Abstract
A 16-year-old girl presented with primary amenorrhea and excess hair growth on her body and face for the last three years, along with pain and a mass in her lower abdomen for last one year. Examination revealed hirsutism and other virilizing features, with an irregular mass in the lower abdomen corresponding to 16 weeks’gestation. Serum testosterone was 320 ng / dl and ultrasonogram of the pelvis revealed a solid mass of 5 × 4 cm in the left adnexa. Suspecting it to be a virilizing tumor of the left ovary, the patient was subjected to staging laparotomy, which revealed stage 1a ovarian involvement amenable to surgical resection alone. Histopathological examination confirmed the diagnosis of granulosa cell tumor of the ovary. Postoperatively the serum testosterone returned to 40 ng / dl and her menstrual cycle started after two months of surgery.
Keywords: Granulosa cell tumor, hirsutism, primary amenorrhea, virilization
INTRODUCTION
Hormone-producing ovarian tumors are a rare cause of hyperandrogenism in women. They account for 5–8% of all ovarian neoplasms[1] and the malignant ones account for less than 10% of all ovarian carcinomas.[2] They are also called sex cord stromal tumors and are composed of cells derived from sex cords or mesenchyme of the embryonic gonads. They contain granulosa cells, sertoli cells, leydig cells, and theca cells either singly or in combination. They account for most of the hormonally active neoplasms.[2] They are classified accordingly, that is, female-directed granulosa-theca cell tumors and male-directed sertoli-leydig cell tumors (arrhenoblastoma). Others include thecomas, fibroma, gynandroblastoma, sex-cord tumor with annular tubular, and unclassified tumors.
Unlike patients with common epithelial tumors, in which 75% are considered to be in the advanced stages at diagnosis, a majority of patients with these tumors are diagnosed at an early stage. And unlike common epithelial tumors, these tumors have more specific symptoms due to the production of hormones and are associated with signs of hyperestrogenism or hyperandrogenism.
Granulosa cell tumors usually produce estrogens, and hence, cause symptoms and signs of estrogen excess such as endometrial hyperplasia in 50% of the cases and adenocarcinoma uterus in 5–15% of the cases; mostly in perimenopausal and postmenopausal women.[3] However, the luteinizing forms may produce androgens leading to virilization.[4] To date, less than 50 cases of virilizing granulosa cell tumors have been reported in literature.[5] About seven cases have been in the prepubertal age.[5,6] In a majority of them it was the International Federation of Gynecology and Obstetrics (FIGO) stage 1 disease and treated surgically. We hereby report a rare case of granulosa cell tumor with primary amenorrhea and virilization presenting in a young individual.
CASE REPORT
A girl aged 16 years presented with complaints of primary amenorrhea and progressive virilization including hirsutism, deep hoarse voice, and acne for the last three years. For the last one year she also noticed pain and a mass per abdomen. She had no weight gain, proximal muscle weakness or other evidences of Cushing's syndrome. On examination, her weight was 45 kg and height 150 cm with a body mass index (BMI) of 20 kg/m2. Her vital parameters were normal. She had mild frontal baldness, a male-type beard, with acne present. There was excessive coarse black hair covering the chest, upper and lower arms, shoulders, back, abdomen, and legs with a Ferriman–Gallwey hirsutism score of 24. She had deepening of voice and her breasts were atrophied. She had no acanthosis nigricans. She had no features of Cushing's syndrome. Abdominal examination revealed irregular fixed mass corresponding to 16 weeks gestation in the pelvis and lower abdomen. The uterus could not be palpated separately. Per speculum examination revealed clitoromegaly, normal vagina, and cervix. There was a left adnexal mass of around 5 × 5 cm found on bimanual examination.
Hemoglobin, hematocrit, fasting blood glucose, lipid profile, serum electrolytes, and renal and liver function tests were normal. Tumor marker levels in the serum, including CA 125, CA 19-9, and alpha-fetoprotein (AFP) were normal. Her chest X-ray and electrocardiogram (ECG) detected no abnormalities. Endocrine evaluation revealed a markedly increased serum testosterone concentration (320 ng/ dL, normal 40 – 50 ng / dl), while the concentration of dehydroepiandrosterone sulfate was within normal range (170 μg / dL, normal 145 – 390 μg / dl). Serum prolactin (10 μg / dl, normal 2 – 25 μg / dl) and thyroid stimulating hormone (3.2 μIU / ml, normal 0.5 – 5.0 μIU / ml) were normal. Serum luteinizing hormone (11.4 mIU / ml, normal 2 – 15 mIU / ml ), follicle stimulating hormone (5.2 mIU / mL, normal 5 – 20 mIU / ml), and estradiol (51.0 pg / mL, normal 30 – 60 pg / ml) concentrations were within normal range.
