Abstract
Neuroendocrine carcinomas (NEC) of the female genital tract are aggressive and uncommon tumors, which usually involve the uterine cervix and ovary, and are seen very rarely in the endometrium. Only less than 10 cases of large cell NEC (LCNEC) of the endometrium have been reported in the literature and their radiological findings are not well described. We report here two cases of pathologically proven LCNEC of the uterine endometrium. In both cases, the uterine body was enlarged and the tumor occupied part of the uterine cavity. Endometrial mass exhibited heterogeneous high intensity on T2-weighted magnetic resonance (MR) images, and diffusion-weighted MR images revealed high intensity throughout the tumor, consistent with malignancy. LCNEC is a highly malignant neoplasm without particular findings in terms of diagnostic imaging and pathology, so its preoperative definitive diagnosis is very difficult. However, when laboratory test, pathologic diagnosis and MR imaging suggest a poorly differentiated uterine malignancy, positron emission tomography-computed tomography scan should be performed as a general assessment to help with diagnosis.
Key words: large cell neuroendocrine carcinoma, endometrium, uterus, magnetic resonance images, PET/CT.
Introduction
Large cell neuroendocrine carcinoma (LCNEC) of the uterus, which is a very rare and aggressive tumor, occurs predominantly in the lung. Primary LCNEC, originating from the uterine endometrium, are extremely rare and the ones from the uterine cervix are even rarer. Only less than 10 cases of LCNEC originating from the uterine endometrium have been reported in the English literature.1–3
To the best of our knowledge, specific findings of LCNEC of the uterus with magnetic resonance (MR) imaging and/or 18F-fluorodeoxy glucose-positron emission tomography and computed tomography (FDG-PET/CT) have never been reported.
In this article, we demonstrate the MR features of 2 cases with pathologically proven LCNEC of the uterine endometrium, without apparent primary tumor in the other organs, including the lung.
Case Report #1
A 73-year-old woman (gravida 2, para 2), having no history of gynecologic disorders, visited a local hospital with lumbago and abdominal distention and was referred to the Kobe University Hospital as soon as the presence of large uterine tumor was detected by computed tomography (CT) imaging. The histopathological examination of the biopsy specimen from the endometrium suggested a diagnosis of LCNEC, and the one from the cervix had no malignant finding. Physical examination revealed that she had enlarged uterus and right supraclavicular lymphadenopathy. The laboratory tests were unremarkable, except for the elevation of serum neuronspecific enolase (NSE; 630 ng/mL; reference level, <12 ng/mL), cancer antigen 125 (202 U/mL; reference level, <35 U/mL), lactate dehydrogenase (3038 IU/L; reference level, 115∼217 IU/L), and interleukin receptor-2 (767 U/mL; reference level, 122∼466 U/mL). MR images of lower abdomen, but not of the upper, was performed. It demonstrated a diffusely swollen endometrial mass exhibiting hypointensity to myometrium on T1-weighted images, and slightly high heterogeneous intensity on T2-weighted images (Figure 1A). The tumor involved both the myometrium and endometrium. Extensive pelvic and paraaortic lymphadenopathies were also identified. On diffusion weighted imaging the mass and lymph nodes showed remarkably hyperintensity (Figure 1B). Following above, whole body FDG-PET/CT was performed; it revealed increased FDG uptake in the uterus, multiple lymph nodes and bones (Figure 1C). Because the metastasis to right supraclavicular lymph node and bones were identified, the clinical stage was determined to be 4b. The surgery was not performed, because of the aggressive nature and advanced stage of the tumor. The patient refused the systemic chemotherapy, so shifted to palliative care. Five weeks after her first visit to our hospital, she died of respiratory failure. In autopsy examination, the uterus was diffusely enlarged, and accompanied with a 6 cm sized submucosal type leiomyoma. Microscopically, neoplastic cells with prominent nucleoli arranged in nesting pattern showing extensive areas of geographic necrosis. In addition, the tumor cells exhibited remarkable increase of mitotic counts and immunohistochemistry revealed diffuse positivity for synaptophysin, chromograninA (Figure 2), NSE and p53. A diagnosis of LCNEC of the endometrium was made.
