, separation of pubic symphysis, and coax vara deformity of neck of left femur
Figure 3.
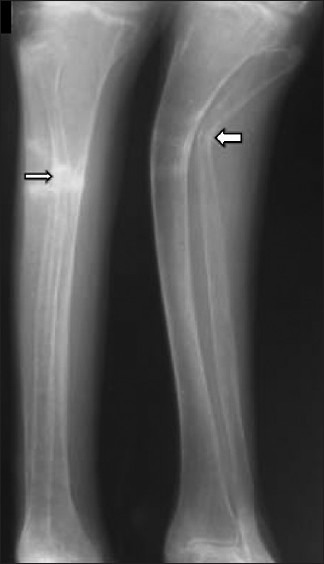
X-ray legs showing healing looser's zone and fractures (arrows)
Case 2: A 35-year-old lady presented with progressively worsening aches and pains over the back and thigh of 1 year duration. She also complained of easy fatigability and difficulty in getting up from squatting posture and walking. She had history of generalized seizures, from 13 years of age and for that she received various antiepileptics (phenobarbitone, carbamazepine, and phenytoin) for a variable period in the past. She was on phenytoin and lamotrigine for the past 7 years. She had no history of hepatic or renal dysfunction. She had adequate sunlight exposure; however, milk intake was inadequate and she was not on any calcium supplements.
On examination, she had significant pallor, tenderness over the thigh and calf, but no skeletal deformities or evidence of malnutrition. Skeletal survey revealed generalized osteopenia. DEXA scan showed T-score of -4.9 over lumbar spine and -3.9 over neck of femur. Her anemia, bone pains, and proximal muscle weakness improved during 1 year of follow-up with CD treatment.
Case 3: A 45-year-old male presented with diffuse body aches, proximal muscle weakness, and progressive deformity of the chest of 3 years duration. He sustained fracture of the left humerus after a trivial fall which had failed to unite. He had difficulty in bearing weight on right lower limb and walked with a limp. He had no gastrointestinal manifestations or chronic drug intake interfering with bone metabolism except for a history of chronic alcohol consumption and poor nutritional intake. He had received calcium and vitamin D supplements for variable period earlier.
On examination, he had kyphosis, loss of chest volume, and pectus carinatum. He had limitation of movement of the right hip joint and tenderness over all muscle groups. Skeletal survey showed diffuse rarefaction of the bones, looser zone in pubic ramii and scapula, fractures over multiple ribs, neck of left humerus, and neck of the right femur. DEXA scan of the lumbar spine showed T-score of -1.8 with increased osteoblastic activity at multiple sites on 99mTc MDP bone scan. He had remarkable relief in bone pains with CD treatment during follow-up of six months. The fracture site showed a good callous response except for right hip joint, for which he underwent total hip replacement [Figure 4].
Figure 4.
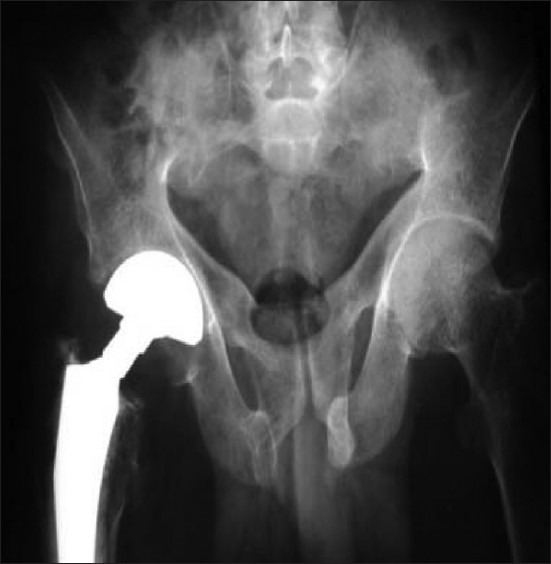
X-ray pelvis showing right hip prosthesis after replacement
Case 4: A 45-year-old male presented with inability to bear weight on his lower limbs and unable to walk since 8 months. Fragility fractures of bilateral neck of femur were detected. He was obese with a BMI of 31.2 kg/m2, non- alcoholic, non-smoker, no history of chronic drug intake, and had no comorbities. He denied any history of trauma, diarrhea, or shortness of breath.
On examination, he had no palpable bony deformities. He had normal liver, renal function tests, and he was euthyroid and eugonadal. Skeletal survey revealed bilateral fractures of neck of femur and 99mTc MDP bone scan suggested increased osteoblastic activity at fracture sites. DEXA scan showed T score of -2.26 over lumbar spine and Z-score of -1.9. He sustained no new fractures with gluten-free diet over 1 year of follow-up and gradually improved with restoration of his weight bearing capacity.