Transvaginal ultrasound examination demonstrated the presence of a well-defined, solid, movable, and homogenous echoic mass (5.5 × 4.9 cm), originating from the left ovary. No pathological findings were found within the right ovary and uterus. No ascites were detected. Abdominal and pelvic computed tomography (CT) scans revealed a left ovarian solid mass measuring 5.5 × 5.0 cm in diameter [Figure 1]. The mass was well-demarcated, round, homogenous, and well-enhanced. No pathological findings of other pelvic organs, ascites or lymphatic enlargements were detected.
Figure 1.
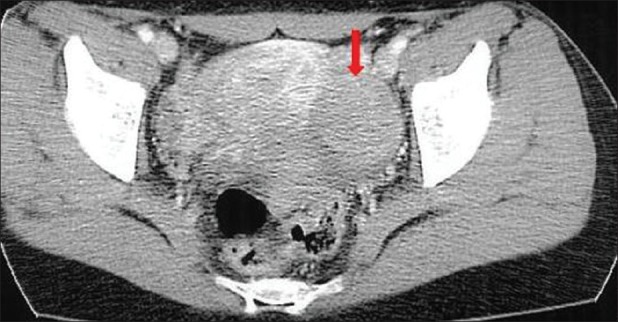
Abdominal and pelvic computed tomography scans demonstrating a left ovarian solid mass (5.5 × 5.0 cm) which was well-demarcated, round, homogenous and well-enhanced. No pathologic findings of other pelvic organs, ascites, and lymphatic enlargements were detected
She underwent staging laparotomy. At laparotomy, a bosselated, yellowish, solid, and movable tumor was found to originate in the left ovary, and measured 6 × 5.5 cm. The uterus and right ovary were macroscopically normal. No pelvic adhesion, ascites or findings of dissemination from the malignant ovarian tumor to the omentum or other organs were observed in the abdominal cavity. This confirmed the stage of the tumor to be Federation of Gynecologists and Obstetricians (FIGO) Stage 1a. Histopathological examination of the left ovarian tumor confirmed the diagnosis of granulosa cell tumor with focal luteinization [Figure 2]. The postoperative course was uncomplicated. She was discharged from the hospital without any complications on the fourth day after surgery. Two weeks after surgery, the serum testosterone level was reduced to 40 ng / dL. She had a normal menstruation two months later. Follow-up after three months revealed no abnormality.
Figure 2.
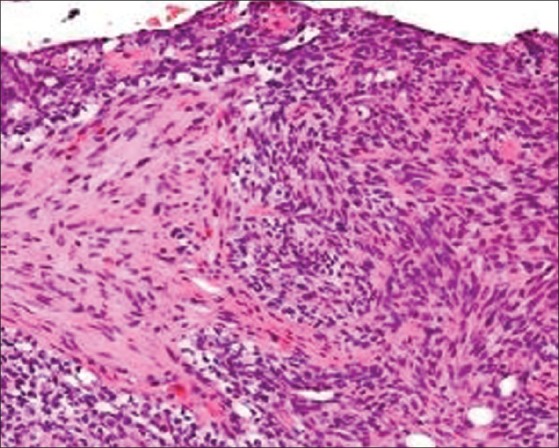
Histopathology study of the biopsy specimen revealing characteristic granulosa cell tumor with focal luteinization (H and E, X300)
DISCUSSION
Two aspects render our case unusual (1) young age of presentation and (2) manifestation with primary amenorrhea and virilization. It is our belief that this is the first such case report from India.
Granulosa cell tumors are the most common variety of sex cord stromal cell tumors. There are two varieties of granulosa cell tumors based on histological characteristics, that is, juvenile and adult granulosa cell tumors. They are characterized by low malignant potential, and late recurrences are known to occur. A majority of them secrete estrogen, with 15% being hormonally inert.[3] Nonmalignant disorders usually follow a more slowly progressive benign course, as compared to the rapid progressive virilization seen with the underlying malignancy. Simple clinical assessment and a single serum testosterone measurement are sufficient in most cases, to differentiate between benign and malignant virilizing tumors in women presenting with hirsutism and androgenic features.[7]
Granulosa cell tumors usually affect females in their 50s.[4] Juvenile granulosa cell tumors occur under 20 years of age. They can present at a very young age with precocious puberty. Ovarian stromal cell tumors may also secrete large amounts of testosterone, which may produce rapidly progressing androgenic effects.[8] Theca cell tumors are other sources leading to hyperandrogenism, although these tumors usually appear to be estrogenic. Androgen secretion of an ovarian tumor before menarche results in heterosexual precocity, with virilizing manifestation, and accelerated somatic growth.[8] During the reproductive age the typical picture of androgen secretion is oligomenorrhea, defeminization, and progressive masculinization (hirsutism, temporal balding, enlargement of the clitoris, deepening of the voice, and muscular development).[9] In the case of a 24-year-old woman described by Takeuchi and colleagues,[10] masculinization developed over a few months. Symptoms of defeminization and masculinization may appear simultaneously, but signs of defeminization usually appear before those of masculinization. Our patient had primary amenorrhea with virilization that developed gradually over a period of three years. In most cases the hormonal abnormalities present in patients with virilizing ovarian tumors include increased serum testosterone levels in the presence of normal levels of serum dehydroepiandrosterone sulfate. Similar was the scenario in our case.