Figure 1.
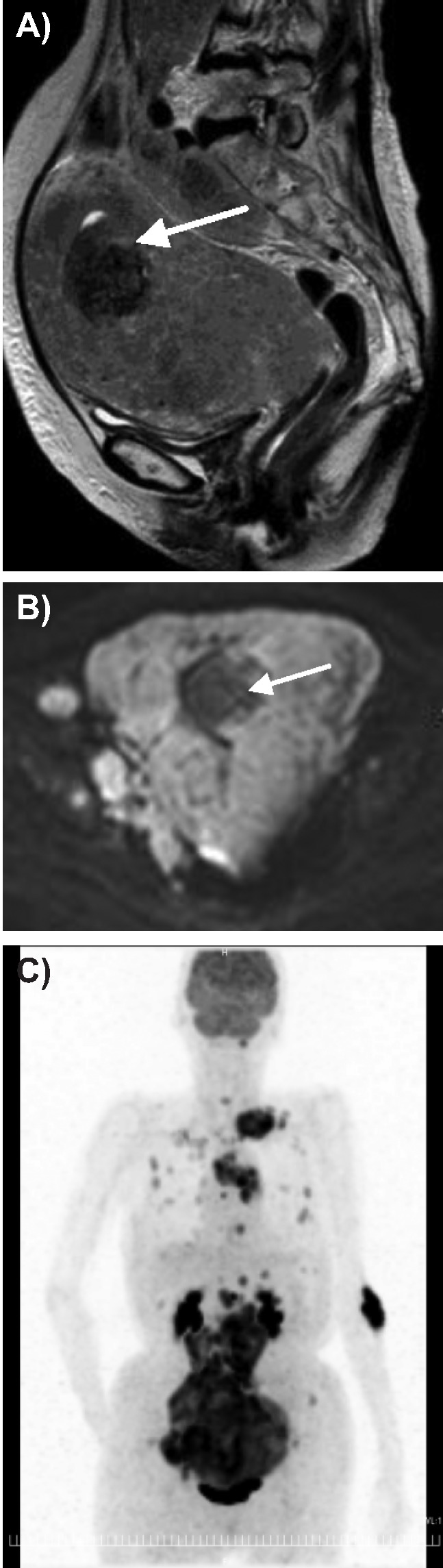
A) T2 weighted magnetic resonance image shows the mass hyperintense compared with myometrium and much more intense of submucosal myoma (arrow); B) diffusion weighted imaging, the mass and lymphnode show remarkable hyperintensity, and submucosal myoma (arrow) with hypointensity; C) positron emission tomography and computed tomography image, uterine mass, many lymph nodes and bones of whole body show strong 18F-fluorodeoxy glucose uptake.
Figure 2.
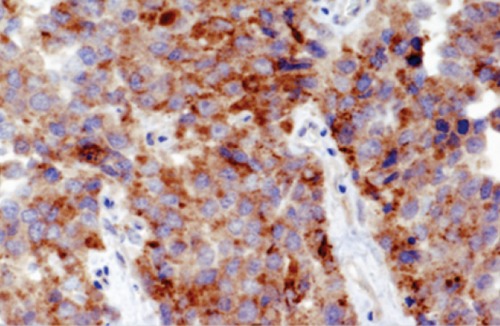
Photomicrograph of synapto-physin immunohistochemical staining (original magnification ×100).
Case Report #2
A 73-year-old woman (gravida 6, para 3), having no history of gynecologic disorders, visited a local hospital with heavy discharge and genital bleeding, and was than referred to our hospital.
The histopathological diagnosis of endometrial biopsy was adenocarcinoma with solid poorly differentiated component, which showed focal positivity of CD56 suggesting neuroendocrine differentiation.