Case 5: A 14-year-old boy presented with multiple recurrent pathological fracture of long bones of lower limbs, over the past 6 years. He had poor exposure to sunlight and poor consumption of dairy products. He had received intermittent calcium and vitamin D supplement without significant improvement. He denied any history of childhood diarrheal illness.
On examination, he was normal built with early pubertal changes suggestive of Tanner's stage 3. He was crippled because of lower extremity fractures with obvious deformities of lower limb. He had normal liver, renal function tests, and he was euthyroid. Skeletal survey revealed bilateral fractures of lower end of femur. His pelvic X-ray revealed bilateral protrusio acetabuli. DEXA scan showed Z-score of -3.76 over lumbar spine and -3.9 at femur neck. After starting treatment he has sustained no new fractures over 1 year of follow-up and was able to walk again without support.
DISCUSSION
We have shown that the patients of CD can present with various musculoskeletal signs and symptoms like bone pains, proximal muscle weakness, osteomalacia, osteopenia, osteoporosis, and even pathological fractures. All these patients had monosymptomatic presentation in the form of severe MBD without any obvious gastrointestinal manifestations. They had decreased BMD with three of them having florid osteomalacia and all of them had osteopenia and/or osteoporosis. None of our patients had gastrointestinal manifestations and were being treated by orthopedicians and internists without a suspicion of CD. Severe MBD (osteoporosis and fractures) has been reported to occur only when manifestation of CD is florid.[2] Various case–control studies and population-based surveys have presented contrasting evidence about the risk for fractures in CD.[11–19] A recent meta-analysis, however, suggested that there is a definite increased risk of fractures by 43% in patients with CD.[20]
Our patients presented with primarily skeletal complaints mainly in the form of fragility fractures without any obvious gastrointestinal manifestations. Four of the five patients had additional risk factors for MBD like the first patient had become symptomatic after menopause and severity of symptoms forced her to remain indoors. The second patient was on antiepileptics for many years before her presentation. The third patient reported chronic alcohol consumption associated with poor dietary intake. The fourth patient had no predisposing factor for MBD. Fifth patient had poor sunlight exposure and milk product intake, though he was vitamin D sufficient due to recent cholecalciferol supplementation. The calcium profile, 25 (OH) D, and iPTH levels in two patients (cases 1 and 2) suggested osteomalacia due to vitamin D deficiency with secondary hyperparathyroidism and a mix of osteomalacia and osteoporotic fractures. The third patient (case 3) presented with severe osteomalacic myopathy and the other two patients presented with pathological fractures. The loss of BMD was maximum in first case but was significantly abnormal in rest of the cases also. In all the patients, serology was suggestive of CD and duodenal biopsy was consistent with CD in all the four patients who underwent doudenoscopy.
CD affects bone mineral metabolism by multiple mechanisms. Etiopathogenesis of osteoporosis may differ according to whether the presentation is classic or atypical. The mild, asymptomatic forms may reduce bone mass by a different mechanism related to the secretion of inflammatory cytokines with increase in interferon-, IL- 6, TNFα, and IL-18 and reduced levels of IL-12. The inflammatory cytokines (IL-1, IL-6, and TNFα) increase bone resorption, by acting directly on the osteoclasts or increasing the RANKL/OPG ratio.[21] However, the classic severe forms of CD with evident malabsorption cause reduction in plasma calcium, vitamin D levels, and vitaminD transporting protein (calbindin). These deficits lead to secondary hyperparathyroidism, which in turn leads to increased bone remodeling resulting in reduced bone mass, alteration of bone quality, with the consequent reduction in bone strength, and risk of fractures. Other endocrine factors that may contribute to this process are a reduction in IGF-1 related to the malabsorption of zinc and reduced levels of leptin.[22]
Our patients had an unusually severe bone involvement despite having no gastrointestinal symptoms. Four of the five patients had one or more additional factors contributing for metabolic bone disorder like post menopausal state, antiepileptic drug use, chronic alcohol consumption and malnutrition, associated vitamin D deficiency, and delay in diagnosis which could have contributed to aggressiveness of the bone disease. DEXA scan was useful to quantitate bone loss in our CD patients. However, considering the high prevalence of CD (1%) and the modestly increased risk of fracture, densitometric studies of all celiac patients, as in menopausal women, are not considered cost-effective.[5] In the absence of evidence-based studies, densitometric studies can be done only in subgroups at high risk of fracture like non-compliant patients or those in whom a gluten-free diet has failed, patients treated with corticosteroids, hypogonadism, age older 70 years, BMI lower 20, and previous fragility fractures.[23] However, as CD may be silent, with osteoporosis as the first manifestation like in our patients, some authors suggest the need for celiac screening in all osteoporotic patients, or at least those who fail treatment.[24]
The specific indications for treatment for osteoporosis in patients with CD are fragility fracture and low BMD with an increased risk of fracture. Gluten-free diet alone in CD patients has been shown to cause significant increase in BMD and results similar to those achieved by bisphosphonates in the treatment of post-menopausal osteoporosis, with a reduction in bone remodeling.[25,26] Gluten-free diet was also associated with reduction in PTH levels, improvement in vitamin D levels, and other biochemical parameters.[27,28] However, there are no systematic data on the efficacy of bisphosphonates for the treatment of osteoporosis in patients with CD. Zoledronate given as a yearly dose showed efficacy in reducing vertebral, non-vertebral and hip fractures in post-menopausal women including few with CD.[29] Recently, Widjaja et al. have reported improvement in T-score and Z-score in a single patient of CD with osteoporosis after 18 months of oral alendronate use in a dose of 10 mg/day.[30] All our patients showed remarkable improvement in clinical, biochemical, and radiological parameters with gluten-free diet, calcium, and vitamin D supplementation.