Surgical treatment is the method of choice. In postmenopausal women total abdominal hysterectomy with bilateral salpingo-oopherectomy is the treatment.[1] In young and premenopausal women desirous of preservation of fertility, unilateral salpingo-oopherectomy and fractional curettage can be done. After surgery, symptoms of defeminization may disappear. Four months after the surgery, menstruation begins, and other symptoms disappear in the order of their appearance, but enlargement of the clitoris may last for 20 years. Our patient began her menstruation two months after the surgery. Prognosis is excellent with 90 – 95% five-year survival with early disease. Prognosis correlates with stage of disease, tumor grade and mitotic index, and bulk of residual disease. The prognosis of our patient is good and we have not observed any relapse during the follow-up. Follow-up must occur at two- to three-month intervals for the first two years, for patients not undergoing chemotherapy. Subsequently, this can be spaced out to every four to six months for the next three years, and then yearly thereafter. The follow-up visits should include history, pelvic examination, serum determination of tumor markers (if these were elevated preoperatively or immediately postoperatively), and abdominopelvic computed tomography (CT) to look for recurrent tumors (if any evidence of recurrence arises during follow-up). Late recurrences are known to occur 20-30 years after initial treatment, and hence, long-term follow-up is needed.
CONCLUSION
Granulosa cell tumors are most commonly associated with estrogenic features. However, they can rarely manifest with virilizing features. Early diagnosis followed by surgical treatment results in good prognosis.
ACKNOWLEDGMENT
All the authors extend their heartfelt thanks to Mrs Sruti Jammula, M Pharm, PhD, Dr. Jagadeesh Tangudu, M Tech, MS, PhD and Mrs Sowmya Jammula, M Tech for their immense and selfless contribution toward the manuscript preparation, language editing, and final approval of text.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Abound EA. Review of granulosa cell tumors and the comas of the ovary. Arch Gynecol Obstet. 1997;259:161–5. doi: 10.1007/BF02505327. [DOI] [PubMed] [Google Scholar]
- 2.Stegner HE, Loning T. Endocrine-active tumors of the ovary. Pathologe. 2003;24:314–22. doi: 10.1007/s00292-003-0622-0. [DOI] [PubMed] [Google Scholar]
- 3.Takemori M, Nishimura R, Hasegawa K. Ovarian thecoma with ascites and high serum levels of CA 125. Arch Gynecol Obstet. 2000;264:42–4. doi: 10.1007/pl00007485. [DOI] [PubMed] [Google Scholar]
- 4.Keeney GL. Ovarian tumors with endocrine manifestations. In: De Groot LJ, editor. Endocrinology. 4th ed. Philadelphia, PA: WB Saunders Company; 2001. p. 2172. [Google Scholar]
- 5.Pautrier P, Lhomme C, Culine S, Duvillard P, Michel G, Bidart JM, et al. Adult granulose cell tumor of the ovary: A retrospective analysis study of 45 cases. Int J Gynecol Cancer. 2003;7:58–65. doi: 10.1046/j.1525-1438.1997.00417.x. [DOI] [PubMed] [Google Scholar]
- 6.Castro CV, Malpica A, Hearne RH, Silva EG. Androgenic adult granulose cell tumor in a 13 year old prepubertal patient. Int J Gynecol Pathol. 2000;19:266–71. doi: 10.1097/00004347-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 7.O’Driscoll JB, Mamtora H, Higginson J, Pollock A, Kane J, Anderson DC. A prospective study of the prevalence of clear-cut endocrine disorders and polycystic ovaries in 350 patients presenting with hirsutism or androgenic alopecia. Clin Endocrinol (Oxf) 1994;41:231–6. doi: 10.1111/j.1365-2265.1994.tb02535.x. [DOI] [PubMed] [Google Scholar]
- 8.Loh KC, Lo JC, Zaloudek CJ, Fitzgerald PA. Occult virilizing ovarian tumours in postmenopausal women: Problems in evaluation with reference to a case. Ann Acad Med Singapore. 1998;27:712–6. [PubMed] [Google Scholar]
- 9.Siekierska-Hellmann M, Sworczak K, Babinńska A, Wojtylak S. Ovarian thecoma with androgenic manifestations in a postmenopausal woman. Gynecol Endocrinol. 2006;22:405–8. doi: 10.1080/09513590600842539. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi S, Ishihara N, Ohbayashi C, Itoh H, Maruo T. Stromal Leydig cell tumor of the ovary.Case report and literature review. Int J Gynecol Pathol. 1999;18:178–82. doi: 10.1097/00004347-199904000-00014. [DOI] [PubMed] [Google Scholar]