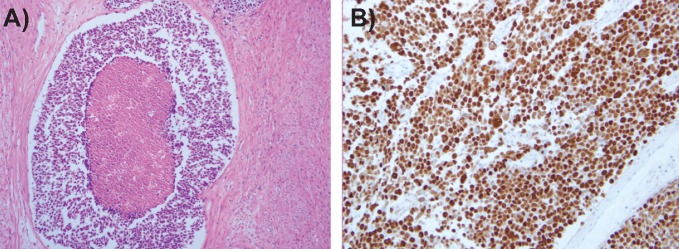
Physical examination revealed that she had an enlarged uterus. The laboratory tests were unremarkable, except for the elevation of serum lactate dehydrogenase (279 IU/L; reference level, 115∼217 IU/L). The MR images demonstrated a large endometrial mass of heterogeneous high intensity expanding in the endometrial cavity on T2-weighted images (Figure 3A), with part of the mass infiltrating to the uterine serosa throughout the myometrium. The mass revealed hypointensity including high intensity foci, suggestive of intratumoral hemorrhage, on T1-weighted images. Contrast-enhanced T1-weighted MR images showed heterogeneously enhanced tumor (Figure 3B), and diffusion-weighted MR images revealed high intensity throughout the tumor (Figure 3C), consistent with a malignant uterine tumor. Paraaortic lymphadenopathies were identified on the abdominal CT. She underwent abdominal total hysterectomy with bilateral salpingo-oophorectomy, omentectomy, and pelvic-paraaortic lymphadenectomy. Grossly, the uterine cavity was dilated and showed a white tumor involving the fundic region, and the tumor infiltrated to the myometrium deeply. The isthmic and cervical areas were uninvolved, and the bilateral adnexa and omentum were free of metastasis. Microscopically, the carcinoma displays a neuroendocrine morphology, including organoid nesting with large zones of necrosis, trabecular growth, rosettes and peripheral palisading patterns (Figure 4A). Immunohistochemistry revealed diffuse positivity for synaptophysin, chromogranin, CD56 and p53 (Figure 4B). The pathologic diagnosis was appropriate for large cell neuroendocrine carcinoma of the endometrium, and because of the metastasis to right internal iliac lymph nodes and left external iliac lymph node, the final stage was 3c. She was thereafter treated by chemotherapy (intravenous cisplatin; 60 mg/m2 and irinotecan; 60 mg/m2). After 6 cycles of chemotherapy, this patient was disease-free during 6 months. However, FDG-PET/CT, which was performed 13 months after the operation, revealed increased FDG uptake in paraaortic lymph nodes, being suspected of the recurrence.
Figure 3.
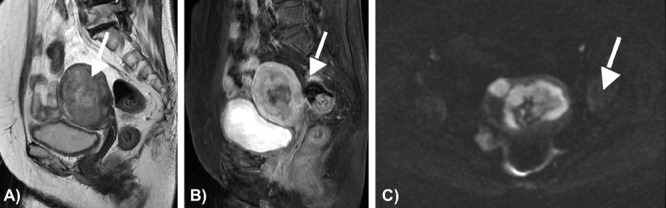
A) T2 weighted image shows the mass with heterogeneous high intensity (arrow); B) contrast enhanced T1 weighted image shows heterogeneous enhancement of the tumor (arrow); C) diffusion-weighted image shows high intensity throughout the tumor (arrow).
Figure 4.
A) Histologic finding of large cell neuroendocrine carcinoma the uterus. The primary tumor showing extensive necrosis and organoid nesting pattern (×100); B) photomicrograph of p53 immunohistochemical staining (original magnification ×200).
Discussion
LCNEC usually develops in the lungs. In the gynecological organs, LCNEC generally affects in the uterine cervix and ovary, and much less rarely in the uterine endometrium.1–3 According to the World Health Organization classification, neuroendocrine tumors in the uterine cervix are categorized into the 4 categories of typical carcinoid, atypical carcinoid, small cell carcinoma (SmCC) and LCNEC. LCNEC in the uterine endometirum is extremely rare and represents less than 1% of all primary endometrial carcinomas.4,5 Mulvany and Allen reported that patients with LCNEC of the uterine endometrium had a mean age of 75 years (50–88 years), and they were older than patients with LCNEC of the uterine cervix.1
As we measured serum NSE values in these two cases, it was found that the value of the Case #1 was raised and that of the Case #2 was within normal limit. Therefore, it is hard to say that these values are useful markers in diagnosis of LCNEC.