To conclude, we have reported five patients of CD with symptomatic MBD without any other gastrointestinal manifestations. Four of the patients had additional risk factors which might have contributed to the severity of metabolic bone disease. Bone manifestations were readily correctable on administration of gluten-free diet. CD should be looked for routinely in patients presenting with unexplained metabolic bone disease.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;257:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 2.Bhadada SK, Bhansali A, Kochhar R, Menon AS, Sinha SK, Dutta P, et al. Does every short stature child need screening for celiac disease? J Gastroenterol Hepatol. 2008;23:e353–6. doi: 10.1111/j.1440-1746.2007.05261.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhadada SK, Bhansali A, Ravikumar P, Kochhar R, Nain CK, Dutta P, et al. Changing scenario in aetiological profile of short stature in India-growing importance of celiac disease: A study from tertiary care centre. Indian J Pediatr. 2011;78:41–4. doi: 10.1007/s12098-010-0227-6. [DOI] [PubMed] [Google Scholar]
- 4.Bhadada SK, Kochhar R, Bhansali A, Dutta U, Kumar PR, Poornachandra KS, et al. Prevalence and clinical profile of celiac disease in type 1 diabetes mellitus in north India. J Gastroenterol Hepatol. 2011;26:378–81. doi: 10.1111/j.1440-1746.2010.06508.x. [DOI] [PubMed] [Google Scholar]
- 5.Pérez-Castrillón JL, Andres-Calvo M, Izquierdo-Delgado E, Mendo M, De Luis D, Dueñas-Laita A. Celiac disease and osteoporosis: A review. Open Bone J. 2009;1:23–7. [Google Scholar]
- 6.Karakan T, Ozyemisci-Takisran O, Gunendi Z, Atalay F, Tuncer C. Prevalence of IgA-antiendomysial antibody in a patient cohort with idiopathic low bone mineral density. World J Gastroenterol. 2007;13:2788–92. doi: 10.3748/wjg.v13.i21.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Study Group on Assessment of Fracture Risk and Its Application to Screening and Postmenopausal Osteoporosis. Report of a WHO Study Group. Technical Report Series (No. 84) 1994 [PubMed] [Google Scholar]
- 8.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of celiac disease: Time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–94. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Walker Smith JA Working Group of European Society of Pediatric Gastroenterology and Nutrition. Revised criteria for diagnosis of celiac disease. Report of working group of European society of pediatric gastroenterology and nutrition. Arch Dis Child. 1990;65:909–11. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravikumara M, Tuthill DP, Jenkins HR. The changing clinical presentation of Celiac disease. Arch Dis Child. 2006;91:969–71. doi: 10.1136/adc.2006.094045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vestergaard P, Mosekilde L. Fracture risk in patients with celiac disease, crohn's disease and ulcerative colitis: A nationwide follow-up study of 16,416 patients in Denmark. Am J Epidemiol. 2002;156:1–10. doi: 10.1093/aje/kwf007. [DOI] [PubMed] [Google Scholar]
- 12.West J, Logan RF, Card TR, Smith C, Hubbard R. Fracture risk in people with celiac disease: A population-based cohort study. Gastroenterology. 2003;125:429–36. doi: 10.1016/s0016-5085(03)00891-6. [DOI] [PubMed] [Google Scholar]
- 13.Vazquez H, Mazure R, Gonzalez D, Flores D, Pedreira S, Niveloni S, et al. Risk of fracture in celiac disease patients: A cross-sectional, case-control study. Am J Gastroenterol. 2000;95:183–9. doi: 10.1111/j.1572-0241.2000.01682.x. [DOI] [PubMed] [Google Scholar]
- 14.Fickling WE, McFarlane XA, Bhalla AK, Robertson DA. The clinical impact of metabolic bone disease in celiac disease. Postgrad Med J. 2001;77:33–6. doi: 10.1136/pmj.77.903.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno ML, Vazquez H, Mazure R, Smecuol E, Niveloni S, Pedreira S, et al. Stratification of bone fracture risk in patients with celiac disease. Clin Gastroenterol Hepatol. 2004;2:127–34. doi: 10.1016/s1542-3565(03)00320-3. [DOI] [PubMed] [Google Scholar]
- 16.Davie MW, Gaywood I, George E, Jones PW, Masud T, Price T. Excess non-spine fractures in women over 50 years with celiac disease: A crosssectional, questionnaire-based study. Osteoporos Int. 2005;16:1150–5. doi: 10.1007/s00198-004-1822-z. [DOI] [PubMed] [Google Scholar]
- 17.Thomason K, West J, Logan RF, Coupland C, Holmes GK. Fracture experience of patients with celiac disease: A population based survey. Gut. 2003;52:518–22. doi: 10.1136/gut.52.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludvigsson JF, Michaelsson K, Ekbom A, Montgomery SM. Coeliac disease and the risk of fractures. A general population based cohort study. Aliment Pharmacol Ther. 2007;25:273–85. doi: 10.1111/j.1365-2036.2006.03203.x. [DOI] [PubMed] [Google Scholar]
- 19.Jafri MR, Nordstrom CW, Murray JA, Van Dyke CT, Dierkhising RA, Zinsmeister AR, et al. Long-term fracture risk in patients with celiac disease: A population-based study in Olmsted County, Minnesota. Dig Dis Sci. 2008;53:964–71. doi: 10.1007/s10620-007-9976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olmos M, Antelo M, Vazquez H, Smecuol E, Mauriño E, Bai JC. Systematic review and meta-analysis of observational studies on the prevalence of fractures in celiac disease. Dig Liver Dis. 2008;40:46–53. doi: 10.1016/j.dld.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 21.McCormick MN, Pacifici R. The role of T lymphocytes in bone metabolism. Immunol Rev. 2005;208:154–68. doi: 10.1111/j.0105-2896.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 22.Jameson S. Coeliac disease, insulin-like growth factor, bone mineral density and zinc. Scand J Gastroenterol. 2000;35:894–6. [PubMed] [Google Scholar]
- 23.Compstom J. Is fracture risk increased in patients with celiac disease? Gut. 2003;52:459–60. doi: 10.1136/gut.52.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchman AL. Population-based screening for celiac disease.Improvement in morbidity and mortality from osteoporosis. Arch Intern Med. 2005;116:370–2. doi: 10.1001/archinte.165.4.370. [DOI] [PubMed] [Google Scholar]
- 25.Valdimarsson T, Löfman O, Toss G, Ström M. Reversal of osteopenia with diet in adult celiac disease. Gut. 1996;38:323–7. doi: 10.1136/gut.38.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mataulen C, Gonzalez D, Mazure R, Vázquez H, Lorenzetti MP, Maurino E, et al. Effect of treatment on bone mass, mineral metabolism and body composition in untreated celiac disease patients. Am J Gastroenterol. 1997;92:313–8. [PubMed] [Google Scholar]
- 27.Kemppainen T, Kröger H, Janatuinen E, Arnala I, Lamberg-Allardt C, Kärkkäinen M, et al. Bone recovery after gluten-free diet: A 5-year follow-up study. Bone. 1999;25:355–60. doi: 10.1016/s8756-3282(99)00171-4. [DOI] [PubMed] [Google Scholar]
- 28.Sategna-Guidetti C, Grosso SB, Grosso S, Mengozzi G, Aimo G, Zaccaria T, et al. The effects of 1- year gluten withdrawal on bone mass, bone metabolism and nutritional status in newly-diagnosed adult celiac disease patients. Aliment Pharmacol Ther. 2000;14:35–43. doi: 10.1046/j.1365-2036.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- 29.Black DM, Delmas P, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zolendronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 30.Widjaja D, Kannegantia KC, Patela M, Chilimuria SS. Role of Alendronate in managing osteoporosis in celiac disease - illustrative case report. Gastroenterol Res. 2011;4:26–9. doi: 10.4021/gr279w. [DOI] [PMC free article] [PubMed] [Google Scholar]