Kiyokawa et al. cited 6 cytological features of LCNEC of the uterine cervix as the following:6 i) presence of cell necrosis, ii) dispersion of tumor cells, some of which forming loosely arranged clusters with irregular stratification or in file-like fashion, iii) occasional nuclear protrusion at the periphery of clusters, iv) high N/C ratio with round to oval nuclei, 3 to 6 times in size of lymphocytes, v) increased coarse granular chromatin and 1 to 3 prominent nucleoli, and vi) which cytoplasm faintly stained light green and ill-defined cell border. These features are similar to those of LCNEC in other organs such as the lungs and closely resemble poorly differentiated adenocarcinoma, making the two types of tumors extremely hard to differentiate especially by small biopsy specimens.6–8 In fact, Case #2 was diagnosed as poorly differentiated carcinoma according to preoperative endometrial sampling. In such cases, immunohistochemical staining for chromogranin A, CD56, or synaptophysin may help to confirm neuroendocrine differentiation.
To the best of our knowledge, specific image findings of LCNEC of the uterus have never been reported. In both of the present cases, the uterine body was enlarged and the tumor occupied part of the uterine cavity. On T2-weighted MR images, the tumor involved both the myometrium and endometrium, and ill-defined endometrial-myometrial border was revealed. Endometrial mass exhibited heterogeneous high intensity on T2-weighted MR images, and this heterogeneity may represent necrosis and diffuse hemorrhage in the tumors.9 Moreover, diffusion-weighted MR images revealed high intensity throughout the tumor, consistent with malignancy. These MRI findings of the 2 cases were somewhat similar to the MR image findings for SmCC, having many similarities to LCNEC in their genetic characteristics of the uterus body and any finding was not specific to LCNEC.8–10 These MRI findings of neuroendocrine carcinoma have been found to mimic those of type cancer including poorly differentiated endometrial adenocarcinoma, which is another malignant tumor of the uterine corpus that invades the endometrium, or malignant lymphoma, uterine sarcoma, and metastatic cancer.9,11,12 In actuality, the image findings in Case #1 led to a diagnosis of malignant lymphoma, while the image findings in Case #2 led to a diagnosis of endometrial cancer with myometrial invasion, respectively. PET/CT is a functional imaging method of metabolic processes and is being used extensively in gynecologic oncology, which offers important information for the pre-, intra-, and postoperative management.13 In the current Case #1, actually, FDG-PET/CT revealed increased FDG uptake in the systemic metastases as well as in the primary uterine tumor, and in Case #2, it detected the recurrent lesion of the para-aortic lymph node. In patients with known malignant gynecologic lesions, therefore, PET may contribute to confirm the presence or absence of distant metastases at other sites and may assist in the selection of an appropriate treatment. NEC including LCNEC of the uterine body is known to have rare incidence, poor prognosis and no established treatment. In the treatment, patients should be given multimodality therapy including surgery, chemotherapy, and radiotherapy. As for the chemotherapy, patients are generally received six cycles of cisplatin (60 mg/m2, day 1) and etoposide (60 mg/m2, day 1, 8, and 15).2,7,10,14 In Case #1, because the patient had numerous metastases throughout her body, it was impossible for her to undergo surgery. Administration of chemotherapy was considered, but due to worsening of the patient's performance status, any active treatment was impossible. So, only pain control was provided, and the patient died 5 weeks after her initial examination. The patient in Case #2 underwent surgery and postoperative adjuvant chemotherapy, but 6 months later PET scans revealed recurrence.
Conclusions
We encountered 2 cases of LCNEC originating from the endometrium, which is extremely rare and has an extremely poor prognosis. Adequate consensus has not been reached on the treatment of the NEC, and more accumulation of the reports is needed to establish treatment methods. LCNEC is a highly malignant neoplasm with no characteristic findings in terms of diagnostic imaging and pathology; so definitive diagnosis of LCNEC preoperatively is difficult. However, when laboratory test, pathologic diagnosis of endometrial tissue, and CT/MRI imaging suggest a poorly differentiated uterine malignancy, PET/CT scans should be performed as a general assessment to help with diagnosis.
References
- 1.Mulvany NJ, Allen DG. Combined large cell neuroendocrine and endometrial carcinoma of the endometrium. Int J Gynecol Pathol. 2008;27:49–57. doi: 10.1097/pgp.0b013e31806219c5. [DOI] [PubMed] [Google Scholar]
- 2.Albores-Saavedra J, Martinez-Benitez B, Luevano E. Small cell carcinomas and large cell neuroendocrine carcinomas of the endometrium and cervix: polypoid tumors and those arising in polyps may have a favorable prognosis. Int J Gynecol Pathol. 2008;27:333–9. doi: 10.1097/PGP.0b013e31815de006. [DOI] [PubMed] [Google Scholar]
- 3.Terada T. Large cell neuroendocrine carcinoma with sarcomatous changes of the endometrium: a case report with immunohistochemical studies and molecular genetic study of KIT and PDGFR. Pathol Res Pract. 2010;206:420–5. doi: 10.1016/j.prp.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Fattanneh A. T., Peter D. World Health Organization Classification of Tumors. Lyon: IARC press; 2003. Pathology and Genetics of Tumor of the Breast and Female Genital Organs. [Google Scholar]
- 5.Abeler VM, Kjørstad KE, Nesland JM. Undifferentiated carcinoma of the endometrium. A histopathologic and clinical study of 31 cases. Cancer. 1991;68(1):98–105. doi: 10.1002/1097-0142(19910701)68:1<98::aid-cncr2820680120>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Kiyokawa T, Yahagi A, Takahashi H, et al. Cervical neuroendocrine tumors of the non-small cell type: a report of 3 cases. Journal of the Japanese Society of Clinical Cytology. 2002;41:164–70. [Google Scholar]
- 7.Deodher KK, Kerkar RA, Suryawanshi P, et al. Large cell neuroendocrine carcinoma of the endometrium: An extremely uncommon diagnosis, but worth the efforts. J Cancer Res Ther. 2011;7:211–3. doi: 10.4103/0973-1482.82942. [DOI] [PubMed] [Google Scholar]
- 8.Okubo C, Minami Y, Tanaka R, et al. Analysis of differentially expressed genes in neuroendocrine carcinomas of the lung. J Thorac Oncol. 2006;1:780–6. [PubMed] [Google Scholar]
- 9.Tamai K, Koyama T, Saga T, et al. Small cell carcinoma of the uterine corpus: MR imaging and pathological correlation. J Comput Assist Tomogr. 2007;31:485–9. doi: 10.1097/01.rct.0000243452.33610.d4. [DOI] [PubMed] [Google Scholar]
- 10.Erhan Y, Dikmen Y, Yucebilgin MS, et al. Large cell neuroendocrine carcinoma of the uterine corpus metastatic to brain and lung: case report and review of the literature. Eur J Gynaecol Oncol. 2004;25:109–12. [PubMed] [Google Scholar]
- 11.Kido A, Togashi K, Koyama T, et al. Diffusely enlarged uterus: evaluation with MR imaging. Radiographics. 2003;23:1423–39. doi: 10.1148/rg.236035033. [DOI] [PubMed] [Google Scholar]
- 12.Goto N, Oishi-Tanaka Y, Tsunoda H, et al. Magnetic resonance findings of primary uterine malignant lymphoma. Magn Reson Med Sci. 2007;6:7–13. doi: 10.2463/mrms.6.7. [DOI] [PubMed] [Google Scholar]
- 13.Kitajima K, Murakami K, Kaji Y, Sugimura K. Spectrum of FDG PET/CT findings of uterine tumors. Am J Roentgenol. 2010;195:737–43. doi: 10.2214/AJR.09.4074. [DOI] [PubMed] [Google Scholar]
- 14.Shahabi S, Pellicciotta I, Hou J, et al. Clinical utility of chromogranin A and octreotide in large cell neuroendocrine carcinoma of the uterine corpus. Rare Tumors. 2011;3:e41–e41. doi: 10.4081/rt.2011.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]